Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Serum Soluble Scavenger Receptor A Levels are Associated with Delayed Cerebral Ischemia and Poor Clinical Outcome After Aneurysmal Subarachnoid Hemorrhage: A Prospective Observational Study
Authors Jiang F, Chen Z, Hu J, Liu Q
Received 25 August 2022
Accepted for publication 20 October 2022
Published 2 November 2022 Volume 2022:18 Pages 2529—2541
DOI https://doi.org/10.2147/NDT.S387487
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Taro Kishi
Feng Jiang,1,2 Zhicheng Chen,1,2 Jiemiao Hu,1,2 Qianzhi Liu1,2
1Department of Neurosurgery, Ningbo Hangzhou Bay Hospital, Ningbo, 315336, People’s Republic of China; 2Department of Neurosurgery, Ningbo Branch, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Ningbo, 315336, People’s Republic of China
Correspondence: Jiemiao Hu, Department of Neurosurgery, Ningbo Hangzhou Bay Hospital, 1155 Binhai, Ningbo, 315336, People’s Republic of China, Tel +86 0574 58981155, Email [email protected]
Objective: Scavenger receptor A (SRA), a pattern recognition molecule, is implicated in immune response after acute brain injury. We strived to identify serum soluble SRA (sSRA) as a potential biomarker of prognosis after aneurysmal subarachnoid hemorrhage (aSAH).
Methods: In this prospective observational study, we quantified serum sSRA levels of 131 aSAH patients and 131 healthy controls. A poor outcome was defined as extended Glasgow outcome scale (GOSE) scores of 1– 4 at 90 days after injury. Relations of serum sSRA levels to severity, delayed cerebral ischemia (DCI) and poor outcome were assessed using multivariate analysis. Predictive efficiency was determined via area under receiver operating characteristic curve (AUC).
Results: Serum sSRA levels were markedly higher in aSAH patients than in controls (median, 2.9 ng/mL versus 1.0 ng/mL; P < 0.001). Serum sSRA levels were independently correlated with Hunt-Hess scores (beta, 0.569; 95% confidence interval (CI), 0.244– 0.894; P = 0.001), modified Fisher scores (beta, 0.664; 95% CI, 0.254– 1.074; P = 0.002) and 90-day GOSE scores (beta, − 0.275; 95% CI, − 0.440– 0.110; P = 0.005). Serum sSRA levels independently predicted DCI (odds ratio, 1.305; 95% CI, 1.012– 1.687; P = 0.040) and a poor outcome (odds ratio, 2.444; 95% CI, 1.264– 4.726; P = 0.008), as well as showed significant accuracy for the discrimination of DCI (AUC, 0.753; 95% CI, 0.649– 0.857; P < 0.001) and a poor outcome (AUC, 0.800; 95% CI, 0.721– 0.880; P < 0.001). Its combination with Hunt-Hess scores and modified Fisher scores displayed significantly improved AUCs for predicting DCI and poor outcome, as compared to any of them (all P < 0.05).
Conclusion: There is a significant elevation of serum sSRA levels after aSAH, which in close correlation with illness severity, are independently associated with DCI and poor clinical outcome after aSAH. Hypothetically, SRA may regulate immune response in acute brain injury after aSAH and serum sSRA is presumed to be a potential prognostic biomarker of aSAH.
Keywords: aneurysm, subarachnoid hemorrhage, scavenger receptor A, delayed cerebral ischemia, prognosis, severity, biomarkers, mechanism
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a common medical urgency event, which has a high rate of morbidity and mortality.1 Clinically, Hunt-Hess scale and modified Fisher scale are the two conventional severity indicators of aSAH.2 Delayed cerebral ischemia (DCI) is a very common adverse event, which is associated with a poor outcome3 and whose prediction is difficult.4 Secondary brain injury following aSAH involves some crucial pathophysiological processes, including immune activation, subsequent inflammation reaction and final neuronal death.5 There is robust evidence showing that microglia are responsible for the innate immune response in the brain and participate in aSAH-induced neuroinflammation.6 Because measurement of biochemical markers in the peripheral blood allows easy obtainment of samples, neuroinflammation-related biomarkers, such as tumor necrosis factor alpha, interleukin-6, chemokine C-C motif ligand 5 and intercellular adhesion molecule-1, have attracted extensive attention in the last decade as prognostic parameters of aSAH.7–9
The scavenger receptor A (SRA, CD204), functioning as a pattern recognition molecule, is a prototypic member of a family of structurally diverse transmembrane receptors.10 SRA is expressed primarily on myeloid cells, such as macrophages and dendritic cells, and harbors both beneficial and detrimental potentials in host defense against microbial pathogens.11–13 Gradually, compelling data have shown that SRA can be expressed by microglia, as well as its expressions were significantly up-regulated in animal brain tissues after middle cerebral artery occlusion.14–16 Evidence has pointed out to regulatory effects of SRA in innate immune and inflammatory responses, thereby contributing to acute brain injury, including acute ischemic stroke and intracerebral hemorrhage.17–19 Collectively, SRA may be a biomarker of acute brain injury. Herein, serum soluble SRA (sSRA) levels were determined in a cohort of aSAH patients and healthy controls, and whereafter, we discovered the prognostic role of serum sSRA in aSAH.
Methods
Study Design and Ethics Approval
In this prospective and observational study performed at Ningbo Hangzhou Bay Hospital from May 2017 to March 2021, study subjects were composed of aSAH patients and controls. Patients with aSAH were consecutively enrolled, and controls were comprised of healthy volunteers. The study was done in compliance with the Declaration of Helsinki. The protocol of the current study was approved by Ethical Committee at Ningbo Hangzhou Bay Hospital (opinion number: 2016026). Written informed consent to participate in the study was acquired from patients’ relatives or controls themselves.
Participant Selection
Patients with aSAH were initially assessed in agreement with the following inclusion criteria: (1) first-ever spontaneous SAH; (2) age of 18 years or greater; (3) SAH as a result of single intracranial aneurysmal rupture; (4) hospital admission within 24 hours after stroke; (5) surgical clipping or interventional embolization of aneurysms within 48 hours after hospitalization. The exclusion criteria were as follows: (1) aneurysm rebleeding; (2) suspected pseudoaneurysm; (3) history of other neurologic diseases, such as stroke, severe craniocerebral trauma and intracranial tumors; (4) other specific diseases or conditions, such as pregnancies, malignancies and serious dysfunction of other important organs. Controls were free of previous diseases and had normal routine laboratory tests.
Data Collection
Recorded information included demographics (age and gender), vascular risk factors (cigarette smoking, alcohol drinking, hypertension, diabetes mellitus and hyperlipidemia), and medication (statins, anticoagulation drugs and antiplatelet drugs). Hunt-Hess scale and modified Fisher scale were estimated for severity assessment. Aneurysm-related radiological parameters included position (posterior/anterior circulation), shape (cystic/others), and diameter (<10 mm/≥10 mm). Patients underwent neurosurgical clipping or endovascular interventional embolization for securing aneurysms. Other collected information included hospital admission time after stroke, acute hydrocephalus, intraventricular hemorrhage and external ventricular drainage.
Outcome Assessment
Prognostic parameters included DCI and functional outcome. DCI was diagnosed based on the previously defined criteria.20 Functional outcome was assessed using extended Glasgow outcome scale (GOSE) at 90 days after stroke. A poor outcome was designated as GOSE scores of 1–4.21
Immune Analysis
Venous blood was collected from patients upon entry into emergency room and those of controls were obtained at entrance into study. Sera from patients and controls were aliquoted and preserved at −80°C until assayed. Commercially available human sSRA enzyme-linked immunosorbent assay kit (Sino Biological Inc., Beijing, China) was used for immune analysis in accordance with the manufacturer’s instructions. Serum sSRA levels were in duplicate measured by the same technician blinded to the clinical data and accordingly, two measurements were averaged for further analysis.
Statistical Analysis
Statistical software used in this study included SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and MedCalc 9.6.4.0 (MedCalc Software, Mariakerke, Belgium). Categorical variables were summarized as counts (percentages). Continuous variables were presented as means (standard deviations) if normally distributed and medians (percentiles 25th-75th) if non-normally distributed. Using the Chi-square test or Fisher exact test, qualitative data were compared between two groups. Using the Mann–Whitney U-test or independent t test, continuous data were compared between two groups. Using the Kruskal–Wallis test, serum sSRA levels were compared among multiple groups divided by GOSE scores, Hunt-Hess scores or modified Fisher scores. Bivariate correlations between serum sSRA levels and other variables, as well as between GOSE scores and other variables were analyzed using the Spearman correlation coefficient. Furthermore, the multivariate linear regression model was built to determine factors which were independently correlated with serum sSRA levels or GOSE scores. In order to identify factors, which were independently associated with 90-day poor outcome and DCI, the binary logistic regression model was established. Under the receiver operating characteristic (ROC) curve, predictive efficiency was assessed by calculating area under curve (AUC). Using Youden method, an optimal cutoff value of serum sSRA levels was selected, which yielded the sensitivities and specificities for prognostic prediction. Two-tailed P values <0.05 were considered statistically significant.
Results
Selection and Characteristics of Participants
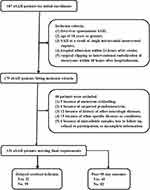
In the current study, 187 aSAH patients were initially recruited during the study period and thereafter 179 patients were retained for further analysis in compliance with the inclusion criteria. Afterwards, 48 patients were excluded because of the reasons displayed in Figure 1. Finally, a total of 131 aSAH patients were analyzed. In addition, 131 healthy controls were enrolled. There were no statistically significant differences in terms of mean age and percentages of gender, cigarette smoking and alcohol drinking between controls and aSAH patients (all P > 0.05; Table 1). Alternatively, controls were free of other previous diseases, and in other words, controls never suffered from hypertension, diabetes mellitus and hyperlipidemia as well as, never used statins, anticoagulation and antiplatelet drugs. Hence, there were substantial differences of the preceding conditions between the two groups (all P < 0.05). Other parameters of patients, including demographics, SAH severity, aneurysmal radiological characteristics and modalities for securing aneurysms, are displayed in Table 1.
 |
Table 1 General Data of Healthy Controls and Patients with Aneurysmal Subarachnoid Hemorrhage |
Serum sSRA Levels and Hemorrhagic Severity
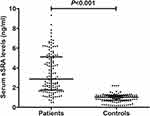
Blood of patients were sampled from 1.0 to 25.0 h (median, 8.6 h; percentiles 25th – 75th, 5.5–12.4 h) after stroke. In Figure 2, serum sSRA levels were substantially higher in patients than in controls (P < 0.001).
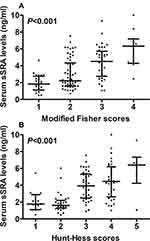
Using the Spearman test, it was verified that serum sSRA levels were intimately correlated with Hunt-Hess scores, modified Fisher scores, acute hydrocephalus, intraventricular hemorrhage, external ventricular drainage, blood leucocyte count and blood glucose levels (all P < 0.05; Table 2). Using the multivariate linear regression model, which contained the preceding significantly correlated factors, it was confirmed that serum sSRA levels were independently correlated with Hunt-Hess scores (beta, 0.569; 95% confidence interval (CI), 0.244–0.894; t = 4.145; P = 0.001) and modified Fisher scores (beta, 0.664; 95% CI, 0.254–1.074; t = 3.946; P = 0.002).
 |
Table 2 Factors Correlated with Serum Soluble Scavenger Receptor A Levels After Aneurysmal Subarachnoid Hemorrhage |
Alternatively, Hunt-Hess scores 1, 2, 3, 4 and 5 were found in 19, 31, 41, 34 and 6 patients respectively; and modified Fisher scores 1, 2, 3 and 4 were revealed in 24, 58, 38 and 11 patients, respectively. Figure 3 shows that serum sSRA levels were substantially highest in patients with Hunt-Hess score 5 or modified Fisher score 4 and were markedly lowest in those with Hunt-Hess score 1 or modified Fisher score 1 (both P < 0.001).
Serum sSRA Levels and DCI
In this group of aSAH patients, a total of 32 patients (24.4%) experienced DCI. Just as listed in Table 3, as compared to patients not presenting with DCI, those suffering from DCI exhibited substantially elevated Hunt-Hess scores, modified Fisher scores, serum sSRA levels, and blood leucocyte count (all P < 0.05); as well as had significantly higher percentages of acute hydrocephalus and external ventricular drainage (both P < 0.05). Thereafter, the above-mentioned variables were forced into the binary logistic regression model and subsequently it was showed that serum sSRA levels (odds ratio, 1.305; 95% CI, 1.012–1.687; P = 0.040), Hunt-Hess scores (odds ratio, 3.174; 95% CI, 1.706–5.907; P = 0.006) and modified Fisher scores (odds ratio, 3.617; 95% CI, 1.788–7.320; P = 0.008) were the three independent predictors of DCI after aSAH.
 |
Table 3 Factors Associated with Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage |
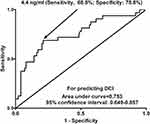
Under ROC curve, serum sSRA levels distinguished patients at risk of DCI significantly (P < 0.001; Figure 4). Using the Youden method, serum sSRA levels >4.4 ng/mL predicted the development of DCI with medium-high sensitivity and specificity values (Figure 4). Moreover, its DCI predictive efficiency was not substantially different from those of modified Fisher scores (AUC, 0.808; 95% CI, 0.732–0.884; P = 0.323) and Hunt-Hess scores (AUC, 0.809; 95% CI, 0.728–0.890; P = 0.277). The combined binary logistic regression model was configured and afterwards, we found that serum sSRA levels numerically improved DCI predictive abilities of Hunt-Hess scores (AUC, 0.837; 95% CI, 0.762–0.913; P = 0.069) and modified Fisher scores (AUC, 0.835; 95% CI, 0.751–0.920; P = 0.147). Furthermore, the DCI predictive ability of serum sSRA levels combined with Hunt-Hess scores and modified Fisher scores (AUC, 0.866; 95% CI, 0.797–0.935) significantly exceeded that of serum sSRA levels (P = 0.010), Hunt-Hess scores (P = 0.011) and modified Fisher scores (P = 0.010) alone.
Serum sSRA Levels and 90-Day Poor Prognosis
At 90 days after stroke, GOSE scores ranged from 1 to 8, with a median value of 6 (lower-upper quartiles, 3–7). In Table 4, there was a close correlation between GOSE scores and diabetes mellitus, between GOSE scores and Hunt-Hess scores, between GOSE scores and modified Fisher scores, between GOSE scores and acute hydrocephalus, between GOSE scores and intraventricular hemorrhage, between GOSE scores and blood leucocyte count, as well as between GOSE scores and serum sSRA levels (all P < 0.05). Using the multivariate linear regression model, where the afore-mentioned variables were incorporated, it was shown that GOSE scores had independent correlation with serum sSRA levels (beta, −0.275; 95% CI, −0.440–0.110; t=−3.045; P = 0.005), Hunt-Hess scores (beta, −0.831; 95% CI, −1.136–0.525; t=−4.423; P = 0.001) and modified Fisher scores (beta, −0.813; 95% CI, −1.197–0.429; t=−4.103; P = 0.001).
 |
Table 4 Factors Correlated with Extended Glasgow Outcome Scale Scores at 90 Days After Aneurysmal Subarachnoid Hemorrhage |
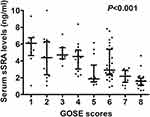
At 90 days after aSAH, a total of 9, 16, 8, 16, 13, 32, 15 and 22 patients displayed GOSE scores 1, 2, 3, 4, 5, 6, 7 and 8. In Figure 5, serum sSRA levels were markedly highest in patients with GOSE score 1, followed by GOSE scores 2, 3, 4, 5, 6 and 7, and were profoundly lowest in those with GOSE score 8 (P < 0.001).
In total, 49 patients experienced a poor outcome at 90 days after aSAH. Just as listed in Table 5, as compared to good outcome patients, those with poor outcome showed the substantially increased blood glucose levels, serum sSRA levels, Hunt-Hess scores and modified Fisher scores, as well as had the significantly raised proportions of diabetes mellitus and hydrocephalus (all P < 0.05). Furthermore, using the binary logistic regression model, where the above significant variables were entered, it was found that Hunt-Hess scores, modified Fisher scores and serum sSRA levels independently predicted the development of 90-day poor outcome after stroke with odds ratio values of 2.444 (95% CI, 1.264–4.726; P = 0.008), 5.778 (95% CI, 2.306–14.477; P = 0.005) and 1.382 (95% CI, 1.057–1.806; P = 0.018) respectively.
 |
Table 5 Factors Associated with 90-Day Poor Outcome (Extended Glasgow Outcome Scale Scores 1–4) After Aneurysmal Subarachnoid Hemorrhage |
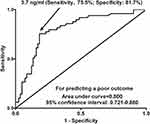
Under ROC curve, serum sSRA levels substantially predicted DCI (P < 0.001; Figure 6). Using the Youden method, serum sSRA levels >3.7 ng/mL differentiated the risk of a poor 90-day outcome with medium-high sensitivity and specificity values (Figure 6). Alternatively, its prognostic predictive capability was equivalent to those of modified Fisher scores (AUC, 0.835; 95% CI, 0.771–0.898; P = 0.435) and Hunt-Hess scores (AUC, 0.857; 95% CI, 0.798–0.915; P = 0.202). The combined binary logistic regression model was built and next, it was demonstrated that serum sSRA levels significantly enhanced prognostic predictive capabilities of Hunt-Hess scores (AUC, 0.870; 95% CI, 0.811–0.929; P = 0.033) and modified Fisher scores (AUC, 0.898; 95% CI, 0.846–0.949; P = 0.010). Furthermore, the prognostic predictive performance of serum sSRA levels combined with Hunt-Hess scores and modified Fisher scores (AUC, 0.915; 95% CI, 0.868–0.961) was substantially superior to that of serum sSRA levels (P < 0.001), Hunt-Hess scores (P < 0.001) and modified Fisher scores (P < 0.001) alone.
Discussion
To the best of my knowledge, this is the first series for investigating serum sSRA levels in humans with acute brain injury disease, which was aSAH in the current study. Our study showed that (1) there was a substantial elevation of serum sSRA levels in patients with aSAH, as compared to healthy controls; (2) when Hunt-Hess scores and modified Fisher scores were regarded as the two categorical variables, there was a significant elevation in serum sSRA levels with increasing Hunt-Hess scores or modified Fisher scores; (3) when Hunt-Hess scores and modified Fisher scores were considered as the two quantitative variables, there was an independent correlation between serum sSRA levels and Hunt-Hess scores, as well as between serum sSRA levels and modified Fisher scores; (4) whether 90-day GOSE score was shown as a qualitative or quantitative variable, serum sSRA levels were still independently associated with poor prognosis, which was reflected by GOSE score; (5) serum sSRA emerged as an independent predictor of DCI; (6) serum sSRA levels displayed substantial predictive ability for poor outcome and DCI, its prognostic discriminatory capability was in range of Hunt-Hess scores and modified Fisher scores, and it significantly improved distinguishable capabilities of Hunt-Hess scores and modified Fisher scores for 90-day poor outcome, but not for DCI; and (7) as compared to any of them, combination of serum sSRA levels, Hunt-Hess scores and modified Fisher scores had markedly raised predictive efficiencies for both DCI and poor outcome. The preceding data indicate that elevated serum sSRA levels after aSAH, in close correlation with hemorrhagic severity, were independently associated with poor clinical outcome, substantializing serum sSRA as a promising prognostic biomarker of aSAH.
The innate immune reaction is implicated in the pathophysiology of acute brain injury diseases.22–24 Compelling data have shown that SRA may play a central role in the induction of innate immunity after acute brain injury.17–19 However, there are some inconsistent data regarding protective or detrimental effect of SRA in acute brain injury. Using gene knockout technique, SRA was demonstrated to increase cerebral infarction size, enhance neuronal apoptosis and induce nuclear factor-kappa B activation, thereby contributing to ischemic brain injury in mouse model with cerebral ischemia–reperfusion injury.17 Contrarily, in mice with intracerebral hemorrhage, SRA significantly lessened microglia activation and protected neuroinflammatory injury, subsequently depressing cerebral water content and improving neurological deficit.18 Similarly, SRA obviously alleviated cerebral water content and decreased neurological deficit of intracerebral hemorrhage mice, possibly via down-regulating inflammatory reaction in microglia by deactivating Toll-like receptor-4.19 Collectively, SRA may participate in neuroinflammation after acute brain injury. However, there is a paucity of data available on actual effect of SRA, protective or detrimental, in acute brain injury.
SRA was significantly expressed in rat microglia after middle cerebral artery occlusion.16 sSRA displayed T cell suppressive activity via functional regulation of innate immune cells, for instance, dendritic cells and myeloid-derived suppressor cells.25,26 However, the significance of sSRA as a potential prognostic biomarker in aSAH has not been explored. Our study not only showed a significant increase of serum sSRA levels after aSAH but also demonstrated some independent relationships between serum sSRA levels and hemorrhagic severity plus prognosis following aSAH. The main characteristics of our study design and statistical analyses were that GOSE score was identified as both a continuous variable and a categorical variable, and therefore, two multivariate models were established to investigate the relationship between serum sSRA levels and poor clinical outcome; alternatively, besides GOSE score, DCI was assessed for analyzing its association with serum sSRA levels. Intriguingly, our data showed that serum sSRA levels substantially improved the prognostic predictive abilities of Hunt-Hess scores and modified Fisher scores, and its combination with Hunt-Hess scores and modified Fisher scores exhibited significantly improved predictive abilities for DCI and poor outcome, as compared to any one among serum sSRA levels, Hunt-Hess scores and modified Fisher scores. Overall, serum sSRA may be a potential prognostic biomarker of aSAH.
There are two limitations in the current study. First, this is a single-center study, characterized by a small sample size. And thus, the conclusions should be validated by a large-scale cohort study. Second, although serum sSRA levels significantly improved the prognostic predictive performances of Hunt-Hess scores and modified Fisher scores and their combination had substantially higher predictive performance than any of them in this study, no comparisons of the prognostic predictive ability between serum sSRA levels and other inflammatory biomarkers have been done. Hence, the clinical role of serum sSRA in prognosis of aSAH should be further verified in future.
Conclusions
To the best of our knowledge, this study, for the first time, measured serum sSRA levels of aSAH patients and further found some interesting results. Elevated serum sSRA levels are independently correlated with illness severity indicated by Hunt-Hess scores and modified Fisher scores, as well as are independently associated with DCI and 90-day poor outcome reflected by GOSE scores. Moreover, serum sSRA levels combined with Hunt-Hess scores and modified Fisher scores show substantially increased predictive performances for DCI and poor outcome. In summary, SRA is assumed to regulate immune response after secondary brain injury after aSAH and serum sSRA may be of clinical value in severity assessment and prognostic prediction of aSAH.
Abbreviations
aSAH, aneurysmal subarachnoid hemorrhage; AUC, area under curve; CI, confidence interval; GOSE, extended Glasgow outcome scale; OR, odds ratio.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethical Review Approval
The study was done according to the principles of the Declaration of Helsinki, and approval for the study protocol was obtained from the ethics committee at Ningbo Hangzhou Bay Hospital (opinion number: 2016026).
Informed Consent
The written informed consent was acquired from relatives of patients and controls themselves.
Acknowledgment
The authors are grateful to all participants for their providing blood samples and participation to this study.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest concerning the materials or methods used in this study or the findings specified in this article.
References
1. Rouanet C, Silva GS. Aneurysmal subarachnoid hemorrhage: current concepts and updates. Arq Neuropsiquiatr. 2019;77(11):806–814. doi:10.1590/0004-282X20190112
2. Fang Y, Lu J, Zheng J, et al. Comparison of aneurysmal subarachnoid hemorrhage grading scores in patients with aneurysm clipping and coiling. Sci Rep. 2020;10(1):9199. doi:10.1038/s41598-020-66160-0
3. Suzuki H, Kanamaru H, Kawakita F, Asada R, Fujimoto M, Shiba M. Cerebrovascular pathophysiology of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Histol Histopathol. 2021;36(2):143–158. doi:10.14670/HH-18-253
4. Katsuki M, Kawamura S, Koh A. Easily created prediction model using automated artificial intelligence framework (Prediction One, Sony Network Communications Inc., Tokyo, Japan) for subarachnoid hemorrhage outcomes treated by coiling and delayed cerebral ischemia. Cureus. 2021;13(6):e15695. doi:10.7759/cureus.15695
5. Osgood ML. Aneurysmal subarachnoid hemorrhage: review of the pathophysiology and management strategies. Curr Neurol Neurosci Rep. 2021;21(9):50. doi:10.1007/s11910-021-01136-9
6. Chen J, Wong GKC. Microglia accumulation and activation after subarachnoid hemorrhage. Neural Regen Res. 2021;16(8):1531–1532. doi:10.4103/1673-5374.303028
7. Chou SH, Macdonald RL. Keller E; Unruptured Intracranial Aneurysms and SAH CDE Project Investigators. Biospecimens and molecular and cellular biomarkers in aneurysmal subarachnoid hemorrhage studies: common data elements and standard reporting recommendations. Neurocrit Care. 2019;30(Suppl 1):46–59. doi:10.1007/s12028-019-00725-4
8. Chaudhry SR, Kinfe TM, Lamprecht A, et al. Elevated level of cerebrospinal fluid and systemic chemokine CCL5 is a predictive biomarker of clinical outcome after aneurysmal subarachnoid hemorrhage (aSAH). Cytokine. 2020;133:155142. doi:10.1016/j.cyto.2020.155142
9. Simon M, Grote A. Interleukin 6 and aneurysmal subarachnoid hemorrhage. a narrative review. Int J Mol Sci. 2021;22(8):4133. doi:10.3390/ijms22084133
10. Kelley JL, Ozment TR, Li C, Schweitzer JB, Williams DL. Scavenger receptor-A (CD204): a two-edged sword in health and disease. Crit Rev Immunol. 2014;34(3):241–261. doi:10.1615/critrevimmunol.2014010267
11. Thakur SA, Hamilton RF, Holian A. Role of scavenger receptor a family in lung inflammation from exposure to environmental particles. J Immunotoxicol. 2008;5(2):151–157. doi:10.1080/15476910802085863
12. Neyen C, Plüddemann A, Roversi P, et al. Macrophage scavenger receptor A mediates adhesion to apolipoproteins A-I and E. Biochemistry. 2009;48(50):11858–11871. doi:10.1021/bi9013769
13. Plüddemann A, Hoe JC, Makepeace K, Moxon ER, Gordon S. The macrophage scavenger receptor A is host-protective in experimental meningococcal septicaemia. PLoS Pathog. 2009;5(2):e1000297. doi:10.1371/journal.ppat.1000297
14. Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40(2):195–205. doi:10.1002/glia.10148
15. Cornejo F, Vruwink M, Metz C, et al. Scavenger receptor-A deficiency impairs immune response of microglia and astrocytes potentiating Alzheimer’s disease pathophysiology. Brain Behav Immun. 2018;69:336–350. doi:10.1016/j.bbi.2017.12.007
16. Xu Z, Han K, Chen J, et al. Vascular endothelial growth factor is neuroprotective against ischemic brain injury by inhibiting scavenger receptor A expression on microglia. J Neurochem. 2017;142(5):700–709. doi:10.1111/jnc.14108
17. Lu C, Hua F, Liu L, et al. Scavenger receptor class-A has a central role in cerebral ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2010;30(12):1972–1981. doi:10.1038/jcbfm.2010.59
18. Yang Z, Zhong S, Liu Y, Shen H, Yuan B. Scavenger receptor SRA attenuates microglia activation and protects neuroinflammatory injury in intracerebral hemorrhage. J Neuroimmunol. 2015;278:232–238. doi:10.1016/j.jneuroim.2014.11.010
19. Yuan B, Shen H, Lin L, Su T, Huang Z, Yang Z. Scavenger receptor SRA attenuates TLR4-induced microglia activation in intracerebral hemorrhage. J Neuroimmunol. 2015;289:87–92. doi:10.1016/j.jneuroim.2015.10.006
20. Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40(6):1963–1968. doi:10.1161/STROKEAHA.108.544700
21. Wilson L, Boase K, Nelson LD, et al. A manual for the Glasgow Outcome Scale-Extended Interview. J Neurotrauma. 2021;38(17):2435–2446. doi:10.1089/neu.2020.7527
22. Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95(6):1246–1265. doi:10.1016/j.neuron.2017.07.010
23. Roa JA, Sarkar D, Zanaty M, et al. Preliminary results in the analysis of the immune response after aneurysmal subarachnoid hemorrhage. Sci Rep. 2020;10(1):11809. doi:10.1038/s41598-020-68861-y
24. Shtaya A, Bridges LR, Williams R, et al. Innate immune anti-inflammatory response in human spontaneous intracerebral hemorrhage. Stroke. 2021;52(11):3613–3623. doi:10.1161/STROKEAHA.121.034673
25. Zuo D, Yu X, Guo C, et al. Scavenger receptor A restrains T-cell activation and protects against concanavalin A-induced hepatic injury. Hepatology. 2013;57(1):228–238. doi:10.1002/hep.25983
26. Chen Y, Huang Z, Ma D, et al. Involvement of soluble scavenger receptor A in suppression of T cell activation in patients with chronic hepatitis B. BMC Immunol. 2015;16:29. doi:10.1186/s12865-015-0088-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.