Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Utility of Serum Growth Arrest-Specific Protein 6 as a Biomarker of Severity and Prognosis After Severe Traumatic Brain Injury: A Prospective Observational Study
Authors Ni BK, Cai JY, Wang XB, Lin Q, Zhang XN, Wu JH
Received 30 April 2022
Accepted for publication 7 July 2022
Published 14 July 2022 Volume 2022:18 Pages 1441—1453
DOI https://doi.org/10.2147/NDT.S372904
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Bu-Kao Ni,1 Jian-Yong Cai,2 Xiao-Bo Wang,1 Qun Lin,2 Xue-Na Zhang,1 Jian-Hua Wu1
1Intensive Care Unit, The Wenzhou Central Hospital, Wenzhou, People’s Republic of China; 2Department of Neurosurgery, The Wenzhou Central Hospital, Wenzhou, People’s Republic of China
Correspondence: Jian-Hua Wu, Tel +86 577 88070335, Email [email protected]
Objective: Growth arrest-specific protein 6 (Gas6) may harbor protective effects in acute brain injury. This study was designed to determine the relation of serum Gas6 levels to severity and prognosis after traumatic brain injury (TBI).
Methods: In this prospective cohort study of 114 controls and 114 patients with severe TBI, multivariate analysis was used to assess relationships between serum Gas6 levels, Glasgow coma scale (GCS) score, Rotterdam computed tomography (CT) score, postinjury 180-day mortality, overall survival and poor prognosis (Extended Glasgow outcome scale score 1– 4).
Results: Significantly increased serum Gas6 levels of patients (median, 10.3 ng/mL versus 32.5 ng/mL; P < 0.001), as compared with controls, were independently correlated with Rotterdam CT score (t = 3.629, P < 0.001) and GCS score (t=− 3.393, P = 0.001), and independently predicted 180-day mortality (odds ratio, 1.078; 95% confidence interval (CI), 1.007– 1.154), overall survival (hazard ratio, 1.074; 95% CI, 1.012– 1.139) and poor prognosis (odds ratio, 1.129; 95% CI, 1.059– 1.205). Areas under receiver operating characteristic curve (AUCs) of serum Gas6 levels for discriminating risks of 180-day mortality and poor prognosis were 0.785 (95% CI, 0.699– 0.857) and 0.793 (95% CI, 0.707– 0.863), respectively; and serum Gas6 levels above 30.9 ng/mL and 28.3 ng/mL predicted 180-day mortality and poor prognosis with maximum Youden indices of 0.451 and 0.468, respectively. The predictive ability of serum Gas6 levels for mortality was similar to those of GCS score (AUC, 0.833; 95% CI, 0.751– 0.896; P = 0.286) and Rotterdam CT score (AUC, 0.823; 95% CI, 0.740– 0.888; P = 0.432). The discriminatory capability of serum Gas6 levels for the risk of poor prognosis was in the range of GCS score (AUC, 0.846; 95% CI, 0.766– 0.906; P = 0.178) and Rotterdam CT score (AUC, 0.831; 95% CI, 0.750– 0.895; P = 0.368).
Conclusion: Serum Gas6 may appear as a promising biochemical parameter for aiding in the assessment of trauma severity and prediction of prognosis among patients with severe TBI.
Keywords: traumatic brain injury, biomarkers, growth arrest-specific protein 6, severity, prognosis
Introduction
Severe traumatic brain injury (sTBI) is one of the most devastating types of trauma associated with high morbidity and mortality rates.1,2 The Glasgow coma scale (GCS) is a clinically valuable prognostic factor of TBI.3–5 Secondary brain injury after TBI involves inflammation, oxidative stress, cellular death, excitatory amino acids toxicity and so on.6–8 In recent decades, some relevant biochemical markers, such as S100B, glial fibrillary acidic protein, ubiquitin carboxyl-terminal hydrolase L1, myelin basic protein and neuron-specific enolase, have obtained interests of neurological researchers for helping clinical prognostic prediction and severity assessment of TBI.9–11 However, circulating levels of those biomarkers have not been conventionally determined for clinical service. Hence, clinical investigation of potential biomarkers is still under way for severity and prognosis analysis of TBI.
Vitamin K‑dependent proteins (VKDPs) are a group of proteins, which need vitamin K to conduct carboxylation.12 Tyro3, Axl and Mertk are collectively termed as TAMs, which are tyrosine kinase cell surface transmembrane receptors and are capable of sensing extracellular ligands, phosphorylating and activating diverse downstream signaling pathways.13 Growth arrest-specific protein 6 (Gas6), which is identified as a member of the VKDP family, can bind the TAM receptors.14 Gas6/TAM signaling may play an important role in apoptotic cell clearance, immune homeostasis and resolution of immune responses.15,16 Gas6 can be extensively expressed in neurons, glial cells and endothelial cells of the central nervous system.17,18 Compelling experimental data have shown that Gas6 facilitated the clearance of apoptotic bodies,19 and inhibited neuroinflammation after acute brain injury,20,21 indicating its brain-protective effects. Interestingly, elevated Gas6 levels in cerebrospinal fluid were intimately correlated with disease duration and severity of Alzheimer’s disease.22 Hence, it has been hypothesized that Gas6 may be a potential biomarker for acute brain injury. In this study, we intended to explore the relationship between serum Gas6 levels and the severity plus outcome after sTBI.
Materials and Methods
Study Design and Participant Selection
In this prospective cohort study performed at the Wenzhou Central Hospital from January 2018 to January 2021, we initially assessed adult (age ≥18 years) patients with isolated and blunt sTBI (GCS score of 3–8), who were hospitalized within post-traumatic twelve hours. Isolated head trauma was referred to as injury severity score below 9 in non-cranial aspects. The exclusion criteria were as follows: 1) history of neurological diseases, for example, hemorrhagic or ischemic stroke, intracranial tumors and severe head trauma; 2) other specific conditions or diseases, for instance severe infections within a month, autoimmune diseases, severe liver, pregnancy, kidney or heart diseases and other known malignancies; 3) loss to follow-up, incomplete information, unavailable blood samples or refusal to participation. Simultaneously, healthy volunteers were enrolled as controls. The current study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Institutional Review Committee at the Wenzhou Central Hospital (No. WCH2017014). The informed consent to participate in this study was signed by the legal representatives of patients and controls themselves.
Data Collection, Outcome Assessment and Biochemical Measurements
Some pertinent information was registered, which included age, gender, cigarette smoking, alcohol consumption, hypertension, diabetes mellitus, indicators of traumatic severity, radiological parameters, traumatic mechanisms, vital signs and clinical outcome. Alcohol abuse was defined as moderate-to severe alcohol consumption in social history (>168g/week).23 Current cigarette smokers were those who smoked regularly at least one cigarette per day at the time of presentation.23 Hypertension and diabetes mellitus were previously diagnosed. Vital signs included heart rate, respiratory rate, blood oxygen saturation and arterial blood pressure. Radiological appearances included abnormal cisterns, midline shift, epidural hematoma, subdural hematoma, subarachnoid hemorrhage, intraventricular hemorrhage, intracerebral hematoma, brain contusion and pneumocephalus. Traumatic mechanisms included automobile/motorcycle, fall/jump and others. Clinical severity was evaluated using GCS and radiological severity was assessed utilizing Rotterdam computed tomography (CT) classification. We used 8-grade Extended Glasgow Outcome Scale (GOSE) to assess clinical outcome at 180 days after head trauma. Patients with GOSE score of 1–4 was considered to have a poor outcome.24
Venous blood samples from controls and patients were obtained and centrifuged thereafter. Acquired serum was promptly preserved at –80°C until measurements. Serum Gas6 levels were in duplicate quantified using a commercially available enzyme-linked immunosorbent assay kit based on the manufacturer’s instructions (Shanghai Kexing Trading Co., Ltd., Shanghai, China). Measurements were completed by the same technician blinded to clinical information.
Statistical Analysis
Data analyses were performed using the SPSS 20.0 statistical package (SPSS Inc., Chicago, Illinois, USA), except for the receiver operating characteristic (ROC) curve analysis, which was completed via MedCalc statistical software version 17.4 (MedCalc Software, Mariakerke, Belgium). After the normal distribution test, quantitative data were reported as means (standard deviations) if normally distributed and as medians (upper – lower quartiles) if non-normally distributed. Qualitative data were summarized as counts (percentages). Normally distributed data were compared between two groups using the independent t-test and non-normally distributed data were compared using the Mann–Whitney test. Intergroup comparisons of categorical data were done using the Pearson’s Chi-square test or Fisher’s exact test as appropriate. The Kruskal–Wallis H-test was done to compare non-normally distributed data among multiple groups. The Log rank test was carried out to compare 180-day overall survival time between the two groups. The ROC curve was drawn to assess the predictive ability of serum Gas6 levels for mortality and poor prognosis, and the area under ROC curve (AUC) was generated to reflect the predictive efficiency. Variables, which were statistically significantly different using univariate analysis, were forced into the multivariate binary logistic regression model or multivariate Cox’s proportional hazard model to determine independent predictors substantially associated with 180-day mortality, poor prognosis or overall survival. Results were reported as odds ratio (OR) or hazard ratio (HR), with the corresponding 95% confidence interval (95% CI). For all tests, the two-sided P-values less than 0.05 were considered statistically significant.
Results
Patient and Control Characteristics
During the study period, we firstly enrolled 146 patients with sTBI and thereafter 32 sTBI patients were excluded for the reasons outlined in Figure 1. The remaining 114 patients consisted of 68 males and 46 females, as well as were aged from 18 to 73 years (mean, 43.6 years; standard deviation, 14.0 years). The excluded 32 patients comprised 17 males and 15 females, as well as had a mean age of 45.0 years (range, 21–76 years; standard deviation, 14.3 years). There were no significant differences in terms of age and gender percentage between the finally assessed patients and the excluded patients (both P > 0.05). Meanwhile, there were 114 healthy controls, who were aged from 18 to 77 years (mean, 44.2 years; standard deviation, 13.8 years), and included 59 males and 55 females. As compared with the eligible patients, controls had similar age and proportion of gender (both P > 0.05).
This group of sTBI patients were analyzed for their related data. There were 68 males and 46 females, and the ratio of male to female was 1.48. They had a mean age of 43.6 years (standard deviation, 14.0 years; range, 18–73 years). There were 35 cigarette smokers, 37 alcohol drinkers, 19 hypertensive patients, 14 patients with diabetes mellitus and 22 patients with hyperlipidemia. Patients were admitted to hospital from 0.5 to 12.0 hours after head trauma (median, 4.7 hours; percentiles 25th–75th, 3.8–6.4 hours). Blood samples were obtained from 1.0 to 14.0 hours (median, 6.4 hours; percentiles 25th–75th, 4.6–8.0 hours) following head trauma. Traumatic causes included automobile/motorcycle (59 cases), fall/jump (44 cases) and others (11 cases). GCS score ranged from 3 to 8, with a median value of 5 and upper-lower quartiles of 4–6. GCS scores 3, 4, 5, 6, 7 and 8 were found in 14, 26, 30, 16, 13 and 15 patients, respectively. The mean systolic and diastolic arterial pressures were 124.9 mmHg (standard deviation, 28.4 mmHg; range, 74–178 mmHg) and 74.1 mmHg (standard deviation, 16.5 mmHg; range, 44–108 mmHg), respectively. Rotterdam CT score ranged from 3 to 6 (median, 4; percentiles 25th–75th, 4–5). Rotterdam CT scores 3, 4, 5 and 6 were revealed among 14, 54, 26 and 20 cases, respectively. Positive radiological appearances included abnormal cisterns (87 cases), midline shift (71 cases), epidural hematoma (61 cases), subdural hematoma (66 cases), subarachnoid hemorrhage (74 cases), intraventricular hemorrhage (12 cases), intracerebral hematoma (65 cases), brain contusion (69 cases) and pneumocephalus (43 cases). Blood glucose levels ranged from 2.8 to 19.7 mmol/l (median, 7.8 mmol/l; lower – upper quartiles, 6.1–10.4 mmol/l). Blood leucocyte count ranged from 3.4 to 18.3 ×109/l (median, 8.5 ×109/l; lower-upper quartiles, 6.3–10.4 ×109/l).
Serum Gas6 Levels and Trauma Severity
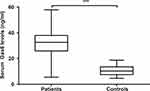
In Figure 2, serum Gas6 levels were substantially higher in sTBI patients than in healthy controls. Regarding the relationship between serum Gas6 levels and traumatic severity indicated by GCS score and Rotterdam CT score, we considered both GCS score and Rotterdam CT score as two categorical or continuous variables. Figure 3A and B show that there was a significant increase in serum Gas6 levels with a decrease in GCS score, and Figure 3C and D display that serum Gas6 levels were substantially increased with rising Rotterdam CT score. Moreover, in Table 1, serum Gas6 levels were highly correlated not only with GCS score and Rotterdam CT score but also with other variables, including abnormal cisterns, midline shift >5 mm, epidural hematoma, subdural hematoma and blood glucose levels. When the preceding significantly correlated variables were incorporated into the multivariate linear regression model, it was demonstrated that serum Gas6 levels were independently correlated with GCS score (t=−3.393, P = 0.001) and Rotterdam CT score (t = 3.629, P < 0.001).
 |
Table 1 Bivariate Correlation Analysis of Serum Growth Arrest-Specific Protein 6 Levels After Severe Traumatic Brain Injury |
Serum Gas6 Levels and 180-Day Mortality
Among this cohort of sTBI patients, a total of 28 patients were deceased within 180 days following head trauma. In Figure 4A, serum Gas6 levels markedly differed between non-survivors and survivors; and moreover, serum Gas6 levels efficiently discriminated the risk of 180-day death (Figure 4B). In Table 2, the variables, which were significantly associated with 180-day death using univariate analysis, included GCS score, Rotterdam CT classification, abnormal cisterns, midline shift >5 mm, epidural hematoma, blood glucose levels and serum Gas6 levels. When the preceding significant variables were forced into the binary logistic regression model, it was confirmed that GCS score (OR, 0.502; 95% CI, 0.265–0.948), Rotterdam CT score (OR, 3.180; 95% CI, 1.360–7.437) and serum Gas6 levels (OR, 1.078; 95% CI, 1.007–1.154) were independently associated with post-traumatic 180-day death. Under ROC curve, the predictive ability of serum Gas6 levels was similar to those of GCS score (AUC, 0.833; 95% CI, 0.751–0.896; P = 0.286) and Rotterdam CT score (AUC, 0.823; 95% CI, 0.740–0.888; P = 0.432).
 |
Table 2 Factors Associated with 180-Day Mortality After Severe Traumatic Brain Injury |
Serum Gas6 Levels and 180-Day Overall Survival
This group of sTBI patients had the mean 180-day overall survival time of 144.1 days (95% CI, 131.9–156.4 days). Based on the cutoff value of serum Gas6 levels, which was yielded using the Youden method under the ROC curve for predicting 180-day death (Figure 4B), all patients were dichotomized into two groups with serum Gas6 levels > and ≤30.9 ng/mL respectively. In Figure 4C, patients with serum Gas6 levels >30.9 ng/mL had substantially shorter 90-day overall survival time than the other remainders. Just as listed in Table 3, GCS score, Rotterdam CT classification, abnormal cisterns, midline shift >5 mm, epidural hematoma, blood glucose levels and serum Gas6 levels were substantially related to 180-day overall survival using univariate Cox’s proportional hazard model. Furthermore, the afore-mentioned significant relevant variables were entered in multivariate Cox’s proportional hazard model and thereafter, it was revealed that GCS score, Rotterdam CT score and serum Gas6 levels independently predicted 180-day overall survival with HR values of 0.630 (95% CI, 0.401–0.992), 2.285 (95% CI, 1.243–4.200) and 1.074 (95% CI, 1.012–1.139), respectively.
 |
Table 3 Factors Associated with 180-Day Overall Survival After Severe Traumatic Brain Injury |
Serum Gas6 Levels and 180-Day Poor Outcome
At 180 days after head trauma, GOSE scores ranged from 1 to 8 (median, 5; percentiles 25th–75th, 2–6); and GOSE scores 1, 2, 3, 4, 5, 6, 7 and 8 were found in 28, 10, 6, 8, 24, 18, 11 and 9 patients, respectively. In total, 52 patients experienced a poor outcome (GOSE scores 1–4). Figure 5A and B display that serum Gas6 levels were tightly and inversely correlated with GOSE scores. Just as depicted in Figure 5C, serum Gas6 levels were significantly higher in patients with the development of poor outcome than in those presenting with good outcome; alternatively, serum Gas6 levels substantially distinguished patients at the risk of poor outcome at post-traumatic 180 days (Figure 5D). In Table 4, as compared to patients who presented with good outcome, those suffering from poor outcome tended to have significantly declined GCS score, were more likely to display substantially increased Rotterdam CT score, blood glucose levels and serum Gas6 levels, and were more prone to exhibit markedly elevated percentages of diabetes mellitus, abnormal cisterns, midline shift >5 mm, subdural hematoma, subarachnoid hemorrhage and epidural hematoma. When the above-mentioned significant variables were contained into the binary logistic regression model, it was shown that factors independently associated with 180-day poor outcome were GCS score (OR, 0.485; 95% CI, 0.267–0.880), Rotterdam CT score (OR, 6.159; 95% CI, 2.242–16.919) and serum Gas6 levels (OR, 1.129; 95% CI, 1.059–1.205). Under ROC curve, the discriminatory capability of serum Gas6 levels was in the range of GCS score (AUC, 0.846; 95% CI, 0.766–0.906; P = 0.178) and Rotterdam CT score (AUC, 0.831; 95% CI, 0.750–0.895; P = 0.368).
 |
Table 4 Factors Associated with 180-Day Poor Outcome After Severe Traumatic Brain Injury |
Discussion
To the best of our knowledge, this is the first series for measuring circulating Gas6 levels in humans after acute brain injury and further determining the relation of serum Gas6 levels to trauma severity and clinical outcome after sTBI. The main findings are that (1) there was a significant elevation in serum Gas6 levels of patients after sTBI; (2) serum Gas6 levels were independently correlated with GCS score and Rotterdam CT score; (3) serum Gas6 levels were independently associated with 180-day death, overall survival and poor outcome; and (4) serum Gas6 levels possessed similar AUCs, as compared to GCS score and Rotterdam CT score. Presumably, serum Gas6 levels should be intimately correlated with trauma severity and be highly associated with clinical outcome after sTBI.
Gas6 is a multifunctional ligand of TAM receptors Tyro3, Axl and Mertk, with the highest affinity to Axl.14 TAM receptors are extensively expressed in the nervous system.25 And Gas6 is abundantly secreted by neurons and endothelial cells.17,18 In vitro experiment showed that the administration of recombinant Gas6 significantly alleviated hippocampal neuronal cell death caused by serum starvation.19 Similarly, in mice with subarachnoid hemorrhage, administration of recombinant Gas6 markedly decreased expressions of interleukin-1β and interleukin-18, lessened brain edema, reduced neuronal degeneration and diminished neurological deficits.21 Alternatively, expressions of Gas6 were greatly up-regulated in mouse brain tissues after intracerebral hemorrhage, as well as administration of recombinant Gas6 significantly diminished brain edema and improved neurobehavioral performances.20 Also, in animal studies, administration of recombinant Gas6 significantly reduced brain infarction, diminished neurological deficits, attenuated blood–brain barrier disruption and depressed hemorrhagic transformation after intravenous thrombolysis.26 Overall, rapidly growing evidence has been supportive of the notion that Gas6 may act as a brain-protective protein.
Circulating Gas6 levels have been found to be significantly increased in some critically ill patients with COVID-19,27 sepsis28 or acute myocardial infarction.29 However, the current study has excluded such critically ill patients from our cohort. Gas6 can be produced greatly from injured brain tissues.20 Thus, it is hypothesized that Gas6 may be released into peripheral blood via disrupted blood–brain barrier after head trauma. So, blood Gas6 may be at least partly derived from injured brain tissues. On the other hand, the release of Gas6 from traumatized brain tissues may be a compensatory increase in order to exert a brain-protective effect.
In order to verify the relationship between serum Gas6 levels and traumatic severity plus prognosis, we configured the four multivariate models, namely, one linear regression model, two binary logistic regression models and one Cox’s proportional hazard model. Interestingly, serum Gas6 levels were found to be independently correlated with GCS score and Rotterdam CT score, as well as to be independently associated with 180-day death, overall survival and poor outcome. Clearly, GCS and Rotterdam CT classification are the two common prognostic determinants of head trauma.3–5,30 Thus, it is deduced that serum Gas6 levels may be tightly related to trauma severity and long-term clinical outcome.
Two limitations warrant to be mentioned in this study. First, although this is the first study to investigate the prognostic role of serum Gas6 levels in sTBI and further find some interesting results, this is only a preliminary study because of its relatively small sample size. Therefore, a large cohort study is needed to validate such conclusions. Second, GCS and Rotterdam CT classification currently serve as the two common prognostic predictors of TBI.3–5,30 Some biomarkers, eg, S100B, glial fibrillary acidic protein, ubiquitin carboxyl-terminal hydrolase L1, myelin basic protein and neuron-specific enolase, are confirmed to be potential prognostic biomarkers of TBI.9–11 The interesting finding in the current study is that serum Gas6 levels showed similar prognostic predictive ability, as compared to GCS score and Rotterdam CT score. Thus, our future mission is to choose a biomarker from the preceding biomarkers, including Gas6, which can efficiently improve the prognostic capability when it is combined with GCS score or Rotterdam CT score.
Conclusions
This is the first series for investigating the relationship between serum Gas6 levels and trauma severity plus long-term clinical outcome of sTBI. The main findings are that elevated serum Gas6 levels after sTBI are independently correlated with clinical and radiological severity indicated by GCS score and Rotterdam CT classification, as well as independently predict long-term outcome, including mortality, overall survival and poor prognosis. Hence, serum Gas6 may serve as a useful prognostic biomarker for sTBI.
Abbreviations
CT, computerized tomography; GCS, Glasgow coma scale; ROC, receiver operating characteristic; sTBI, severe traumatic brain injury; AUC, area under receiver operating characteristic curve; OR, odds ratio; HR, hazard ratio; VKDPs, vitamin K‑dependent proteins; Gas6, growth arrest-specific protein 6; GOSE, extended Glasgow Outcome Scale.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
The authors thank all participants for their willingness to participate in this study. The study was performed at Wenzhou Central Hospital.
Disclosure
The authors have no conflicts of interest in this work.
References
1. Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol. 2015;127:3–13.
2. Davanzo JR, Sieg EP, Timmons SD. Management of traumatic brain injury. Surg Clin North Am. 2017;97(6):1237–1253. doi:10.1016/j.suc.2017.08.001
3. Reith FCM, Lingsma HF, Gabbe BJ, Lecky FE, Roberts I, Maas AIR. Differential effects of the Glasgow coma scale score and its components: an analysis of 54,069 patients with traumatic brain injury. Injury. 2017;48(9):1932–1943. doi:10.1016/j.injury.2017.05.038
4. In-Suk Bae MD, Hyoung-Joon Chun MD, Hyeong-Joong Y, Kyu-Sun C. Using components of the Glasgow coma scale and Rotterdam CT scores for mortality risk stratification in adult patients with traumatic brain injury: a preliminary study. Clin Neurol Neurosurg. 2020;188:105599. doi:10.1016/j.clineuro.2019.105599
5. Nik A, Sheikh Andalibi MS, Ehsaei MR, Zarifian A, Ghayoor Karimiani E, Bahadoorkhan G. The efficacy of Glasgow Coma Scale (GCS) score and Acute Physiology and Chronic Health Evaluation (APACHE) II for Predicting Hospital Mortality of ICU patients with acute traumatic brain injury. Bull Emerg Trauma. 2018;6(2):141–145. doi:10.29252/beat-060208
6. Wang KK, Yang Z, Zhu T, et al. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn. 2018;18:165–180. doi:10.1080/14737159.2018.1428089
7. Khellaf A, Khan DZ, Helmy A. Recent advances in traumatic brain injury. J Neurol. 2019;266(11):2878–2889. doi:10.1007/s00415-019-09541-4
8. Dixon KJ. Pathophysiology of traumatic brain injury. Phys Med Rehabil Clin N Am. 2017;28:215–225. doi:10.1016/j.pmr.2016.12.001
9. Golden N, Mahadewa TGB, Aryanti C, Widyadharma IPE. S100B serum level as a mortality predictor for traumatic brain injury: a meta-analysis. Open Access Maced J Med Sci. 2018;6:2239–2244. doi:10.3889/oamjms.2018.432
10. Shemilt M, Boutin A, Lauzier F, et al. Prognostic value of glial fibrillary acidic protein in patients with moderate and severe traumatic brain injury: a systematic review and meta-analysis. Crit Care Med. 2019;47(6):e522–e529. doi:10.1097/CCM.0000000000003728
11. Berger RP, Adelson PD, Pierce MC, Dulani T, Cassidy LD, Kochanek PM. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J Neurosurg. 2005;103:61–68. doi:10.3171/ped.2005.103.1.0061
12. Xiao H, Chen J, Duan L, Li S. Role of emerging vitamin K‑dependent proteins: growth arrest‑specific protein 6, Gla‑rich protein and periostin (Review). Int J Mol Med. 2021;47:2. doi:10.3892/ijmm.2020.4835
13. Akalu YT, Rothlin CV, Ghosh S. TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev. 2017;276(1):165–177. doi:10.1111/imr.12522
14. Nagata K, Ohashi K, Nakano T, et al. Identification of the product of growth arrest specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271(47):30022–30027. doi:10.1074/jbc.271.47.30022
15. Burstyn-Cohen T, Maimon A. TAM receptors, Phosphatidylserine, inflammation, and Cancer. Cell Commun Signal. 2019;17(1):156. doi:10.1186/s12964-019-0461-0
16. Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33(1):355–391. doi:10.1146/annurev-immunol-032414-112103
17. Stitt TN, Conn G, Goret M, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80(4):661–670. doi:10.1016/0092-8674(95)90520-0
18. Prieto AL, Weber JL, Tracy S, Heeb MJ, Lai C. Gas6, a ligand for the receptor protein-tyrosine kinase Tyro-3, is widely expressed in the central nervous system. Brain Res. 1999;816:646–661. doi:10.1016/S0006-8993(98)01159-7
19. Funakoshi H, Yonemasu T, Nakano T, Matumoto K, Nakamura T. Identification of Gas6, a putative ligand for Sky and Axl receptor tyrosine kinases, as a novel neurotrophic factor for hippocampal neurons. J Neurosci Res. 2002;68:150–160. doi:10.1002/jnr.10211
20. Tong LS, Shao AW, Ou YB, et al. Recombinant Gas6 augments Axl and facilitates immune restoration in an intracerebral hemorrhage mouse model. J Cereb Blood Flow Metab. 2017;37:1971–1981. doi:10.1177/0271678X16658490
21. Du Y, Lu Z, Yang D, et al. MerTK inhibits the activation of the NLRP3 inflammasome after subarachnoid hemorrhage by inducing autophagy. Brain Res. 2021;1766:147525. doi:10.1016/j.brainres.2021.147525
22. Sainaghi PP, Bellan M, Lombino F, et al. Growth arrest specific 6 concentration is increased in the cerebrospinal fluid of patients with Alzheimer’s disease. J Alzheimers Dis. 2017;55:59–65. doi:10.3233/JAD-160599
23. Kim BS, Jung HS, Bang OY, Chung CS, Lee KH, Kim GM. Elevated serum lipoprotein(a) as a potential predictor for combined intracranial and extracranial artery stenosis in patients with ischemic stroke. Atherosclerosis. 2010;212(2):682–688. doi:10.1016/j.atherosclerosis.2010.07.007
24. Stein DM, Hu PF, Brenner M, et al. Brief episodes of intracranial hypertension and cerebral hypoperfusion are associated with poor functional outcome after severe traumatic brain injury. J Trauma. 2011;71(2):364–373. doi:10.1097/TA.0b013e31822820da
25. Pierce AM, Keating AK. TAM receptor tyrosine kinases: expression, disease and oncogenesis in the central nervous system. Brain Res. 2014;1542:206–220. doi:10.1016/j.brainres.2013.10.049
26. Guo ZN, Liu J, Chang J, et al. GAS6/Axl signaling modulates blood-brain barrier function following intravenous thrombolysis in acute ischemic stroke. Front Immunol. 2021;12:742359. doi:10.3389/fimmu.2021.742359
27. Morales A, Rojo Rello S, Cristóbal H, et al. Growth arrest-specific factor 6 (GAS6) is increased in COVID-19 patients and predicts clinical outcome. Biomedicines. 2021;9:335. doi:10.3390/biomedicines9040335
28. Yeh LC, Huang PW, Hsieh KH, et al. Elevated plasma levels of Gas6 are associated with acute lung injury in patients with severe sepsis. Tohoku J Exp Med. 2017;24:187–193. doi:10.1620/tjem.243.187
29. Caldentey G, García De Frutos P, Cristóbal H, et al. Serum levels of growth arrest-specific 6 protein and soluble AXL in patients with ST-segment elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2019;8:708–716. doi:10.1177/2048872617740833
30. Munakomi S. A comparative study between Marshall and Rotterdam CT scores in predicting early deaths in patients with traumatic brain injury in a major tertiary care hospital in Nepal. Chin J Traumatol. 2016;19(1):25–27. doi:10.1016/j.cjtee.2015.12.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.