Back to Journals » International Journal of General Medicine » Volume 16
Serum Sestrin2 Emerges as a Prognostic Biomarker of Human Aneurysmal Subarachnoid Hemorrhage: A Prospective Observational Cohort Single-Center Study
Authors Wang D, Ma L, Li Z, Ye G, Chen M
Received 28 June 2023
Accepted for publication 23 August 2023
Published 28 August 2023 Volume 2023:16 Pages 3869—3887
DOI https://doi.org/10.2147/IJGM.S428011
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Dongfeng Wang, Lei Ma, Zhenqiang Li, Gengfan Ye, Maosong Chen
Department of Neurosurgery, Ningbo Medical Center Lihuili Hospital, Ningbo, 315100, People’s Republic of China
Correspondence: Dongfeng Wang, Department of Neurosurgery, Ningbo Medical Center Lihuili Hospital, Ningbo, 315100, People’s Republic of China, Email [email protected]
Background: Sestrin2 functions as a neuroprotective factor. Herein, serum sestrin2 was investigated with respect to its associations with severity, delayed cerebral ischemia (DCI) and prognosis of aneurysmal subarachnoid hemorrhage (aSAH).
Methods: In this prospective, observational, cohort, single-center study, serum sestrin2 levels were measured at entry into the study in 45 healthy controls and at admission in 135 aSAH patients. Also, they were gauged in other time points (namely, at days 1, 2, 3, 5 and 7) among 45 patients. Unfavorable prognosis was defined as extended Glasgow Outcome Scale (GOSE) scores of 1– 4 at six months after aSAH.
Results: Serum sestrin2 levels were immediately raised at admission in patients, increased thereafter, peaked at day 2, declined afterwards till day 7, and were significantly higher than those in controls (all P< 0.001). Serum sestrin2 levels had independent correlation with Hunt-Hess scores (beta, 1.715; 95% confidence interval (CI), 0.595– 2.835; P=0.003) and modified Fisher scores (beta, 2.505; 95% CI, 1.102– 3.907; P=0.001). Alternatively, serum sestrin2 levels, which were independently correlated with 6-month GOSE scores (beta, − 0.050; 95% CI, − 0.099– 0.001; P=0.044), were independently associated with DCI (odds ratio, 1.079; 95% CI, 1.008– 1.156; P=0.029) and unfavorable prognosis (odds ratio, 1.093; 95% CI, 1.020– 1.172; P=0.012). DCI and prognosis prediction models, which were composed of serum sestrin2, Hunt-Hess scores and modified Fisher scores, were comparatively stable and clinically beneficial under calibration curve and decision curve. Prognosis prediction model showed significantly higher area under receiver operating characteristic curve than serum sestrin2, Hunt-Hess scores and modified Fisher scores alone (all P< 0.05).
Conclusion: A significant enhancement of serum sestrin2 levels after aSAH is independently related to severity, DCI and poor prognosis following aSAH. The models incorporating serum sestrin2 perform well in predicting the DCI and prognosis of aSAH patients. Presumably, determination of serum sestrin2 may be of clinical significance in aSAH.
Keywords: aneurysm, subarachnoid hemorrhage, Sestrin2, delayed cerebral ischemia, prognosis, severity
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH), which is characterized as a hemorrhagic stroke resulting from aneurysmal rupture, is the principal cause of death and disability among survivors around the world.1 When bleedings accumulate inside the subarachnoid space, some subsequent phenomena, such as early brain injury (EBI) and delayed cerebral ischemia (DCI), occur.2,3 Both EBI and DCI are the two key pathophysiological processes pertaining to acute brain injury following aSAH.4 Oxidative stress and inflammation have been acknowledged as the two main molecular mechanisms contributing to acute brain injury after aSAH.5,6 The excessive generation of reactive oxygen species and cytokines results in cellular toxicity, thereby inducing neuronal death, brain edema and blood–brain barrier disruption; and such brain damage elicited neurologic dysfunction and even death of patients with aSAH.7 Clinically, several severity assessment scales, such as the Hunt-Hess scale and modified Fisher scales, have been universally adopted as the prognostic predictors of aSAH.8 Noteworthily, biochemical markers in peripheral blood, such as glial fibrillary astrocyte protein, myelin basic protein, S100B, C-reactive protein and interleukin-6, are ubiquitously studied and exploration of apposite prognostic biomarkers has attracted widespread attentions in aSAH-related neurologic science during recent decades.9,10
Sestrin2 is recognized as hypoxia-inducible gene 95, which is a very important member of the sestrin family.11 Its production can be induced by various insults, such as hypoxia, oxidative stress, inflammation and ischemia.12 Accumulating evidence has pointed to a consensus concept that sestrin2 may be a protective shield because of its anti-oxidant and anti-inflammatory properties in numerous diseases, such as chronic obstructive pulmonary disease, coronary artery disease and type 2 diabetes mellitus.13–15 Also, there has been a common view that high blood sestrin2 levels in those patients may reflect a compensatory response to enhanced insults.16 In central nervous system, sestrin2 may be mainly expressed by neurons and microglia.17–19 It is believably demonstrated that sestrin2 may be neuroprotective based on experimental studies concerning ischemic stroke,17 traumatic brain injury18 and subarachnoid hemorrhage.19 Intriguingly, serum sestrin2 levels had been found to be were significantly elevated in patients with Parkinson’s disease,20 Alzheimer’s disease or mild cognitive impairment.21 It is referred that serum sestrin2 may be a biomarker for reflecting acute brain injury. Herein, we measured serum sestrin2 levels and further explored its significance as a prognostic biochemical marker of aSAH.
Methods
Study Design
This was a prospective, observational, cohort study, which was implemented at an academic medical center in Ningbo, Zhejiang Province, China from May 2018 to October 2021. This study was assigned into the two parts. In the first part, we intended to observe dynamic change of serum sestrin2 levels after aSAH and thus peripheral blood samples were collected at entry into the study from controls and at admission and days 1, 2, 3, 5 and 7 from all patients, who agreed with blood-drawings at multiple time-points. In the second part, we attempted to investigate the prognostic role of serum sestrin2 in aSAH and therefore peripheral blood was acquired at admission from all patients, who gave consent to blood-obtainment at admission. The 2008 Revision of the Declaration of Helsinki and its later amends were obeyed when the current study went on. The study protocol was endorsed by the Institutional Review Committee at Ningbo Medical Center Lihuili Hospital (No. LHH23209). Prior to blood-collection, informed consent was signed by patients’ legal representatives or controls themselves.
Participant Enrollment
The consecutively recruited subjects in the current study included patients and healthy controls. Inclusion criteria for patients were depicted as follows: (1) Consent to participation in this study; (2) Hospital admission with first-ever stroke; (3) A computerized tomography (CT) confirmation of SAH; (4) An age of 18 years or older; (5) A CT angiography or digital subtraction angiography diagnosis of intracranial aneurysm; (6) Rupture of single aneurysm; (7) Hospitalization of 24 hours after symptom onset; and (8) Aneurysm clipping or interventional treatment of 48 hours following hospital admission. Exclusion criteria for patients were shown below: (1) Rerupture of the aneurysm; (2) Pseudoaneurysm; (3) Previous or present other neurological diseases, such as cerebrovascular disorders, brain degenerative diseases and intracranial tumors; (4) Previous or present certain diseases, such as malignancies, immune system diseases and severe dysfunction of other organs; and (5) Other specific conditions, such as loss to follow-up, incomplete information and hemolysis. Controls were chosen from healthy volunteers. They did not have chronic comorbidities, such as hypertension, diabetes mellitus and coronary heart disease, there were normal data in some routine blood tests, such as blood sugar detection, blood cell analyses and blood electrolyte measurements, and some specific auxiliary examinations, such as chest x-ray check and routine electrocardiogram, did not show obvious abnormal results.
Data Collection
We collected some conventional information, such as demographical data, adverse lifestyle habits, chronic comorbidities, medication histories and vital signs. The Hunt-Hess scale, varying from 1 to 5, was adopted to evaluate neurologic status and the modified Fisher grading scale, varying from 0 to 4, was applied as a radiological severity indicator. We recorded conformation, location and size of aneurysms. For the sake of securing aneurysms, neurosurgical clipping or endovascular intervention was employed. Head CT scans were implemented on the first day, the first week, the second week and the first month postoperatively, and diagnosis of DCI was verified in accordance with the previous criteria.22 Intraventricular hemorrhage and acute hydrocephalus were experienced in some patients. As necessary, external ventricular drainage was performed. The recorded complications included pneumonia and seizure. Extended Glasgow Outcome Scale (GOSE) scores varied from 1 to 8 and GOSE scores of 1–4 at six months after the onset of symptom was deemed as an unfavorable prognosis.23
Immune Analysis
Venous blood was drawn, once controls were permitted into the study. Among a portion of patients, venous blood samples were obtained at admission. And among another portion of patients, venous blood samples were collected at days 0 (at admission) 1, 2, 3, 5 and 7 after aSAH. All blood samples were immediately placed in 5 mL gel-containing biochemistry tubes. Blood was centrifugated and thereby serum was separated. Afterwards, an aliquot of extracted serum sample was stored at a −80°C refrigerator for later measurements. Every at most three months, a batch of serum samples were melted and serum sestrin2 levels were gauged. All measurements were in duplicate done by the same skilled technician, who was inaccessible to the clinical materials. The enzyme-linked immunosorbent assay kits (catalog number, E3437Hu) were bought from Bioassay Technology Laboratory (Shanghai, China). The detection sensitivity was 0.01 ng/mL, detection range was from 0.05 to 15 ng/mL, intra-assay coefficients of variation were <8% and inter-assay coefficients of variation were <10%.
Statistical Analysis
As for data analysis, we used the three statistical software packages as follows: SPSS (version 22.0; SPSS Inc., Chicago, IL, USA), MedCalc 20.1 (MedCalc Software, Mariakerke, Belgium) and R software (version 3.5.1; https://www.r-project.org). Qualitative variables, which are shown in the form of the counts (percentages), were compared between the group using the chi-square test or Fisher’s exact test as appropriate. As regard to quantitative variables, the Shapiro–Wilk test was done to evaluate data distributions. Subsequently, they were presented as the mean (standard deviation, SD) or the median (25th-75th percentiles) as appropriate. Also, the Mann–Whitney U-test or the independent t-test was adopted for intergroup comparisons. The Kruskal–Wallis test or Friedman test was employed for multiple-group comparisons of serum sestrin2 levels. The Spearman correlation coefficient test and the multivariable linear regression analysis was sequentially used for bivariate correlation analyses. The binary logistic regression models were constructed, in which DCI and 6-month poor prognosis were regarded as dependent variables. The independent predictors were incorporated into nomograms, and then calibration curves, decision curves and restricted cubic splines were drawn to assess the prediction model. Area under receiver operating characteristic (ROC) curve (AUC) was estimated to assess discriminative efficiency. The Z-test was carried out for comparing AUCs. The cutoff criterion was selected via the Youden method. When two-tailed P-value was less than 0.05, the difference was deemed as statistical significance.
Results
Participant Recruitments
Based on the specified inclusion criteria, an aggregate of 186 patients, who were inflicted with aSAH, underwent an initial observational assessment. Afterwards, fifty-one patients were removed from the current study owing to the reasons displayed in Figure 1. Eventually, one hundred and thirty-five patients were chosen for the data analysis. Among them, there were 48 cases with the development of six-month unfavorable prognosis, 35 cases presenting with DCI and 45 cases who accepted blood-sample collections at admission and at days 1, 2, 3, 5 and 7 after aSAH. Additionally, forty-five controls were selected.
Participants’ Basic Characteristics
Controls were aged from 33 to 77 years (mean, 47.4 years; SD, 9.8 years), comprised 23 females and 22 males, and consisted of 15 alcohol drinkers and 14 cigarette smokers, as well as their body mass index ranged from 19.3 to 30.2 kg/m2 (mean, 24.1 kg/m2; SD, 2.8 kg/m2). Among this special group of 45 patients, there were 28 females and 17 males, the age ranged from 32–73 years (mean, 49.9 years; SD, 10.5 years), 13 alcohol drinkers and 13 cigarette smokers were revealed, and body mass index ranged from 19.6 to 28.7 kg/m2 (mean, 23.3 kg/m2; SD, 2.4 kg/m2). As for the differences of mean age and body mass index, as well as gender, alcohol drinker and cigarette smoker percentages between controls and that special group of 45 aSAH patients, statistical significances were not confirmed (all P>0.05).
This cohort of 135 aSAH patients (76 females and 59 males) were aged from 28 to 73 years (mean, 52.1 years; SD, 10.7 years). Their body mass index ranged from 19.0 to 30.1 kg/m2 (mean, 23.6 kg/m2; SD, 2.5 kg/m2). There were 41 cigarette smoker, 36 alcohol drinkers, 25 hypertensive individuals, 14 diabetic subjects, 30 dyslipidemic suffers and 7 patients sustaining coronary heart disease. In total, 20, 7, 13 and 21 patients orally took statins, anticoagulants, antiplatelet and antihypertensive agents, respectively. And eleven patients were orally administrated with hypoglycemic drugs or were subcutaneously injected with insulin. Systolic arterial blood pressure, diastolic arterial blood pressure and mean arterial blood pressure ranged from 84 to 192 mmHg (mean,131.1 mmHg; SD, 21.3 mmHg), from 48 to 110 mmHg (mean,81.9 mmHg; SD, 13.3 mmHg) and from 66 to 151 mmHg (mean, 106.5 mmHg; SD, 16.2 mmHg), respectively. The Hunt-Hess scores varied from 1 to 5, with a median value of 3 (lower-upper quartiles, 2–4) and the modified Fisher scores varied from 1 to 4, with a median value of 2 (lower-upper quartiles, 2–3). Intracranial aneurysms in 104 patients were located in anterior circulation and those in the remainders were in posterior circulation; 113 patients occupied cystic aneurysms and other shapes were displayed in aneurysms of the remaining patients. Aneurysms with diameter <10 mm and ≥10 mm were revealed in 74 and 61 patients, respectively. Surgical clipping was done for securing aneurysms among 53 patients and 82 patients underwent endovascular intervention for embolizing aneurysms. Acute hydrocephalus, intraventricular hemorrhage, pneumonia and seizure were observed in 24, 22, 26 and 17 patients, respectively. External ventricular drainage was implemented for 22 patients. Admission time varied from 0.4 to 24.0 hours (median, 8.6 hours; 25th-75th percentiles, 4.5–13.8 hours). Blood-sample collection time varied from 0.6 to 26.5 hours (median, 9.4 hours; lower-upper quartiles, 5.1–14.6 hours).
Dynamic Change of Serum Sestrin2 Levels After aSAH
In the current study, 45 patients gave permission to blood-collection at the multiple time points. As plotted in Figure 2, serum sestrin2 levels were immediately raised at admission, afterwards were gradually increased at day 1, peaked at day 2, and were declined at day 3 until day 7; and serum sestrin2 levels were substantially higher in patients at admission and at days 1, 2, 3, 5 and 7 than controls (all P<0.001).
Relationship Between Serum Sestrin2 Levels and Illness Severity After aSAH
As displayed in Table 1, there was a tight correlation between serum sestrin2 levels and Hunt-Hess scores (P<0.001), between serum sestrin2 levels and modified Fisher scores (P<0.001), between serum sestrin2 levels and external ventricular drainage (P<0.05), between serum sestrin2 levels and intraventricular hemorrhage (P<0.01), between serum sestrin2 and acute hydrocephalus (P<0.05), as well as between serum sestrin2 levels and blood glucose levels (P<0.01). In the Multivariate linear regression model incorporating the above-mentioned six variables of significant correlation, serum sestrin2 levels had independent correlation with Hunt-Hess scores (beta, 1.715; 95% CI, 0.595–2.835; VIF, 1.749; t=3.029; P=0.003) and modified Fisher scores (beta, 2.505; 95% CI, 1.102–3.907; VIF, 1.722; t=3.534; P=0.001). In total, 24, 24, 51, 31 and 5 patients had Hunt-Hess scores 1, 2, 3, 4 and 5, respectively. In aggregate, modified Fisher scores 1, 2, 3 and 4 were found in 24, 51, 48 and 12 patients, respectively. As depicted in Figure 3, as compared to patients with Hunt-Hess score 1, serum sestrin2 levels were substantially elevated in those with Hunt-Hess score 2, followed by those with Hunt-Hess scores 3 and 4, and were significantly highest in those with Hunt-Hess score 5 (P<0.001); and its levels were markedly raised in the order of modified Fisher scores from 1 to 4 (P<0.001; Figure 4).
 |
Table 1 Relationship Between Serum Sestrin2 Levels and Other Variables in Patients with Aneurysmal Subarachnoid Hemorrhage |
Relation of Serum Sestrin2 Levels to Six-Month GOSE Scores After aSAH
At six months following aSAH, GOSE scores varied from 1 to 8, its median value was 5, as well as its lower and upper quartiles were 4 and 7 respectively. Totally, 8, 11, 12, 17, 25, 18, 19 and 25 patients experienced GOSE scores 1, 2, 3, 4, 5, 6, 7 and 8, respectively. In Figure 5, serum sestrin2 levels were significantly declined in the order of GOSE scores from 1 to 8 (P<0.001). As listed in Table 2, GOSE scores were intimately correlated with serum sestrin2 levels (P<0.001), Hunt-Hess scores (P<0.001), modified Fisher scores (P<0.001), external ventricular drainage (P<0.05), intraventricular hemorrhage (P<0.01), acute hydrocephalus (P<0.01) and blood glucose levels (P<0.05). Using the multivariate linear regression analysis, the factors, which were retained as the independent correlated variables, were serum sestrin2 levels (beta, −0.050; 95% CI, −0.099–0.001; VIF, 1.583; t=−2.037; P=0.044), Hunt-Hess scores (beta, −0.670; 95% CI, −0.993–0.347; VIF, 1.874; t=−4.108; P<0.001) and modified Fisher scores (beta, −0.665; 95% CI, −1.074–0.256; VIF, 1.890; t=−3.214; P=0.002).
 |
Table 2 Relationship Between Postinjury 6-Month Extended Glasgow Outcome Scale Scores and Other Variables in Patients with Aneurysmal Subarachnoid Hemorrhage |
Association of Serum Sestrin2 Levels with Poor Outcome at Postinjury Six Months
A total of 48 patients experienced six-month poor outcome (GOSE scores 1–4) following aSAH. Using the restricted cubic spline (Figure 6), serum sestrin2 levels were linearly related to the risk of poor outcome (P=0.439). Under the ROC curve (Figure 7), serum sestrin2 levels significantly discriminated patients at risk of poor outcome and sestrin2 levels above 7.2 ng/mL predicted poor outcome with the maximum Youden index.
Table 3 shows that, patients with the development of poor outcome, in comparison to those presenting with good outcome, had substantially elevated Hunt-Hess scores (P<0.001), modified Fisher scores (P<0.001), serum sestrin2 levels (P<0.001) and blood glucose levels (P<0.05), as well as displayed significantly increased proportions of intraventricular bleeding (P<0.05), acute hydrocephalus (P<0.05) and external ventricular drainage (P<0.05). When the binary logistic regression model, in which the preceding seven significant variables were forced, was configured, Hunt-Hess scores (OR, 3.439; 95% CI, 1.649–7.171; P=0.001), modified Fisher scores (OR, 3.325; 95% CI, 1.526–7.244; P=0.003) and serum sestrin2 levels (OR, 1.093; 95% CI, 1.020–1.172; P=0.012) were verified to be the three independent predictors of poor six-month outcome following aSAH.
 |
Table 3 Factors Associated with 6-Month Functional Outcome After Aneurysmal Subarachnoid Hemorrhage |
The Hunt-Hess scores, modified Fisher scores and serum sestrin2 levels were integrated to form a prediction model using the combined logistic regression method. A nomogram (Figure 8), in which the prediction model was visually displayed, was configured to describe the risk of poor outcome. Using the Hosmer–Lemeshow goodness-of-fit test, the model was relatively stable (P=0.688). Under the calibration curve (Figure 9), the model was valid. Using the decision curve analysis (Figure 10), the model was of clinical benefit. Under the ROC curve (Figure 11), the predictive model had substantially higher discriminatory efficiency for poor prognosis than the Hunt-Hess scores, modified Fisher scores and serum sestrin2 levels alone (all P<0.05).
Relation of Serum Sestrin2 Levels to DCI Following aSAH
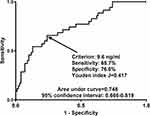
A total of 35 patients suffered from DCI after aSAH. Using the restricted cubic spline (Figure 12), there was a linear relationship between serum sestrin2 levels and the DCI risk (P=0.397). As for the discriminatory efficiency under the ROC curve (Figure 13), serum sestrin2 levels substantially distinguished the DCI risk and sestrin2 levels more than 9.6 ng/mL discriminated patients at risk of DCI with the maximum Youden index.
As displayed in Table 4, as opposed to patients without DCI development, those experiencing DCI exhibited dramatically increased Hunt-Hess scores (P<0.001), modified Fisher scores (P<0.001), serum sestrin2 levels (P<0.001) and blood glucose levels (P<0.05), as well as showed markedly raised percentages of intraventricular bleeding (P<0.05), acute hydrocephalus (P<0.05) and external ventricular drainage (P<0.05). The above-mentioned seven significant variables were entered into the binary logistic regression model, and subsequently it was found that Hunt-Hess scores (OR, 2.432; 95% CI, 1.225–4.856; P=0.011), modified Fisher scores (OR, 2.402; 95% CI, 1.130–5.230; P=0.023) and serum sestrin2 levels (OR, 1.079; 95% CI, 1.008–1.156; P=0.029) emerged as the three independent predictors of DCI following aSAH.
 |
Table 4 Factors Associated with Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage |
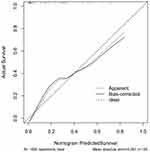
A model, in which the Hunt-Hess scores, modified Fisher scores and serum sestrin2 levels were combined using the combined logistic regression method, was built to predict DCI. A nomogram (Figure 14) was used to show the model visually. Using the Hosmer–Lemeshow goodness-of-fit test, the model was comparatively stable (P=0.561). The model was rather valid using the calibration curve analysis (Figure 15). Under the decision curve (Figure 16), the model was clinically beneficial. As regards predictive ability under the ROC curve (Figure 17), the model displayed significantly higher DCI predictive ability than serum sestrin2 levels alone (P<0.05), but not than the Hunt-Hess scores and modified Fisher scores alone (both P>0.05).
Discussion
To the best of our knowledge, this epidemiological study may be for the first time done to investigate serum sestrin2 levels after human aSAH and subsequently some interesting findings were discovered. There was a significant enhancement in serum sestrin2 levels after aSAH, which peaked at day 2 and thereafter gradually decreased. And serum sestrin2 levels of patients with aSAH at all time points were substantially higher than those of controls. Also, serum sestrin2 levels were independently correlated with Hunt-Hess scores, modified Fisher scores and postinjury six-month GOSE scores, as well as were independently associated with DCI and poor prognosis at six months after aSAH. Noteworthily, serum sestrin2 levels significantly improved discriminatory ability of Hunt-Hess scores and modified Fisher scores alone, especially in prognostic prediction. The prediction model composed of serum sestrin2 levels, Hunt-Hess scores and modified Fisher scores showed high stability, accuracy, validity and clinical benefit. Presumably, serum sestrin2 may be a prognostic biomarker of clinical value.
Sestrin2 has been recognized as a stress response protein, which may be in possession of cytoprotective characteristics via anti-inflammation, anti-oxidation and anti-apoptosis.11,12 Therefore, its expressions may be easily elicited in acute or chronic neurologic disorders with inflammatory or oxidative feature.17–21 As hypothesized, in rats subjected to acute focal cerebral ischemia, sestrin2 expressions were obviously up-regulated in infarcted zone.24 Similarly, sestrin2 expression was progressively enhanced in the hippocampal CA1 subfield within 24 hours in rats following transient global ischemia, reaching the maximal levels at 24 hours, and afterwards decreased till 48 hours.25 Also, sestrin2 was markedly induced in mouse cerebral cortices exposed to traumatic brain injury.18 Consistently, sestrin2 was dramatically raised in injured brain tissues of mice after SAH and moreover, it was mainly located in neurons and microglia.19 Thus, acute brain injury could lead to production or release of sestrin2 from injured brain tissues. In some chronic neurologic diseases, such as Parkinson’s disease, Alzheimer’s disease and mild cognitive impairment, serum sestrin2 levels have been found to be significantly higher than those of healthy controls.20,21 Our study not only observed the change of serum sestrin2 levels in humans after aSAH, but also investigated its temporal change. Specifically, peripheral blood of aSAH patients was collected from 0.6 to 26.5 hours, with a median value of 9.4 hours. Among a small portion of patients, blood samples were obtained also in other time points, namely days 1, 2, 3, 5 and 7 after aSAH. Our data showed that serum sestrin2 levels were immediately increased during the early phase, peaked at day 2 and then gradually diminished. Its high levels still existed during 7 days after aSAH, when compared to healthy controls. In consideration of a significant elevation of its expressions in animal brain tissues after acute brain injury,18,19,24,25 it is theoretically possible that serum sestrin2 may at least partially originate from central nervous system.
A growing body of evidence suggests that sestrin2 may confer neuroprotective effects against various noxious insults. In experiments regarding ischemic brain injury, sestrin2 has been demonstrated to be brain-protective via promoting angiogenesis, lessening cerebral edema, diminishing cerebral infarct area and improving neurological function.24 Also, using in vivo and in vitro models of traumatic brain injury, sestrin2 was confirmed to inhibit oxidative stress, reduce apoptosis, depress brain edema and diminish neurological deficit.18 Similarly, in an endovascular perforation SAH model of mice, sestrin2 obviously attenuated neuroinflammatory injury and oxidative stress, thereby ameliorating neurologic function.19 Contrarily, in another experiment, in which both oxygen-glucose deprivation/reoxygenation model of primary neurons and rat model of middle cerebral artery occlusion were built, sestrin2 significantly led to neuronal injury and enhanced the cerebral ischemia-reperfusion injury.26 Although there is an inconsistency in experimental data with respect to protective or detrimental effects of sestrin2 in acute brain injury, the accumulating evidence has been strongly supportive of the notion that sestrin2 may be cytoprotective in acute brain injury diseases.17–19,24,25 In view of the present assumption of sestrin2’s protective function, it is conceivably believed that increased sestrin2 levels should probably be a compensatory response to noxious stimuli.
In the current study, the registered prognostic parameters were DCI and poststroke six-month functional outcome; and the recorded severity indicators were Hunt-Hess scores and modified Fisher scores. In terms of statistical methods, univariate and multivariate analyses were sequentially employed to analyze all correlations and associations. Also, GOSE, Hunt-Hess scale and modified Fisher scale were not only regarded as the continuous variables, but also as the categorical variables. Statistical results pointed to the assumption that serum sestrin2 levels were independently correlated with illness severity indicated by Hunt-Hess scores and modified Fisher scores and were independently associated with poor prognosis reflected by DCI, 6-month GOSE scores after aSAH.
In our study, a potential prediction model of DCI or poor prognosis at six months after aSAH was established, which was visually displayed using a nomogram. The model was composed of serum sestrin levels, Hunt-Hess scores and modified Fisher scores, all of which have been demonstrated to be the independent predictors of DCI and poor prognosis in the current study. For the sake of assessment of model stability, efficiency and clinical benefit, a series of statistical methods were applied, including the Hosmer–Lemeshow goodness-of-fit test, calibration curve analysis, ROC curve analysis and decision curve analysis. Our data showed that the model was highly efficient, comparatively stable and clinically beneficial in prognostic discrimination. Thus, serum sestrin2 may aid in severity assessment and prognostic prediction of aSAH, when combined with other severity scales, such as Hunt-Hess scale and modified Fisher scale, indicating that serum sestrin2 may be a prognostic biomarker of human aSAH.
Several strengths and weaknesses warrant to be mentioned in the current study. The strengths are that (1) Serum sestrin2, for the first time, was investigated with respect to its prognostic value in human aSAH and therefore was verified to be a potential prognostic biomarker of aSAH; (2) In view of statistical methods, all relations of serum sestrin2 levels to severity, DCI and prognosis of aSAH were demonstrated using multivariate analysis; and (3) Besides correlation analysis, two combination models were established to well predict DCI and long-term clinical outcomes of aSAH. The weaknesses are that (1) Although serum sestrin2 levels were determined at multiple time-points after aSAH, this study was devoid of enough patients to reveal optimal time-point, where serum sestrin2 levels would display highest prognostic predictive ability; (2) Although this study included more than one hundred patients for clinical investigation, this is still a single-center study. Thus, the final conclusions need to be validated via a larger cohort study in future.
Conclusions
To the best of our knowledge, this is a first series for determining relationship between serum sestrin2 levels and severity along with functional outcome of aSAH. Our study showed that (1) Serum sestrin2 levels are significantly elevated during the seven days after aSAH, reaching a highest level at day 2; (2) Admission serum sestrin2 levels are independently related to disease severity, DCI and functional outcome of aSAH; (3) The additive effects of serum sestrin2 levels on conventional severity scales (namely, Hunt-Hess scale and modified Fisher scale) are dominant in terms of DCI and outcome prediction. Hypothetically, serum sestrin2 may be considered as a prognostic determinant of clinical significance.
Abbreviations
aSAH, aneurysmal subarachnoid hemorrhage; AUC, area under the curve; CI, confidence interval; GOSE, Extended Glasgow Outcome Scale; OR, odds ratio; ROC, receiver operating characteristic; DCI, delayed cerebral ischemia.
Acknowledgments
The authors thank all staff for their technical support and are grateful to all participants for their blood samples.
Funding
The study was supported by grants from the Ningbo Municipal Bureau of Science and Technology (No. 2021S172) and NINGBO Medical & Health Leading Academic Discipline Project (No. 2022-F04).
Disclosure
The authors report no conflicts of interest concerning the materials or methods used in this study, or the findings specified in this article.
References
1. Connolly ES
2. Lauzier DC, Jayaraman K, Yuan JY, et al. Early brain injury after subarachnoid hemorrhage: incidence and mechanisms. Stroke. 2023;54(5):1426–1440. doi:10.1161/STROKEAHA.122.040072
3. Ikram A, Javaid MA, Ortega-Gutierrez S, et al. Delayed cerebral ischemia after subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2021;30(11):106064. doi:10.1016/j.jstrokecerebrovasdis.2021.106064
4. Osgood ML. Aneurysmal subarachnoid hemorrhage: review of the pathophysiology and management strategies. Curr Neurol Neurosci Rep. 2021;21(9):50. doi:10.1007/s11910-021-01136-9
5. Lucke-Wold BP, Logsdon AF, Manoranjan B, et al. Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci. 2016;17(4):497. doi:10.3390/ijms17040497
6. Yang Y, Chen S, Zhang JM. The updated role of oxidative stress in subarachnoid hemorrhage. Curr Drug Deliv. 2017;14(6):832–842. doi:10.2174/1567201813666161025115531
7. Wu F, Liu Z, Li G, et al. Inflammation and oxidative stress: potential targets for improving prognosis after subarachnoid hemorrhage. Front Cell Neurosci. 2021;15:739506. doi:10.3389/fncel.2021.739506
8. Fang Y, Lu J, Zheng J, et al. Comparison of aneurysmal subarachnoid hemorrhage grading scores in patients with aneurysm clipping and coiling. Sci Rep. 2020;10(1):9199. doi:10.1038/s41598-020-66160-0
9. Zheng YK, Dong XQ, Du Q, et al. Comparison of plasma copeptin and multiple biomarkers for assessing prognosis of patients with aneurysmal subarachnoid hemorrhage. Clin Chim Acta. 2017;475:64–69. doi:10.1016/j.cca.2017.10.009
10. Rodríguez-Rodríguez A, Egea-Guerrero JJ, Ruiz de Azúa-López Z, Murillo-Cabezas F. Biomarkers of vasospasm development and outcome in aneurysmal subarachnoid hemorrhage. J Neurol Sci. 2014;341(1–2):119–127. doi:10.1016/j.jns.2014.04.020
11. Che X, Chai J, Fang Y, et al. Sestrin2 in hypoxia and hypoxia-related diseases. Redox Rep. 2021;26(1):111–116. doi:10.1080/13510002.2021.1948774
12. Pasha M, Eid AH, Eid AA, Gorin Y, Munusamy S. Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxid Med Cell Longev. 2017;2017:3296294. doi:10.1155/2017/3296294
13. Zhang DW, Wei YY, Ji S, Fei GH. Correlation between sestrin2 expression and airway remodeling in COPD. BMC Pulm Med. 2020;20(1):297. doi:10.1186/s12890-020-01329-x
14. Kishimoto Y, Aoyama M, Saita E, et al. Association between plasma sestrin2 levels and the presence and severity of coronary artery disease. Dis Markers. 2020;2020:7439574. doi:10.1155/2020/7439574
15. Chung HS, Hwang HJ, Hwang SY, et al. Association of serum Sestrin2 level with metabolic risk factors in newly diagnosed drug-naïve type 2 diabetes. Diabetes Res Clin Pract. 2018;144:34–41. doi:10.1016/j.diabres.2018.07.024
16. Kishimoto Y, Kondo K, Momiyama Y. The protective role of sestrin2 in atherosclerotic and cardiac diseases. Int J Mol Sci. 2021;22(3):1200. doi:10.3390/ijms22031200
17. Shi X, Doycheva DM, Xu L, Tang J, Yan M, Zhang JH. Sestrin2 induced by hypoxia inducible factor 1 alpha protects the blood-brain barrier via inhibiting VEGF after severe hypoxic-ischemic injury in neonatal rats. Neurobiol Dis. 2016;95:111–121. doi:10.1016/j.nbd.2016.07.016
18. Liu X, Li M, Zhu J, Huang W, Song J. Sestrin2 protects against traumatic brain injury by reinforcing the activation of Nrf2 signaling. Hum Exp Toxicol. 2021;40(7):1095–1111. doi:10.1177/0960327120984224
19. Yang Y, Ding H, Yang C, et al. Sestrin2 provides cerebral protection through activation of Nrf2 signaling in microglia following subarachnoid hemorrhage. Front Immunol. 2023;14:1089576. doi:10.3389/fimmu.2023.1089576
20. Rai N, Upadhyay AD, Goyal V, Dwivedi S, Dey AB, Dey S. Sestrin2 as serum protein marker and potential therapeutic target for Parkinson’s disease. J Gerontol a Biol Sci Med Sci. 2020;75(4):690–695. doi:10.1093/gerona/glz234
21. Rai N, Kumar R, Desai GR, et al. Relative alterations in blood-based levels of sestrin in Alzheimer’s disease and mild cognitive impairment patients. J Alzheimers Dis. 2016;54(3):1147–1155. doi:10.3233/JAD-160479
22. Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391–2395. doi:10.1161/STROKEAHA.110.589275
23. Jiang F, Chen Z, Hu J, Liu Q. Serum soluble scavenger receptor A levels are associated with delayed cerebral ischemia and poor clinical outcome after aneurysmal subarachnoid hemorrhage: a prospective observational study. Neuropsychiatr Dis Treat. 2022;18:2529–2541. doi:10.2147/NDT.S387487
24. Wang P, Zhao Y, Li Y, et al. Sestrin2 overexpression attenuates focal cerebral ischemic injury in rat by increasing Nrf2/HO-1 pathway-mediated angiogenesis. Neuroscience. 2019;410:140–149. doi:10.1016/j.neuroscience.2019.05.005
25. Chuang YC, Yang JL, Yang DI, Lin TK, Liou CW, Chen SD. Roles of sestrin2 and ribosomal protein S6 in transient global ischemia-induced hippocampal neuronal injury. Int J Mol Sci. 2015;16(11):26406–26416. doi:10.3390/ijms161125963
26. Zhang LL, Zhang ZJ. Sestrin2 aggravates oxidative stress of neurons by decreasing the expression of Nrf2. Eur Rev Med Pharmacol Sci. 2018;22(11):3493–3501. doi:10.26355/eurrev_201806_15176
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.