Back to Journals » Journal of Inflammation Research » Volume 16
Pretreatment with Eupatilin Attenuates Inflammation and Coagulation in Sepsis by Suppressing JAK2/STAT3 Signaling Pathway
Authors Lu Y , Li D , Huang Y, Sun Y, Zhou H, Ye F, Yang H , Xu T, Quan S, Pan J
Received 25 October 2022
Accepted for publication 24 February 2023
Published 10 March 2023 Volume 2023:16 Pages 1027—1042
DOI https://doi.org/10.2147/JIR.S393850
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Adam D Bachstetter
Yilun Lu,1– 3,* Ding Li,1– 3,* Yueyue Huang,1– 3 Yuanyuan Sun,1– 3 Hongmin Zhou,1– 3 Fanrong Ye,1– 3 Hongjing Yang,1– 3 Tingting Xu,1– 3 Shichao Quan,3– 6 Jingye Pan1– 5
1Department of Intensive Care Unit, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 2Key Laboratory of Intelligent Treatment and Life Support for Critical Diseases of Zhejiang Province, Wenzhou, People’s Republic of China; 3Wenzhou Key Laboratory of Critical Care and Artificial Intelligence, Wenzhou, People’s Republic of China; 4Collaborative Innovation Center for Intelligence Medical Education, Wenzhou, People’s Republic of China; 5Zhejiang Engineering Research Center for Hospital Emergency and Process Digitization, Wenzhou, People’s Republic of China; 6Department of General Medicine, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jingye Pan, Department of Intensive Care Unit, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China, Email [email protected] Shichao Quan, Department of General Medicine, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China, Email [email protected]
Purpose: Sepsis is an aggressive and life-threatening organ dysfunction induced by infection. Excessive inflammation and coagulation contribute to the negative outcomes for sepsis, resulting in high morbidity and mortality. In this study, we explored whether Eupatilin could alleviate lung injury, reduce inflammation and coagulation during sepsis.
Methods: We constructed an in vitro sepsis model by stimulating RAW264.7 cells with 1 μg/mL lipopolysaccharide (LPS) for 6 hours. The cells were divided into control group, LPS group, LPS+ Eupatilin (Eup) group, and Eup group to detect their cell activity and inflammatory cytokines and coagulation factor levels. Cells in LPS+Eup and Eup group were pretreated with Eupatilin (10μM) for 2 hours. In vivo, mice were divided into sham operation group, cecal ligation and puncture (CLP) group and Eup group. Mice in the CLP and Eup groups were pretreated with Eupatilin (10mg/kg) for 2 hours by gavage. Lung tissue and plasma were collected and inflammatory cytokines, coagulation factors and signaling were measured.
Results: In vitro, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and tissue factor (TF) expression in LPS-stimulated RAW264.7 cells was downregulated by Eupatilin (10μM). Furthermore, Eupatilin inhibited phosphorylation of the JAK2/STAT3 signaling pathway and suppressed p-STAT3 nuclear translocation. In vivo, Eupatilin increased the survival rate of the mice. In septic mice, plasma concentrations of TNF-α, IL-1β and IL-6, as well as TF, plasminogen activator inhibitor 1 (PAI-1), D-dimer, thrombin-antithrombin complex (TAT) and fibrinogen were improved by Eupatilin. Moreover, Eupatilin alleviated lung injury by improving the expression of inflammatory cytokines and TF, fibrin deposition and macrophage infiltration in lung tissue.
Conclusion: Our results revealed that Eupatilin may modulate inflammation and coagulation indicators as well as improve lung injury in sepsis via the JAK2/STAT3 signaling pathway.
Keywords: Eupatilin, sepsis, inflammation, coagulation, JAK2/STAT3
Introduction
Sepsis is a life-threatening organ dysfunction caused by infection, which results from dysregulated host response.1 A study reported in Lancet showed that sepsis remains a major cause of health loss worldwide, with 48.9 million cases each year and approximately 11 million deaths, which accounts around one-fifth of the total global deaths.2 According to Nature Reviews Disease Primers, more than half of sepsis patients are complicated with coagulation system imbalance.3 Moreover, in patients who have sepsis with disseminated intravascular coagulation (DIC) the mortality rate is almost twice that of sepsis patients without DIC.4 Therefore, reducing inflammation and coagulation disorders could be the key to reducing sepsis mortality.
Macrophages are involved in various pathophysiological processes in sepsis, including inflammation, coagulation, immunity and metabolism, and play a crucial role in sepsis-induced acute lung injury.5,6 In the early stages of sepsis, macrophages engulf pathogens and activate the innate immune system. Macrophages express pattern recognition receptors (PRRs), which can detect pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), initiating a series of immune cell activations and upregulating the expression of inflammation-related genes, that produce a series of inflammatory cytokines.7 In this process, the body targets pathogens to contain, kill and/or expel them. However, there is also a defense strategy, called disease tolerance, which refers to limiting the negative impact of infection on the host without affecting the pathogen load.8 The expression of high levels of PRRs and other receptors, including cytokines by tissue-resident macrophages contributes to the establishment of disease tolerance.9 In addition, signal transduction pathways that are activated in response to PAMPs or DAMPs also contribute to tissue damage control and establishment of disease tolerance, both of which limit the negative effects of infection.
Lipopolysaccharide (LPS), an important cell wall constituent of Gram-negative bacteria, is a potent inducer of inflammatory responses.10 After exposure to LPS, macrophages produce large amounts of inflammatory cytokines, interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF).11 Recent studies have shown that monocytes and macrophages, especially those from myeloid origin, are main cell types that express tissue factor (TF), which causes pathological coagulation in sepsis.12,13 In severe sepsis, macrophages secrete inflammatory mediators and the coagulation system is activated, which promotes fibrin production and thrombosis.14,15 In addition, coagulation abnormalities contribute to the lethality of sepsis and septic acute lung injury (ALI).16 Many in vivo studies use cecal ligation and puncture (CLP) model in mice, which can simulate the pathological condition of abdominal infection through cecal contamination and, to some extent, mimic the response of clinical patients with sepsis.17,18 Thus, we conducted sepsis model using LPS-stimulated RAW264.7 macrophages and CLP-induced mice in our study.
Eupatilin (Eup) is a lipophilic flavonoid isolated from Artemisia argyi, which has the effect of warming the meridian to stop bleeding, dispersing cold and relieving pain. In recent years, Eupatilin has been found to have biological activities against inflammation, oxidation, and tumors.19 It has antioxidant and anti-apoptotic effects against cisplatin-induced ototoxicity, as well as inhibiting inflammatory responses and aging in nucleus pulposus (NP) cells.20,21 In addition, a derivative of Eupatilin to treat dry eye disease is being developed and is undergoing Phase I study.22 Eupatilin can also reduce the inflammatory response in LPS-induced rats, decreasing the level of surfactant protein (SP)-A, SP-D, and inflammatory factors.23 Moreover, Eupatilin can reduce the number of inflammatory cells in OVA-induced asthmatic mice and inhibit the NF-κB and MAPK pathways in both mice and RAW264.7 cells.24 Besides, by inhibiting STAT3, Eupatilin attenuates airway remodeling and inhibits gastric cancer cell growth.25,26 A recent study has shown that Eupatilin is associated with coagulation. It can inhibit arachidonic acid (AA)-induced platelet aggregation and thromboxane A2 (TXA2) and 5-hydroxytryptophan (5-HT) generation.27 Nevertheless, the underlying mechanisms and whether it can be used to protect sepsis-induced coagulation disorders is unclear. Besides, our previous study has shown that in sepsis, phosphorylation of STAT3 at Tyr705 can affect the coagulation and inflammation systems, resulting in fatal damage.28
Thus, we hypothesized that Eupatilin can exert anti-inflammatory and anti-coagulation effects in LPS-induced RAW264.7 macrophages and CLP mouse model by suppressing the JAK2/STAT3 signaling pathway.
Materials and Methods
Reagents
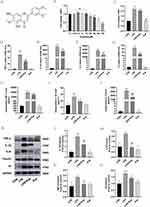
LPS, from Escherichia coli (O127:B8),29 was acquired from Sigma (USA). Eupatilin (Catalog No: HY-N0783), AG490 (Catalog No: HY-12000) and Cell Counting Kit-8 kit (Catalog No: HY-K0301) were purchased from MedChem Express (Shanghai, China). The molecular formula of Eupatilin was shown in Figure 1A. Total RNA from cells was isolated using RNA kit (Catalog No: DP419, Tiangen, China). SYBR Green (Catalog No: QST-100, TOROIVD, China) and 5 × All-In-One RT MasterMix (Catalog No: G492, ABM, Canada) were used for Real-time quantitative PCR (RT-qPCR). Fetal bovine serum (FBS; Catalog No: 16000–004) and DMEM (high glucose; Catalog No: C11995500BT) were gained from Gibco (Life Technologies, Darmstadt, Germany). RIPA buffer (Catalog No: P0013B), Phenylmethanesulfonyl fluoride (PMSF, Catalog No: ST506), DTT (Catalog No: ST041) and Endogenous Peroxidase Blocking Buffer (Catalog No: P0100B) were obtained from Beyotime, China. The BCA kit and ECL chemiluminescence reagent were procured from Thermo Fisher (USA). The PVDF membrane was from EDM Millipore (Billerica, MA, USA). Phosphate-buffered saline (PBS, Catalog No: P1020), 20 × TBST buffer (Catalog No: T1082), 4% paraformaldehyde (Catalog No: P1110), Triton X-100 (Catalog No: T8200), 5% BSA (Catalog No: SW3015) and H&E staining kit (Catalog No: G1120) were from Solarbio (Beijing, China). The DAB kit was from Zhongshan Golden Bridge Biotechnology (Beijing, China).
Preparation of Eupatilin
In the in vitro experiments, Eupatilin was dissolved in DMSO to form a stock solution with a final concentration of 10 mM, and stored at −80°C. In the in vivo experiments, Eupatilin was dissolved in 80% corn oil and 20% DMSO at a concentration of 10 mg/mL; the solution was used immediately after preparation.
In vitro
Cell Culture and Intervention
RAW264.7 macrophages were purchased from ATCC (Manassas, VA, USA) and cultivated in DMEM with 10% FBS. The cells were maintained in an incubator at 37°C with a 5%CO2 level. Cells were randomly classified into four groups: control (CON) group, LPS group, LPS + Eup group, and Eup group. The LPS group was stimulated with 1 μg/mL LPS for 6 hours,30 while the LPS + Eup group was treated with 10 μM Eupatilin for 2 hours, followed by LPS for 6 hours. The Eup group was treated only with 10 μM Eupatilin, which was added the same time as LPS+ Eup group.
Cell Counting Kit-8 (CCK-8) Assay
The effects of Eupatilin on the proliferation of RAW264.7 macrophages were assessed using the CCK-8 assay. The day before stimulation, RAW264.7 cells were seeded in a 96-well plate at a density of 1.0×104 cells/well. After 24 hours, the supernatant was replaced with fresh medium. The CCK-8 solution was then added into each well, and the mixture was incubated at 37°C. After 2 hours, the optical density at 450 nm was measured using a microplate reader (Molecular Devices, San Jose, CA, USA).
Real Time Quantitative PCR
In accordance with the instructions, the total RNA from RAW264.7 cells was extracted and 1000 ng of RNA was transformed into 20 μL cDNA using the reagents listed above. The cDNA was then stored at ‑80°C. The RT-qPCR was performed using SYBR Green on a PCR detection system (7500fast, USA). The cycling conditions were as follows: initial denaturation at 95°C for 60s, then denaturation at 95°C for 15s, annealing at 60°C for 30s, and extension at 72°C for 45s, the three steps above cycled for 40 times. The results were normalized to GAPDH and the statistics were calculated by the 2−ΔΔCt method. The primer pair sequences for mice were from Generay Biotech (Shanghai, China) and listed in Table 1.
 |
Table 1 The Primer Pair Sequences of Real-Time (RT)-qPCR |
In vivo
Experimental Animals
We obtained male C57BL/6 mice at the age of 6–8 weeks and the weight of 18–25 g from Shanghai SLAC Laboratory, Animal Limited Liability Company (SCXK (ZHE) 2019–0001). According to the local animal welfare policy, the mice were raised in a specific pathogen-free laboratory animal environment, in which the temperature maintained at 23 ± 3°C, the humidity kept at 55% ± 10%, and the light/dark cycle controlled at 12:12. During the study, the mice were fed a normal diet. All animal experiments were complied with the requirements of the Animal Care and Use Committee of Wenzhou Medical University. The animal experimentation protocol was granted by the Ethics Committee of Wenzhou Medical University (YS2018-088, 2018.02.26).
Cecal Ligation and Puncture (CLP) and Animal Tests
The mice were randomly assigned into three groups according to the random number table method: sham operation (SHAM) group, CLP group, CLP + Eup group. After the processing time, each group contained more than six samples. The mice were anesthetized by 1% sodium pentobarbital (Solarbio, China) according to their weight (100 μL/10 g) before operation. After the mice were anesthetized, we opened the abdominal cavity and carefully exposed the cecum, which was then ligated at 1 cm from the end and punched through by a needle (21-gauge). Subsequently, the feces were squeezed out into the abdomen and the cecum was placed back. Finally, the abdominal cavity was closed.31 Similar procedures without ligation and puncture were used in the SHAM group. For the CLP+Eup group, the mice were administered Eupatilin (10mg/kg) by gavage 2 hours before operation. After surgery, each mouse was immediately resuscitated by subcutaneous injection of 1 mL warm saline. The experimental samples were collected 24 hours after surgery. Besides, survival analysis was performed and lasted for 72 hours, with 10 mice per group, during which the mice were monitored every 6 hours and euthanized at the point of moribundity.
Hematoxylin and Eosin (H&E) Staining
The lung tissue from three groups of mice were collected, fixed in 4% paraformaldehyde, dehydrated, embedded, and sliced into 4-μm-thick sections. Following the manufacturer’s instructions, the slices were stained by H&E staining kit. Then, the severity of lung injury was evaluated based on inflammatory cell infiltration, edema and congestion. The pulmonary injury was scored as follows: 0 point for no injury, 1 point for <25% of the field injured, 2 points for 25% to 50% of the field injured, 3 points for 50% to 75% of the field injured, and 4 points for >75% of the field injured.32 Each slide had six microscopic fields analyzed, and the average was taken as the final score for each lung tissue. Lung histological analyses were performed in a blinded manner.
Immunohistochemical Assay
The lung slices were dewaxed and rehydrated. Then, the antigen in slices was retrieved in citrate buffer (0.01 M, pH 6.0) for 10 minutes using a microwave oven, followed by quenching of endogenous peroxidase for 15 minutes. Next, the sections were blocked with 5% BSA for an hour. The slices were then incubated with rabbit anti-fibrinogen antibody (Abcam, Catalog No: ab34269) overnight at 4°C. The next day, the slices were incubated at 37°C for 1h with HRP-conjugated goat anti-rabbit antibody. Subsequently, the slices were stained using a DAB kit and observed under a microscope.
Elisa Test
The supernatants from RAW264.7 cell were collected, centrifuged at 1000 × g for 10 minutes and stored at −20°C. Twenty-four hours after surgery, the plasma was collected from mice using anticoagulant tubes with 3.2% sodium citrate. The tubes were centrifuged at 1000 × g for 15 minutes and stored at − 80°C. The experiment was performed according to the manufacturer’s instructions. The catalog numbers for the reagent kits were as follows: IL-1β (Catalog No:70-EK201B), IL-6 (Catalog No:70-EK206) and TNF-α (Catalog No:70-EK282) from MultiScience, China. TF (Catalog No: DY3178-05) and Plasminogen activator inhibitor (PAI)-1 (Catalog No: DY3828-05) from R&D Systems, USA. D-dimer (Catalog No: CSB-E13584m), Fibrinogen (Catalog No: CSB-E08202m) and thrombin-antithrombin complex (TAT) (Catalog No: CSB-E08433m) from Cusabio, China.
Western Blotting
RAW264.7 macrophages and lung tissue from mice were dissociated by RIPA buffer with 10% protein phosphatase inhibitor and 1% PMSF. After measuring the protein concentrations using the BCA kit, lysates containing 20 µg protein samples were separated on 10% or 12.5% SDS-PAGE gels. After that, the proteins were electrotransferred onto PVDF membranes in a cold condition; these membranes were then blocked using 5% skim milk in 1 × TBST buffer for 1.5 hours at room temperature. Then, the membranes were incubated with the respective primary antibodies at 4°C overnight. On the second day, the membranes were incubated with goat HRP-conjugated IgG antibodies (Catalog No: SA00001-2 for anti-rabbit or Catalog No: SA00001-1 for anti-mice, Proteintech, China) for 1 hour at room temperature. The proteins were then visualized using an ECL chemiluminescence reagent. The primary antibodies were as follows: STAT3 (Catalog No: 9139S) and p-STAT3 (Try705) (Catalog No: 9145S) from Cell Signaling Technology, USA. TF (Catalog No: DF6400), IL-6 (Catalog No: DF6087), TNF-α (Catalog No: AF7014), IL-1β (Catalog No: AF5103), GAPDH (Catalog No: AF7021), Tubulin (Catalog No: AF7011), and Histone H3 (Catalog No: BF9211) are from Affinity, China. p-JAK2 (pY1007+Y1008) (Catalog No: ab32101), JAK2 (Catalog No: ab108596), thrombin (Catalog No: ab92621) and PAI-1 (Catalog No: ab222754) from Abcam (Cambridge, United Kingdom). The internal reference proteins in this study included GAPDH, Tubulin, and Histone H3. ImageJ software (NIH, USA) was used to quantify the protein bands.
Immunofluorescence
For cell immunofluorescence, macrophages were fixed with 4% paraformaldehyde for 10 min, permeabilized in 0.2% Triton X-100 for 15 minutes, and blocked in 5% BSA for 30 minutes. All the operations were performed at room temperature. Then, the cells are incubated with p-STAT3 primary antibodies overnight at 4°C, followed by incubation with FITC secondary antibodies against the appropriate sources for 1 hour at 37°C (Catalog No: BL031A for anti-mice or Catalog No: BL033A for anti-rabbit, Biosharp). Thereafter, the sections were mounted using an anti-fluorescence quencher containing DAPI (Catalog No:36308ES11, Yeasen, China).
For tissue immunofluorescence staining, lung sections were treated the same way as for immunohistochemistry, then incubated with the F4/80 (Catalog No: sc-377009, Santa Cruz) and TF (Catalog No: DF6400, Affinity) primary antibodies overnight at 4°C. The following day, the slides were performed in the same way as for cell immunofluorescence. Finally, we visualized the fluorescence signal in the tissue and cells using a laser confocal microscope (Nikon, Japan). The statistical significance was estimated using the mean fluorescence intensity (MFI).
Statistical Analyses
GraphPad Prism 9.0.0 (GraphPad Software, USA) was used for the statistical analysis. Each experiment was independently carried out in at least three duplicates. Values were presented as mean ± SD. P < 0.05 was considered to be statistically significant. After evaluating normality and variance in each group, the group differences were examined using one-way ANOVA, followed by Tukey’s post-hoc multiple comparison test. The analysis was carried out to determine the statistical methods for comparison between groups. The Log rank test was used to compare the survival time in each group of mice.
Results
Effects of Eupatilin on Cell Viability
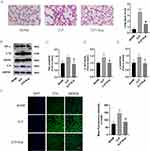
To assess the effect of Eupatilin on cell viability and cytotoxicity, we examined the effect of Eupatilin on RAW 264.7 macrophages using the CCK8 assay. As shown in Figure 1B, the cell viability of RAW 264.7 macrophages was little affected within 24 hours at concentrations less than 10μM. Thus, in the following experiments, we used a 10μM concentration of Eupatilin to eliminate any potential cytotoxic effect. Besides, the result of CCK8 also showed that LPS induced cell proliferation in macrophages, while Eupatilin could inhibit the LPS-induced cell proliferation (Figure 1C).
Eupatilin Decreased the Expression of Inflammatory Cytokines and TF in LPS-Induced RAW264.7 Cells
To explore the effect of Eupatilin, we measured inflammatory cytokines and TF levels in RAW264.7 cells six hours after LPS stimulation. As shown in Figure 1D-F, after the stimulation with LPS, the mRNA expression raised sharply in TNF-α (25.15±4.10), IL-1β (3753.55±114.04) and IL-6 (36976.14±1121.44). However, pretreatment with Eupatilin apparently attenuated the LPS-induced increase in inflammatory cytokine expression. TF is a major promoter of coagulation. As shown in Figure 1G, Eupatilin apparently inhibited LPS-induced mRNA expression of TF (84.66±4.86 in LPS group vs 48.49±3.39 in LPS+Eup group).
Western blot and ELISA were performed to examine the expression of inflammatory cytokines and TF at the protein level. In the supernatants from the LPS group, the concentrations of TNF-α, IL-1β and IL-6 were higher than the control group, whereas this increase was significantly reduced in the LPS + Eup group (Figure 1H-J, 19,615.96±714.41pg/mL, 26.24±2.44pg/mL and 2590.58±109.74pg/mL in LPS group vs 17,095.77±140.07pg/mL, 19.10±1.79pg/mL and 1796.74±256.63pg/mL in LPS+Eup group, respectively). Moreover, the Western blot results illustrated that pretreatment with Eupatilin significantly reduced LPS-induced inflammatory cytokine expression in RAW264.7 cells (Figure 1K-N). Both the Western blot and ELISA experiments indicated the reduction in the expression of inflammation indicators in the RAW 264.7 cells after using Eupatilin. Western blot analysis also revealed a decrease in TF expression in the LPS + Eup group (Figure 1K and O).
Eupatilin Regulated LPS-Induced Inflammation and Coagulation by Suppressing JAK2/STAT3 Signaling Pathway in RAW264.7 Cells
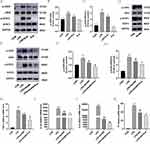
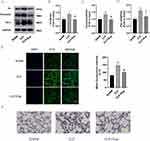
To observe whether Eupatilin-mediated effects on inflammation and coagulation were linked to the JAK2/STAT3 signaling pathway, we performed Western blot in RAW264.7 cells to detect the phosphorylation of target proteins. Compared with the CON group, the LPS group had significantly higher expression levels of both p-JAK2 and p-STAT3. However, after pretreatment with Eupatilin, the expression levels of both p-JAK2 and p-STAT3 were diminished (Figure 2A-C, 1.48±0.02 and 3.10±0.14 in LPS group vs 1.21±0.06 and 2.38±0.11 in LPS+Eup group, respectively). AG490 is an inhibitor of JAK2, which reduced the phosphorylation of JAK2 and STAT3 (Figure 2D). Western blot analysis revealed that AG490 inhibited the phosphorylation of both STAT3 and JAK2, while Eupatilin pretreatment strengthened this inhibition (Figure 2E-G). Moreover, comparing with CLP group, the use of AG490 could reduce the expression of TNF-α, IL-1β, IL-6 and TF (Figure 2H-K, 15.09±1.61, 1843.46±85.28, 18,345.62±427.00, and 55.24±1.82 in LPS group vs 11.71±0.17, 1170.53±41.54, 4321.65±241.26, and 38.15±3.08 in LPS+AG490 group, respectively), while the use of Eupatilin strengthened this inhibition.
To verify whether Eupatilin pretreatment can reduce p-STAT3 nuclear translocation in LPS-stimulated RAW264.7 cells, both Western blot and immunofluorescence were performed. Western blot analysis showed that the pretreatment with Eupatilin attenuated the expression of p-STAT3 in nucleus (Figure 3A). In addition, cell immunofluorescence showed a reduction in the nuclear translocation of p-STAT3 after the pretreatment with Eupatilin in RAW264.7 cells (Figure 3B).
Eupatilin Increased Survival Rate and Improved Inflammation and Coagulation in Septic Mice
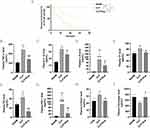
To investigate the effect of Eupatilin on the survival rate of septic mice, we established a model using CLP with C57BL/6 mice and calculated the 72-hour survival rate. The results showed that compared with SHAM group, the survival rate in CLP group dropped sharply (Figure 4A, 1/10,10% in CLP group vs 10/10,100% in SHAM group). Moreover, compared to mice in CLP group, the survival rate in CLP+Eup group increased (5/10, 50%).
To explore whether Eupatilin improves inflammation and coagulation in mouse model, ELISA was performed. As anticipated, the level of inflammatory cytokines, such as TNF-α, IL-1β and IL-6 (241.72±30.17pg/mL, 33.59±2.25pg/mL, 213.99±75.66ng/mL in CLP group vs 141.52±19.96pg/mL, 25.80±4.05pg/mL, 41.36±17.86ng/mL in CLP+Eup group, respectively), as well as coagulation factors, such as TF, TAT, PAI-1 and D-dimer (82.24±4.89pg/mL, 42.79±8.39ng/mL, 233.75±82.02ng/mL, 31.80±3.14ng/mL in CLP group vs 68.21± 2.50pg/mL, 12.60±2.90ng/mL, 23.91±21.07ng/mL, 23.33±1.78ng/mL in CLP+Eup group, respectively), were lower in the plasma for mice in CLP+Eup group (Figure 4B-H) than those in the CLP group. In addition, compared with the SHAM group, the fibrinogen level in plasma was decreased in the CLP group, while the pretreatment with Eupatilin reversed this trend (Figure 4I, 497.13±21.69µg/mL in CLP group vs 705.19±138.68µg/mL in CLP+Eup group, respectively).
Eupatilin Alleviated Pulmonary Inflammation and Lung Injury in Septic Mice
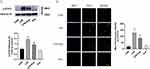
We performed H&E staining to detect lung injury. H&E staining revealed that in the CLP models, the alveolar wall was thickened, hyaline membranes were formed, and neutrophils had accumulated in the interstitial space. As anticipated, the CLP+Eup group exhibited slighter lung tissue injury (Figure 5A). Moreover, the expression of inflammatory cytokines in lung tissue among the three groups was analyzed. As shown in Figure 5B-E, Eupatilin reduced the expression of TNF-α, IL-1β and IL-6 in lung tissue of mice. Macrophages initiate the primary inflammatory process, and F4/80 is a biomarker for them. Immunofluorescence staining demonstrated that the CLP group had stronger F4/80 fluorescent signals than the sham group, while Eupatilin pretreatment decreased these signals (Figure 5F).
Eupatilin Reduced Coagulation and Fibrin Deposition in the Lung Tissue of Septic Mice
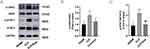
We used Western blot, immunohistochemistry and immunofluorescence staining to investigate whether Eupatilin reduces coagulation in lung tissue. The exposure of TF is the way to promote coagulation. The results of Western blot and immunofluorescence showed that Eupatilin could lower the exposure of TF in the lung tissue of septic mice (Figure 6A, B and E). Furthermore, Western blot showed decreased expression levels of thrombin, and PAI-1 in the lung tissue of septic mice after Eupatilin pretreatment (Figure 6A, C and D). Additionally, according to immunohistochemistry results, fibrin deposition (brown stain) was observed in the CLP group. However, the amount of fibrin deposition was less in the lung tissue from mice pretreated with Eupatilin (Figure 6F).
Eupatilin Suppressed the Activation of JAK2/STAT3 Signaling Pathway in the Lung Tissue of Septic Mice
To explore the effect of Eupatilin on JAK2/STAT3 pathway, we extracted proteins from the lung tissue of septic mice. The Western blot analysis revealed that the expression of p-JAK2 and p-STAT3 was considerably higher in the CLP group than in the SHAM group, and decreased with Eupatilin pretreatment (Figure 7A-C, 1.79±0.24 and 2.21±0.20 in CLP group vs 1.24±0.16 and 1.11±0.15 in CLP+Eup group, respectively).
Discussion
One of the pathological features of sepsis is the hyperactivation of inflammation and coagulation systems; both can promote and impact upon each other.33 In the present study, we found that Eupatilin reduced the expression of inflammatory factors and tissue factors in LPS-induced RAW264.7 cells in vitro and increased the survival of CLP mice. In vivo, Eupatilin attenuated lung injury and reduced inflammatory infiltration and coagulation activation in CLP mice. In summary, our research revealed that Eupatilin could protect septic mice from lung injury, by improving inflammation and coagulation, and that this protection was linked to JAK2/STAT3 signaling pathway.
Ample evidence suggests extensive interaction between inflammation and coagulation, with inflammation activating coagulation and coagulation greatly influencing inflammatory activity.34 In sepsis patients, high concentrations of cytokines can be found in blood circulation.15 The first mediator identified is TNF, which is then followed by various IL with noticeably elevated levels—IL-6 and IL-1 being the most significant.35 Our research showed that, in both in vivo and in vitro sepsis models, the levels of inflammatory cytokines were increased.
Pro-inflammatory cytokines have the ability to stimulate coagulation in vitro. TF is the main factor in the coagulation process and is also a link between coagulation and inflammation.36–39 Under normal physiological conditions, monocytes, endothelial cells and epithelial cells have low TF expression.40 However, during sepsis, these cells express and release TF into circulation after being stimulated by inflammatory factors and LPS.13,41 Besides, after LPS stimulation, caspase-11 is activated, followed by gasdermin D (GSDMD) pores formed and phosphatidylserine exposure, triggering the coagulation cascade.42 Previous research has also demonstrated that blocking IL-6 inhibits endotoxin-induced coagulation activation.43 Numerous investigations have shown that the stimulation of human monocytes with LPS increases TF expression.40,44 Moreover, healthy humans who received a low-dose endotoxemia injection had a 125-fold increase in the amount of TF mRNA in their circulating monocytes.45,46 In a model of endotoxemia, early research showed that inhibiting the expression of TF attenuated septic shock, and animals with low TF levels had decreased coagulation, inflammation, and mortality.47 In our study, we discovered that TF expression was noticeably higher in sepsis models, while the pretreatment with Eupatilin reversed these changes.
After the release of TF, it binds to activated factor VII(VIIa), forming the TF–FVIIa complex, which can induce a pro-inflammatory response in macrophages.48 The TF–VIIa complex then activates factor X and initiates coagulation activation. Factor Xa, together with factor Va, forms the prothrombinase complex, which subsequently converts prothrombin to thrombin.49 It can amplify inflammation by binding to platelets and protease activated receptor 1 (PAR-1).50 Thrombin then cleaves fibrinogen into fibrin,51 and fibrin degradation products can induce IL-6 production by monocytes in vitro.52 Moreover, fibrin degradation can elevate the levels of D-dimer. This process produces higher levels of PAI-1, and the overproduction of which will impair fibrinolysis.53 Increased fibrin formation due to impaired fibrinolysis causes organ injury and mortality in sepsis.54
According to a number of studies, the STAT3 signaling pathway can regulate inflammatory responses in LPS-induced macrophages and acute lung injury, including the production and release of inflammatory factors.55–58 In our previous study, we found that activation of the nuclear transcription factor STAT3 through phosphorylation at the tyrosine 705 site induced TF expression and facilitated the onset of coagulation dysfunction in sepsis.28 In addition, the use of the STAT3 inhibitor BP-1-102 decreased the expression of LPS-induced inflammatory cytokines such as IL-1β, IL-6, TNF-α, and TF in RAW264.7 cells. Similarly, our present experiments detected phosphorylation of STAT3 in both mice lung tissue and LPS-stimulated macrophages sepsis models, and increased intranuclear phosphorylation was found in macrophages, both of which are in agreement with previous studies.29
However, other mechanisms may also be involved in Eupatilin’s effects. Studies have shown that Eupatilin is a selective PPARα-receptor agonist, the activation of PPARα may be related to the alleviation of inflammation and oxidative stress in LPS-induced ALI.23,59 Eupatilin has also been found to reduce the expression of inflammatory cytokines by inhibiting toll-like receptor 4 (TLR4).60 However, whether it has specific receptors requires further study. In addition, Eupatilin may also play a role in other pathways, such as PI3K/AKT, NF-κB, MAPK and Nrf2 pathways.24,61 Thus, whether these pathways also play a role in regulating inflammation and coagulation in sepsis needs further investigation. According to one study, Eupatilin can lower the expression of inflammatory mediators by inhibiting the NF-κB signaling pathway in endotoxin-stimulated macrophages.62 However, the specific mechanism of its regulation of macrophages and its effect on pathogens has not been elucidated yet.
In our study, we found that the effect of Eupatilin pretreatment on the expression of inflammatory cytokines and tissue factors was consistent in both in vitro and in vivo experiments. Eupatilin-mediated effects in vivo were maintained in both plasma and lung tissues. Moreover, the inhibition of the JAK2/STAT3 signaling pathway was observed in both lung tissue and cells after Eupatilin pretreatment. Similar to Eupatilin, another plant-based molecule, piperlongumin, also has regulatory effects on inflammation and coagulation in severe infections, and further studies are needed to compare the effects of these compounds.29
However, our study also has some limitations. First of all, endothelial cells also play an important role in inflammation and coagulation disorders caused by sepsis. However, we did not perform analysis on endothelial cells in the present study. Secondly, the study was performed only in C57BL/6 mice and was not validated in other murine species. Finally, we only used a single dose and intervention time in our study, so further studies should be conducted to investigate the optimum concentration and intervention time for Eupatilin-related effects.
Conclusion
Our results confirmed that Eupatilin can reduce the expression of inflammatory cytokines and coagulation factors in both RAW264.7 macrophages and mouse sepsis models. It also increased survival and attenuated lung injury in mice with sepsis. The mechanism for this may involve the inhibition of JAK2/STAT3 activation and the reduction of p-STAT3 nuclear translocation, thereby regulating inflammatory cytokines and coagulation factors. Additionally, the potential mechanisms and effects of Eupatilin on other organs in sepsis require further investigation.
Abbreviation
JAK2, Janus kinase 2; p-JAK2, phosphorylated JAK2; STAT3, signal transducer and activator of transcription 3; p-STAT3, phosphorylated STAT3; LPS, lipopolysaccharide; CLP, cecal ligation and puncture; IL, interleukin; TNF, tumor necrosis factor; TF, tissue factor; PAI-1, plasminogen activator inhibitor 1; TAT, thrombin-antithrombin complex; DIC, disseminated intravascular coagulation; ALI, acute lung injury; ELISA, enzyme-linked immunosorbent assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Ethics Approval
All animal studies were conducted by the Guide for the Care and Use of Laboratory Animals (2011) and approved by Wenzhou Medical University Institutional Animal Care and Use Committees. The animal experimentation protocol was granted by the Ethics Committee of Wenzhou Medical University (YS2018-088, 2018.02.26). The licence number for the mice was SCXK (ZHE) 2019-0001.
Acknowledgments
We sincerely appreciate the financial support from the National Natural Science Foundation of China (Grant No.81873949), Medical Innovation Discipline of Zhejiang Province (Critical Care Medicine, Y2015), Major project co-founded by Zhejiang Province and Ministry of Science and Technology (Grant No.WKJ-ZJ-1909), Major Science and Technology Project of Wenzhou Science and Technology Bureau (Grant No.2018ZY002), Basic Scientific Research Project of Wenzhou Science and Technology Bureau (Grant No.Y20210097), The 5G Network-based Platform for Precision Emergency Medical Care in Regional Hospital Clusters. MIIT. PRC (2020NO.78).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211.
3. Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:16037.
4. Levi M, Scully M. How I treat disseminated intravascular coagulation. Blood. 2018;131(8):845–854.
5. Rothmeier AS, Marchese P, Petrich BG, et al. Caspase-1-mediated pathway promotes generation of thromboinflammatory microparticles. J Clin Invest. 2015;125(4):1471–1484.
6. Rosseau S, Hammerl P, Maus U, et al. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;279(1):L25–35.
7. Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019;20(21):58.
8. Wiersinga WJ. Current insights in sepsis: from pathogenesis to new treatment targets. Curr Opin Crit Care. 2011;17(5):480–486.
9. Soares MP, Teixeira L, Moita LF. Disease tolerance and immunity in host protection against infection. Nat Rev Immunol. 2017;17(2):83–96.
10. Mazgaeen L, Gurung P. Recent advances in lipopolysaccharide recognition systems. Int J Mol Sci. 2020;21(2):2. doi:10.3390/ijms21020379
11. Cavaillon JM, Adib-Conquy M. Monocytes/macrophages and sepsis. Crit Care Med. 2005;33(12 Suppl):S506–509.
12. Kral-Pointner JB, Schrottmaier WC, Horvath V, et al. Myeloid but not epithelial tissue factor exerts protective anti-inflammatory effects in acid aspiration-induced acute lung injury. J Thromb Haemost. 2017;15(8):1625–1639.
13. Pawlinski R, Wang JG, Owens AP, et al. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010;116(5):806–814.
14. Zhang H, Zeng L, Xie M, et al. TMEM173 drives lethal coagulation in sepsis. Cell Host Microbe. 2020;27(4):556–570.e556.
15. Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38(2 Suppl):S26–34.
16. Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol. 2000;22(4):401–404.
17. Corral J, Yélamos J, Hernández-Espinosa D, et al. Role of lipopolysaccharide and cecal ligation and puncture on blood coagulation and inflammation in sensitive and resistant mice models. Am J Pathol. 2005;166(4):1089–1098.
18. Zanotti-Cavazzoni SL, Goldfarb RD. Animal models of sepsis. Crit Care Clin. 2009;25(4):703–719, vii–viii.
19. Nageen B, Sarfraz I, Rasul A, et al. Eupatilin: a natural pharmacologically active flavone compound with its wide range applications. J Asian Nat Prod Res. 2020;22(1):1–16.
20. Lu, X, Deng, T, Dong, H et al. Novel Application of Eupatilin for Effectively Attenuating Cisplatin-Induced Auditory Hair Cell Death via Mitochondrial Apoptosis Pathway. Oxid Med Cell Longev. 2022;2022:1090034.
21. Yang H, Yang X, Rong K, et al. Eupatilin attenuates the senescence of nucleus pulposus cells and mitigates intervertebral disc degeneration via inhibition of the MAPK/NF-κB signaling pathway. Front Pharmacol. 2022;13:940475.
22. Jung J, Huh KY, Jin X, et al. A phase I study to evaluate the safety, tolerability, pharmacodynamic and pharmacokinetic profiles of ocular GLH8NDE in healthy male adults. Clin Transl Sci. 2022;15(2):343–352.
23. Liu H, Hao J, Wu C, et al. Eupatilin alleviates lipopolysaccharide-induced acute lung injury by inhibiting inflammation and oxidative stress. Med Sci Monit. 2019;25:8289–8296.
24. Bai D, Sun T, Lu F, et al. Eupatilin suppresses OVA-induced asthma by inhibiting NF-κB and MAPK and activating Nrf2 signaling pathways in mice. Int J Mol Sci. 2022;23:3.
25. Li Y, Ren R, Wang L, Peng K. Eupatilin alleviates airway remodeling via regulating phenotype plasticity of airway smooth muscle cells. Biosci Rep. 2020;40:1.
26. Cheong JH, Hong SY, Zheng Y, Noh SH. Eupatilin inhibits gastric cancer cell growth by blocking STAT3-mediated VEGF expression. J Gastric Cancer. 2011;11(1):16–22.
27. Ryu R, Jung UJ, Kim HJ, et al. Anticoagulant and antiplatelet activities of artemisia princeps pampanini and its bioactive components. Prev Nutr Food Sci. 2013;18(3):181–187.
28. Xu S, Pan X, Mao L, et al. Phospho-Tyr705 of STAT3 is a therapeutic target for sepsis through regulating inflammation and coagulation. Cell Commun Signal. 2020;18(1):104.
29. Fang Z, Zhang X, Huang Y, et al. Piperlongumin improves survival in the mouse model of sepsis: effect on coagulation factors and lung inflammation. Inflammation. 2022;1:1–16.
30. Xiang P, Chen T, Mou Y, et al. NZ suppresses TLR4/NF-κB signalings and NLRP3 inflammasome activation in LPS-induced RAW264.7 macrophages. Inflamm Res. 2015;64(10):799–808.
31. Toscano MG, Ganea D, Gamero AM. Cecal ligation puncture procedure. J Vis Exp. 2011;51.
32. Li B, Zeng M, He W, et al. Ghrelin protects alveolar macrophages against lipopolysaccharide-induced apoptosis through growth hormone secretagogue receptor 1a-dependent c-Jun N-terminal kinase and Wnt/β-catenin signaling and suppresses lung inflammation. Endocrinology. 2015;156(1):203–217.
33. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045.
34. Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–2704.
35. Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44.
36. Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30(43):10363–10370.
37. Witkowski M, Landmesser U, Rauch U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc Med. 2016;26(4):297–303.
38. Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(4):709–725.
39. Zelaya H, Rothmeier AS, Ruf W. Tissue factor at the crossroad of coagulation and cell signaling. J Thromb Haemost. 2018;16(10):1941–1952.
40. Egorina EM, Sovershaev MA, Bjørkøy G, et al. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25(7):1493–1498.
41. McVey JH. The role of the tissue factor pathway in haemostasis and beyond. Curr Opin Hematol. 2016;23(5):453–461.
42. Yang X, Cheng X, Tang Y, et al. Bacterial endotoxin activates the coagulation cascade through gasdermin D-dependent phosphatidylserine exposure. Immunity. 2019;51(6):983–996.e986.
43. van der Poll T, Levi M, Hack CE, et al. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179(4):1253–1259.
44. Gregory SA, Morrissey JH, Edgington TS. Regulation of tissue factor gene expression in the monocyte procoagulant response to endotoxin. Mol Cell Biol. 1989;9(6):2752–2755.
45. Franco RF, de Jonge E, Dekkers PE, et al. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood. 2000;96(2):554–559.
46. Aras O, Shet A, Bach RR, et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103(12):4545–4553.
47. Pawlinski R, Pedersen B, Schabbauer G, et al. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103(4):1342–1347.
48. Cunningham MA, Romas P, Hutchinson P, Holdsworth SR, Tipping PG. Tissue factor and factor VIIa receptor/ligand interactions induce proinflammatory effects in macrophages. Blood. 1999;94(10):3413–3420.
49. Amaral A, Opal SM, Vincent JL. Coagulation in sepsis. Intensive Care Med. 2004;30(6):1032–1040.
50. Conway EM. Thrombin: coagulation’s master regulator of innate immunity. J Thromb Haemost. 2019;17(11):1785–1789.
51. Mosesson MW. The roles of fibrinogen and fibrin in hemostasis and thrombosis. Semin Hematol. 1992;29(3):177–188.
52. Spronk HM, van der Voort D, Ten Cate H. Blood coagulation and the risk of atherothrombosis: a complex relationship. Thromb J. 2004;2(1):12.
53. Zeerleder S, Hack CE, Wuillemin WA. Disseminated intravascular coagulation in sepsis. Chest. 2005;128(4):2864–2875.
54. Watanabe R, Wada H, Watanabe Y, et al. Activity and antigen levels of thrombin-activatable fibrinolysis inhibitor in plasma of patients with disseminated intravascular coagulation. Thromb Res. 2001;104(1):1–6.
55. Bode JG, Albrecht U, Häussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins--regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur J Cell Biol. 2012;91(6–7):496–505.
56. Gao Y, Zhao H, Wang P, Wang J, Zou L. The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases. Scand J Immunol. 2018;88(6):e12727.
57. Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol. 2016;54:28–41.
58. Zhao J, Yu H, Liu Y, et al. Protective effect of suppressing STAT3 activity in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2016;311(5):L868–l880.
59. Choi Y, Jung Y, Kim SN. Identification of Eupatilin from Artemisia argyi as a Selective PPARα Agonist Using Affinity Selection Ultrafiltration LC-MS. Molecules. 2015;20(8):13753–13763.
60. Fei X, Chen C, Kai S, et al. Eupatilin attenuates the inflammatory response induced by intracerebral hemorrhage through the TLR4/MyD88 pathway. Int Immunopharmacol. 2019;76:105837.
61. Lee DC, Oh JM, Choi H, et al. Eupatilin inhibits reactive oxygen species generation via Akt/NF-κB/MAPK signaling pathways in particulate matter-exposed human bronchial epithelial cells. Toxics. 2021;9:2.
62. Choi EJ, Lee S, Chae JR, Lee HS, Jun CD, Kim SH. Eupatilin inhibits lipopolysaccharide-induced expression of inflammatory mediators in macrophages. Life Sci. 2011;88(25–26):1121–1126.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.