Back to Journals » Drug Design, Development and Therapy » Volume 17
Melatonin Attenuates Sepsis-Induced Acute Lung Injury via Inhibiting Excessive Mitophagy
Authors Ling J, Yu S, Xiong F, Xu T, Li S
Received 25 June 2023
Accepted for publication 31 August 2023
Published 11 September 2023 Volume 2023:17 Pages 2775—2786
DOI https://doi.org/10.2147/DDDT.S423264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Jianmin Ling,1,2 Shanshan Yu,1,2 Feng Xiong,1,2 Tingting Xu,1,2 Shusheng Li1,2
1Department of Emergency Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430000, People’s Republic of China; 2Department of Critical Care Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430000, People’s Republic of China
Correspondence: Shusheng Li, Department of Emergency Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095 Jiefang Dadao, Wuhan, 430000, People’s Republic of China, Email [email protected]
Background: Epidemiological studies have indicated that lung injury is a frequent complication of sepsis. Mitophagy is vital to multiple pathological processes and diseases; however, its influence on sepsis-induced acute lung injury remains elusive. Melatonin has multiple antioxidant action and anti-inflammatory effects, including regulating mitophagy and inflammatory cytokine expression. Whereas, little is known about the affection of melatonin and mitophagy on CLP-induced ALI.
Methods: The in vivo effect of melatonin on OPTN-mediated mitophagy was studied by CLP-induced ALI in a mouse model using C57BL/6 followed by treatment with vehicle and melatonin (30 mg/kg/d, intraperitoneal injection). ALI was assayed by lung wet /dry ratio, hematoxylin and eosin staining, and immunohistochemical staining. Signaling pathway changes were subsequently determined by Western blotting and immunofluorescence staining. The effects of melatonin on STAT3 activation and TNF-α production were detected by Western blotting, PCR, and immunohistochemical staining.
Results: Our results indicated that OPTN, mitophagy adaptors were significantly repressed in CLP-induced ALI, accompanied by overactivation of mitophagy and inflammation. At the same time, we found that melatonin treatment alleviated ALI caused by CLP, and the effect was highly correlated with OPTN-related mitophagy. Furthermore, we demonstrated that OPTN-related mitophagy, which was normalized by melatonin, blocked STAT3 involved epithelial barrier and inflammation in vivo.
Conclusion: Overall, our results confirm that mitophagy is adjusted by melatonin in the CLP-induced ALI. Moreover, manipulation of mitophagy through melatonin could be a possible treatment to reduce sepsis-associated lung injury.
Keywords: melatonin, sepsis, acute lung injury, mitophagy, inflammation
Introduction
Sepsis associated with a high incidence of morbidity and mortality is caused by an uncontrolled host response to infection.1 The lungs are the most vulnerable organs in patients suffering from sepsis in the intensive care unit.2 Acute lung injury (ALI) caused by sepsis is one of the leading causes of mortality, refractory hypoxemia and pulmonary edema in affected patients3 However, sepsis-related ALI remains lacks specific therapy.
ALI is accompanied by severe mitochondrial dysfunction caused by excessive reactive oxygen species (ROS).4 Furthermore, loss of mitochondrial function results in epithelial barrier breakdown.5 Mitochondrial dysfunction is linked to survival in sepsis-related ALI.6 Mitochondrial quality is tightly linked to the clearance of damaged mitochondria via the process of mitophagy.7 Optineurin (OPTN) is a multifunctional protein which is associated with various immune disorders.8–10 The characterized function of OPTN is mitophagy through its protein 1 light-chain 3B-II (LC3-II) interaction region.11 Mitophagy receptor OPTN plays an important role in committing damaged mitochondria to autophagy.12,13 It has been shown that OPTN inhibits signal transducer and activator of transcription 3 (STAT3) activation via blocking Janus kinase 2 (JAK2) dimerization.14 OPTN negatively regulates tumor necrosis factor-α (TNF-α) through decreased ubiquitination and association of TNF receptor.15
Melatonin is an indole-heterocyclic compound mainly secreted by the pineal gland. Melatonin regulates the process of the sleep, anti-inflammation, anti-oxidant, anti-aging, anti-viral properties and so forth.16–20 Previous studies have found melatonin levels correlated strongly with mortality risk in patients with sepsis.21 A protective role of melatonin in sepsis associated insufficiency has also been identified.22 Recently, several studies have shown the moderating effect that melatonin has on mitophagy.23 Melatonin inhibits excessive mitophagy through the Pink1/Parkin pathway,24 and also represses mitophagy to protect cells from oxidative damage.25 However, mitophagy displays individual roles in different illnesses.26 The causal relationship between melatonin and OPTN-mediated mitophagy in sepsis-related ALI remains unknown. In the study, under the condition of cecum ligation and puncture (CLP), we found that CLP induced excessive mitophagy by upregulated microtubule-associated LC3-II, downregulated OPTN and translocase of outer mitochondrial membrane 20 (TOMM20). Furthermore, melatonin suppressed CLP-induced excessive mitophagy and attenuated pulmonary edema, epithelial barrier and inflammation by inhibiting downstream molecules STAT3 and TNF-α, indicating that targeting OPTN might be helpful in the treatment of sepsis-related ALI.
Materials and Methods
Drug and Antibodies
Melatonin ≥99.47% was purchased from Med Chem Express (MCE, USA, HY-B0075). Antibodies were listed in Supplementary Table 1.
Animals
C57BL/6 mice (WT) obtained from Beijing Sibeifu Biotechnology were kept in SPF environment with abundant supplement of food and water. The mice were treated with melatonin (30 mg/kg, intraperitoneal injection),27,28 or the same volume of corresponding vehicle for 3 days and 1 hour before CLP surgery. The mice were used at 8 weeks old (20–22g). The animal experiments were conducted according to the Guidelines for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People’s Republic of China and approved by the Institutional Animal Care and Use Committee at Tongji Medical College, Huazhong University of Science and Technology.
Cecum Ligation and Puncture (CLP)
CLP-induced sepsis model was developed as described before.29 Mice were anesthetized with isoflurane (3% induction and 2% maintenance in pure oxygen). After skin preparation and disinfection, a 10-mm-wide opening was made on the ventral midline. The cecum was isolated and punctured with a 16-gauge needle to induce sepsis. A small amount of feces was extruded, and the peritoneum was closed. Mice were resuscitated with sterile normal saline (1 mL). The sham group underwent the same surgical procedures without CLP.
Histological Evaluation of Hematoxylin and Eosin (HE) Staining
The lung tissues and bronchoalveolar lavage fluid were collected, washed with PBS, and fixed with a 4% paraformaldehyde solution. The lung tissues were then dehydrated with gradient ethanol, embedded in paraffin, sliced into 5-μm-thick sections. The tissues and BALF were stained with hematoxylin/eosin and examined under a microscope. The damage to the tissues was scored using a previously described scoring system.30
Bronchoalveolar Lavage Fluid (BALF)
Total cells in the BALF were evaluated by cell counting plate following the manufacturer’s protocols. Briefly, after sacrificed, the mice were treated with PBS (0.3 mL) into the lungs injected by 22-gauge catheter, and then pumped it back after 3 sec. The same injection was repeated 3 times as previously described. After recovered and centrifugated, protein contents of BALF were measured by bicinchoninic acid (BCA) methods.31
Lung Wet and Dry (W/D) Ratio
The left lung was dissected and weighted immediately to determine the wet lung weight. All samples were dried at 65 °C for 48 h till reaching a constant weight and were weighed again to determine the dry weight.32
Immunohistochemical (IHC) Staining
The lung sections in 5 µm thick were baked at 65°C for 1 h and followed by immersing into xylene for 60 min. And then the slices were dehydrated in graded ethanol (100%, 95%, 85%, and 75%) for 10 min each time. The slices were boiled for 20 min with citric acid buffer for tissue antigen recovery. Next, sections were incubated with 3% H2O2 for 0.5 h and then slices were blocked in 5% BSA with 0.5% Triton X-100 for 40 min. For IHC, sections were incubated with primary antibodies (listed in the Supplementary Table 1) in 0.3% Triton X-100 phosphate buffer saline at 4°C for 24 h. Rabbit-specific HRP/DAB (ABC) Detection IHC Kit was used for IHC. After incubation with rabbit-specific secondary antibody at 37°C for 60min, slices were stained with DAB kit for 2–5min and then were washed with water. Finally, sections were rehydrated through graded ethanol, cleared with xylene and observed with a microscope.33
Immunofluorescence (IF) Staining
IF staining experiment was carried out according to the established method.34 After the formalin-fixed lung tissues were embedded, sliced, deparaffinage, dehydration, and antigen recovery, IF staining was conducted. Briefly, sections were blocked in goat serum with 0.3% Triton X-100 in PBS for 1 h. Then, primary antibodies (listed in the Supplementary Table 1) were incubated at 4 °C overnight. The next day, sections were incubated with Alexa Fluor secondary antibodies at 37°C for 1 h and were washed with PBS. Finally, sections were stained with DAPI for 10 min. After washing, sections were sealed with anti-fluorescence quencher. The images were captured by the Olympus microscope SV120.
Western Blotting (WB)
Lung tissues were lysed with radio immunoprecipitation assay (RIPA), phenylmethanesulfonyl fluoride (PMSF), and protease inhibitors. Then, samples were mixed with 1× loading buffer and were boiled for 10 min. About 10–25ug of protein extracts were subjected to 10–15% SDS-PAGE gels and blotted onto nitrocellulose (NC) blotting membranes. After blocking with 5% BSA, the membranes were incubated with primary antibodies (listed in the Supplementary Table 1) for 16 h. Then, the NC membranes were washed by TBST for 3 × 10 min and incubated with IRDyeTM (800CW)-conjugated 800M or 800R secondary antibody for 1.5 h. Odyssey Imaging System and ImageJ software were used for visualization and Quantitative analysis of protein bands.35
Polymerase Chain Reaction (PCR)
Total RNA was extracted from the tissues by TaKaRa RNAiso Reagent Trizol as previously described.36 cDNA synthesis was performed using Takara PrimeScriptTMRT reagent kit. qRT-PCR was performed using SYBR Green Master Mix and StepOnePlus real-time PCR System according to the instructions. The following primer sequences were used: TNFα forward 5’-CACGCTCTTCTGTCTACTGAACTTC-3’, reverse 5’- ATGATCTGAGTGTGAGGGTCTGG -3’. β-actin forward 5’- GGCTGTATTCCCCTCCATCG -3’, reverse 5’- CCAGTTGGTAACAATGCCATGT -3’.
Statistics
Data were performed by GraphPad Prism 8.0 software and were presented as means ± SEM. Comparisons between two groups were analyzed using the student’s t-test. Multiple group comparisons were performed by one-way ANOVA followed by Tukey’s multiple comparisons test. P <0.05 was considered statistically significant.
Results
CLP-Induced Sepsis Models Present the OPTN-Mediated overactivated Mitophagy
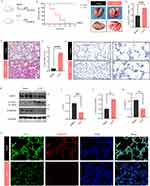
We established the lung injury model through CLP and observed that the mice in the CLP group were in poor condition with a high survival rate. The survival rate of mice showed that the CLP group started to die by 12 h and 100% of CLP mice died by 60 h, compared with 0% of sham mice dying by 72 h (Figure 1A and B). Mice that underwent CLP showed diffuse pathological pulmonary damage (Figure 1C). W/D was also detected to estimate pulmonary interstitial hyperemia and edema severity. Moreover, the W/D ratio of the left lung in the CLP group was higher than that in the sham group (Figure 1D). Meanwhile, HE staining showed that alveolar epithelium disruption, alveolar congestion, and massive inflammatory cell infiltration in the CLP group (Figure 1E and F). The BALF analysis revealed that, by CLP, cell counts and protein contents were significantly increased (Figure S1A–C), implicating inflammation in lung tissue. The MPO staining in lung tissue was also increased after CLP (Figure 1G), suggesting aggravated neutrophil infiltration. All these results revealed that modeling was successful. Next, Western blotting for RIPA total OPTN demonstrated decrease in OPTN protein in the CLP group compared to the sham controls (Figure 1H and I). As an essential receptor for mitophagy, OPTN is needed for the degradation of the damaged mitochondria by mitophagy. In order to investigate the change of OPTN-mediate mitophagy in the lung of CLP-induced septic mice, the expressions of LC3 and TOMM20 proteins were investigated. In the CLP mice, the level of mitophagy-related protein LC3 was higher than those in the control group (Figure 1H and J). Furthermore, the protein level of the mitochondrial membrane protein TOMM20 was significantly decreased in the CLP group (Figure 1H and K). To confirm these results in a different way, the immunofluorescence staining was used to investigate the changes of OPTN-mediate mitophagy in the lung of CLP-induced septic mice. By immunostaining the lung with OPTN together with TOMM20, we found that the expression of OPTN and TMM20 were downregulated (Figure 1L), as observed in the homogeneous lung tissue. These results suggest that CLP-induced sepsis models present the OPTN-mediated overactivated mitophagy.
Melatonin Administration Alleviates CLP-Induced Lung Injuries and Increases Survival Time in Septic Mice
To investigate whether melatonin regulates lung injury induced by CLP, the CLP group was given 30 mg/kg melatonin (Figure 2A). Subsequently, the impact of melatonin on the survival rates of mice was explored. The survival rate of mice that underwent Melatonin treatment was found to be significantly higher compared with the CLP group (Figure 2B). Moreover, these experiments revealed that CLP-induced lung injury was attenuated by the administration of Melatonin, as shown by a moderate level of W/D ratio (Figure 2C and D), interstitial edema (Figures 2E and S2A), and decreased level of inflammatory cell infiltration (Figures 2F, G and S2B, C). Overall, these data confirm that melatonin has an ability to alleviate CLP-induced lung injury.
Melatonin Administration Inhibits Excessive Mitophagy Through OPTN in Septic Mice
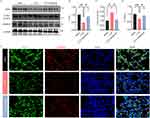
The expression of OPTN, LC3 and TOMM20 was explored. The protein levels of the mitophagy adaptor OPTN and mitochondrial marker TOMM20 were increased, while the mitophagy markers LC3 was decreased in the CLP mice treated with 30mg/kg Melatonin for 96 h (Figure 3A–D). Furthermore, immunohistochemistry for OPTN and TOMM20 were performed with lung tissues of Sham, CLP, and CLP+ Melatonin. We found that lung sections from CLP+Melatonin exhibited more OPTN and TOMM20 immunoreactivity compared to those from CLP mice (Figure 3E). The results suggested that melatonin inhibited excessive OPTN-mediated mitophagy in the CLP-induced septic mice models.
Melatonin Inhibits STAT3 Activation
After the CLP, the total STAT3 and phosphorylation of STAT3 (p-STAT3) were obviously upregulated. However, melatonin downregulated the phosphorylation of this signaling pathway factors, as shown in Figure 4A–C. Activation of STAT3 signaling regulates ZO-1. To explore endothelial barrier function in CLP-induced septic lungs, we examined expression of tight junction proteins ZO-1 by immunofluorescence in CLP treated mice. As shown in Figure 4D, immunoactivity expression of ZO-1 was markedly downregulated after CLP compared to Sham, while melatonin significantly restored protein abundance of ZO-1 in CLP + Melatonin group.
Melatonin Treatment Inhibits Inflammatory Response in CLP- Treated Lung Tissues
As a member of the STAT family, STAT3 is phosphorylated, which enhances its transcriptional activity and is vital for the production of proinflammatory cytokine tumor necrosis factor-alpha. TNF-α mRNA was increased in lung tissues under CLP conditions compared with the Sham group (Figure 5A). Treatment with melatonin inhibited TNF-α expression in lung tissues compared to CLP group. To confirm this result in a different way, the Western blot was used to investigate the changes of TNF-α in the lung of Melatonin treated septic mice. We found that the expression of TNF-α was downregulated (Figure 5B and C), as observed in the PCR (Figure 5A). Furthermore, the IHC staining of TNF-α in lung tissues showed that immunoactivity expression of TNF-α was markedly downregulated after treatment with melatonin compared to CLP alone (Figure 5D).
Discussion
ALI accounted for 10% of all ICU patients in 50 countries in 2016.37,38 The dysregulated host response to bacterial infection caused by sepsis disrupts the pulmonary alveolar-capillary barrier and leads to ALI hallmarked by refractory hypoxemia, pulmonary edema, and diffuses alveolar inflammatory response.39 It has been noted that the incidence of sepsis-associated ALI increases annually, resulting in serious health and economic burden.40 Unfortunately, the current therapy for sepsis-induced ALI is limited and is incapable of reducing its mortality.41 Thus, it is vital to find an effective therapeutic therapy for sepsis-induced ALI. Herein, we developed a mice model of sepsis by CLP surgery and found that CLP-induced sepsis led to ALI in mice, with characteristic symptoms, such as alveolar exudate, damage of epithelial cells and interstitial, alveolar cellular infiltrates and inflammatory cell infiltration by histopathology. Our results demonstrated that CLP induced excessive mitophagy of mice by upregulating LC3, downregulating OPTN and TOMM20 levels, but this effect was reversed by melatonin pretreatment through Western blotting and immunofluorescence staining. The current result was consistent with the previous study which CLP induced excessive mitophagy via increasing PINK1/Parkin levels,24 but melatonin pretreatment could decrease it. Moreover, other studies revealed that STAT3, ZO-1 and TNF-α were involved in sepsis-induced vascular injury and inflammation;42–44 we found that CLP upregulated the expression of STAT3 and TNF-α, downregulated the expression of ZO-1 during the process of sepsis-induced ALI, but these effects were also reversed by melatonin pretreatment.
Mitophagy serves as an important player in sepsis-induced ALI.45 Mitophagy is a dynamic process in which damaged mitochondria in the cytoplasm are degraded.46 Mitophagy is a special type of selective autophagy, which is dependent on the mediation of a family of cargo receptors, and the OPTN is a well-known cargo receptor.47 OPTN contains an LC3-interacting region (LIR) and a cargo binding motif, which binds to LC3 and ubiquitinated substrates, respectively, thereby allowing the recruitment of mitochondria to autophagosomes.11 However, the mechanism of its activity in ameliorating CLP-induced lung injury is still unclear. Here, we show that OPTN is downregulated by septic associated lung injury and that administration of Melatonin increases OPTN in mice via inhibiting excessive mitophagy. Our results confirm that OPTN-related mitophagy is actually modulated by melatonin in the CLP-induced ALI.
There are few clinical treatments targeting the significant therapeutic role of melatonin against lung disease.22,48,49 Melatonin has been associated with anti-excessive mitophagy and anti-inflammatory effects in sepsis, leading to improved survival in animal models.50,51 Melatonin suppresses normal cell death caused by hyperreactive mitophagy under excessive oxidative stress.23 OPTN recruit participants in the Pink1-Parkin mitochondrial ubiquitylation pathway to promote mitophagy.52 Our results are consistent with the fact that Melatonin ameliorates excessive mitophagy by regulating PINK1/Parkin pathway.53
Previous studies have shown that OPTN has the ability to bind to JAK2 and OPTN participates in the regulation of JAK2/STAT3 signaling pathway.14 In parallel, it has been reported that OPTN acted as an inhibitor of STAT3. STAT3 is a transcription activator that can mediate the expression of various genes in response to oxidative stimulations. It can be activated via phosphorylation and translocated to the cell nucleus.54 STAT3 plays a critical part in many physiological functions, including mitophagy/autophagy, maintenance of epithelial barrier, and inflammation.55 Our results revealed that pSTAT/STAT3 was strongly augmented with CLP-induced ALI. Our data also showed that the expression of STAT3 was decreased in Melatonin pretreated CLP-induced lung injury. It has been reported that STAT3 bound to the promoter of ZO-1 and acted as a vital signaling molecule in the process of maintaining the integrity of epithelial barrier function.56 It is worth noting that the pretreatment of Melatoninpromoted expression of ZO-1.
Furthermore, we demonstrated that OPTN and STAT3, which was normalized by melatonin, blocked TNF-α-involved inflammation in vivo. The proinflammatory cytokines, TNF-α, are among the promising as a biomarker for predicting morbidity and mortality in ALI and ARDS.37 One of the main signaling pathways of ALI is TNF-α-mediated inflammation amplification effect of NF-κB signal pathway.57 STAT3 activation can enhance the transcription of TNF-α genes, thus, creating a vicious feedback loop that amplifies early inflammatory signals and exacerbates the initial inflammatory effect. Previous findings also indicated that downregulating expression of OPTN elevates inflammation.58,59 Evidence has suggested that OPTN probably utilizes TNF-α to mediate inflammation and apoptosis.60
There were some limitations in the study. First, we only studied OPTN, which is a well-known significant molecule for playing an important role in regulating mitophagy, and some other members of the cargo receptors, such as SQSTM1, NDP52, TAX1BP1 and NBR1,61 also should be investigated in the future. Second, some other members of the STAT family, such as STAT1 and STAT5, may contribute to the work of melatonin in the study. Third, further investigations that overexpress OPTN in vivo and in vitro may be necessary.
Conclusion
In summary, we demonstrated that OPTN-related mitophagy is actually overactive in the lung tissues of septic mice. Moreover, pretreatment of melatonin restrained the activation of inflammation via inhibiting excessive mitophagy, thereby mitigating sepsis-induced lung injury. Our results manifested that manipulation of the OPTN-related mitophagy via melatonin may be a novel therapeutic approach to reduce sepsis-associated lung injury.
Acknowledgments
We thank Dr. Yanqing Wu and Qian Liu for insightful discussions and assistance. We appreciate the excellent technical assistance from Dr. Xin Wang and Ke Liu.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Jiang W, Ma C, Bai J, Du X. Macrophage SAMSN1 protects against sepsis-induced acute lung injury in mice. Redox Biol. 2022;56:102432. doi:10.1016/j.redox.2022.102432
3. Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi:10.1001/jama.2017.21907
4. Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107(1):57–64. doi:10.1093/bja/aer093
5. Chang AL, Ulrich A, Suliman HB, Piantadosi CA. Redox regulation of mitophagy in the lung during murine Staphylococcus aureus sepsis. Free Radic Biol Med. 2015;78:179–189. doi:10.1016/j.freeradbiomed.2014.10.582
6. Haden DW, Suliman HB, Carraway MS, et al. Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2007;176(8):768–777. doi:10.1164/rccm.200701-161OC
7. Patoli D, Mignotte F, Deckert V, et al. Inhibition of mitophagy drives macrophage activation and antibacterial defense during sepsis. J Clin Invest. 2020;130(11):5858–5874. doi:10.1172/jci130996
8. Markovinovic A, Cimbro R, Ljutic T, et al. Optineurin in amyotrophic lateral sclerosis: multifunctional adaptor protein at the crossroads of different neuroprotective mechanisms. Prog Neurobiol. 2017;154:1–20. doi:10.1016/j.pneurobio.2017.04.005
9. Albagha OM, Visconti MR, Alonso N, et al. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget’s disease of bone. Nat Genet. 2010;42(6):520–524. doi:10.1038/ng.562
10. Smith AM, Sewell GW, Levine AP, et al. Disruption of macrophage pro-inflammatory cytokine release in Crohn’s disease is associated with reduced optineurin expression in a subset of patients. Immunology. 2015;144(1):45–55. doi:10.1111/imm.12338
11. Ryan TA, Tumbarello DA. Optineurin: a coordinator of membrane-associated cargo trafficking and autophagy. Front Immunol. 2018;9:1024. doi:10.3389/fimmu.2018.01024
12. Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14. doi:10.1038/nrm3028
13. Georgakopoulos ND, Wells G, Campanella M. The pharmacological regulation of cellular mitophagy. Nat Chem Biol. 2017;13(2):136–146. doi:10.1038/nchembio.2287
14. Wang J, Wang J, Hong W, et al. Optineurin modulates the maturation of dendritic cells to regulate autoimmunity through JAK2-STAT3 signaling. Nat Commun. 2021;12(1):6198. doi:10.1038/s41467-021-26477-4
15. Nakazawa S, Oikawa D, Ishii R, et al. Linear ubiquitination is involved in the pathogenesis of optineurin-associated amyotrophic lateral sclerosis. Nat Commun. 2016;7:12547. doi:10.1038/ncomms12547
16. Reiter RJ, Tan DX, Rosales-Corral S, et al. Mitochondria: central organelles for melatonin’s antioxidant and anti-aging actions. Molecules. 2018;23(2):509. doi:10.3390/molecules23020509
17. Tarocco A, Caroccia N, Morciano G, et al. Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019;10(4):317. doi:10.1038/s41419-019-1556-7
18. Hardeland R. Aging, melatonin, and the pro- and anti-inflammatory networks. Int J Mol Sci. 2019;20(5):1223. doi:10.3390/ijms20051223
19. Tamtaji OR, Mirhosseini N, Reiter RJ, Behnamfar M, Asemi Z. Melatonin and pancreatic cancer: current knowledge and future perspectives. J Cell Physiol. 2019;234(5):5372–5378. doi:10.1002/jcp.27372
20. Anderson G, Reiter RJ. Melatonin: roles in influenza, Covid-19, and other viral infections. Rev Med Virol. 2020;30(3):e2109. doi:10.1002/rmv.2109
21. Lorente L, Martín MM, Abreu-González P, et al. Serum melatonin levels are associated with mortality in severe septic patients. J Crit Care. 2015;30(4):860.e861–866. doi:10.1016/j.jcrc.2015.03.023
22. Liu R, Luo X, Li J, et al. Melatonin: a window into the organ-protective effects of sepsis. Biomed Pharmacother. 2022;154:113556. doi:10.1016/j.biopha.2022.113556
23. Coto-Montes A, Boga JA, Rosales-Corral S, et al. Role of melatonin in the regulation of autophagy and mitophagy: a review. Mol Cell Endocrinol. 2012;361(1–2):12–23. doi:10.1016/j.mce.2012.04.009
24. Zeng L, He J, Liu C, et al. Melatonin attenuates ropivacaine-induced apoptosis by inhibiting excessive mitophagy through the Parkin/PINK1 pathway in PC12 and HT22 cells. Inflammation. 2022;45(2):725–738. doi:10.1007/s10753-021-01579-9
25. Jiang Y, Shen M, Chen Y, et al. Melatonin represses mitophagy to protect mouse granulosa cells from oxidative damage. Biomolecules. 2021;11(7):968. doi:10.3390/biom11070968
26. Shen M, Jiang Y, Guan Z, et al. FSH protects mouse granulosa cells from oxidative damage by repressing mitophagy. Sci Rep. 2016;6:38090. doi:10.1038/srep38090
27. Kang JY, Xu MM, Sun Y, et al. Melatonin attenuates LPS-induced pyroptosis in acute lung injury by inhibiting NLRP3-GSDMD pathway via activating Nrf2/HO-1 signaling axis. Int Immunopharmacol. 2022;109:108782. doi:10.1016/j.intimp.2022.108782
28. Rahim I, Sayed RK, Fernández-Ortiz M, et al. Melatonin alleviates sepsis-induced heart injury through activating the Nrf2 pathway and inhibiting the NLRP3 inflammasome. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(2):261–277. doi:10.1007/s00210-020-01972-5
29. Jiao Y, Zhang T, Zhang C, et al. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit Care. 2021;25(1):356. doi:10.1186/s13054-021-03775-3
30. Li J, Chen Q, He X, et al. Dexmedetomidine attenuates lung apoptosis induced by renal ischemia-reperfusion injury through α(2)AR/PI3K/Akt pathway. J Transl Med. 2018;16(1):78. doi:10.1186/s12967-018-1455-1
31. Xu JY, Chang W, Sun Q, Peng F, Yang Y. Pulmonary midkine inhibition ameliorates sepsis induced lung injury. J Transl Med. 2021;19(1):91. doi:10.1186/s12967-021-02755-z
32. Sue RD, Belperio JA, Burdick MD, et al. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol. 2004;172(6):3860–3868. doi:10.4049/jimmunol.172.6.3860
33. Jiang XJ, Wu YQ, Ma R, et al. PINK1 alleviates cognitive impairments via attenuating pathological tau aggregation in a mouse model of tauopathy. Front Cell Dev Biol. 2021;9:736267. doi:10.3389/fcell.2021.736267
34. Tao Z, Jie Y, Mingru Z, et al. The Elk1/MMP-9 axis regulates E-cadherin and occludin in ventilator-induced lung injury. Respir Res. 2021;22(1):233. doi:10.1186/s12931-021-01829-2
35. Wang Y, Chen D, Xie H, et al. AUF1 protects against ferroptosis to alleviate sepsis-induced acute lung injury by regulating NRF2 and ATF3. Cell Mol Life Sci. 2022;79(5):228. doi:10.1007/s00018-022-04248-8
36. Ling J, Wu Y, Zou X, et al. (−)-Epicatechin reduces neuroinflammation, protects mitochondria function, and prevents cognitive impairment in sepsis-associated encephalopathy. Oxid Med Cell Longev. 2022;2022:2657713. doi:10.1155/2022/2657713
37. Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140(4):345–350. doi:10.5858/arpa.2015-0519-RA
38. Zhang H, Liu J, Zhou Y, et al. Neutrophil extracellular traps mediate m(6)A modification and regulates sepsis-associated acute lung injury by activating ferroptosis in alveolar epithelial cells. Int J Biol Sci. 2022;18(8):3337–3357. doi:10.7150/ijbs.69141
39. Englert JA, Bobba C, Baron RM. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome. JCI Insight. 2019;4(2):e124061. doi:10.1172/jci.insight.124061
40. Wan B, Xu WJ, Chen MZ, et al. Geranylgeranyl diphosphate synthase 1 knockout ameliorates ventilator-induced lung injury via regulation of TLR2/4-AP-1 signaling. Free Radic Biol Med. 2020;147:159–166. doi:10.1016/j.freeradbiomed.2019.12.024
41. Rello J, Valenzuela-Sánchez F, Ruiz-Rodriguez M, Moyano S. Sepsis: a review of advances in management. Adv Ther. 2017;34(11):2393–2411. doi:10.1007/s12325-017-0622-8
42. Li W, Li D, Chen Y, et al. Classic signaling pathways in alveolar injury and repair involved in sepsis-induced ALI/ARDS: new research progress and prospect. Dis Markers. 2022;2022:6362344. doi:10.1155/2022/6362344
43. Spadaro S, Park M, Turrini C, et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J Inflamm. 2019;16:1. doi:10.1186/s12950-018-0202-y
44. Assimakopoulos SF, Akinosoglou K, de Lastic AL, et al. The Prognostic value of endotoxemia and intestinal barrier biomarker ZO-1 in bacteremic sepsis. Am J Med Sci. 2020;359(2):100–107. doi:10.1016/j.amjms.2019.10.006
45. Mannam P, Shinn AS, Rivastava AS, et al. MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;306(7):L604–L619. doi:10.1152/ajplung.00272.2013
46. Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K. Molecular mechanisms and physiological functions of mitophagy. EMBO j. 2021;40(3):e104705. doi:10.15252/embj.2020104705
47. Wong YC, Holzbaur EL. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci U S A. 2014;111(42):E4439–E4448. doi:10.1073/pnas.1405752111
48. Wang W, Gao J. Effects of melatonin on protecting against lung injury (Review). Exp Ther Med. 2021;21(3):228. doi:10.3892/etm.2021.9659
49. Mahalanobish S, Saha S, Dutta S, Ghosh S, Sil PC. Melatonin counteracts necroptosis and pulmonary edema in cadmium-induced chronic lung injury through the inhibition of angiotensin II. J Biochem Mol Toxicol. 2022;36(10):e23163. doi:10.1002/jbt.23163
50. Acuña-Castroviejo D, Rahim I, Acuña-Fernández C, et al. Melatonin, clock genes and mitochondria in sepsis. Cell Mol Life Sci. 2017;74(21):3965–3987. doi:10.1007/s00018-017-2610-1
51. Hardeland R. Melatonin and inflammation-Story of a double-edged blade. J Pineal Res. 2018;65(4):e12525. doi:10.1111/jpi.12525
52. Heo JM, Ordureau A, Paulo JA, Rinehart J, Harper JW. The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol Cell. 2015;60(1):7–20. doi:10.1016/j.molcel.2015.08.016
53. Yi S, Zheng B, Zhu Y, et al. Melatonin ameliorates excessive PINK1/Parkin-mediated mitophagy by enhancing SIRT1 expression in granulosa cells of PCOS. Am J Physiol Endocrinol Metab. 2020;319(1):E91–E101. doi:10.1152/ajpendo.00006.2020
54. Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016;31:1–15. doi:10.1016/j.cytogfr.2016.05.001
55. You L, Wang Z, Li H, et al. The role of STAT3 in autophagy. Autophagy. 2015;11(5):729–739. doi:10.1080/15548627.2015.1017192
56. Zhou X, Qi W, Hong T, et al. Exopolysaccharides from Lactobacillus plantarum NCU116 regulate intestinal barrier function via STAT3 signaling pathway. J Agric Food Chem. 2018;66(37):9719–9727. doi:10.1021/acs.jafc.8b03340
57. Liu D, Wen L, Wang Z, et al. The mechanism of lung and intestinal injury in acute pancreatitis: a review. Front Med. 2022;9:904078. doi:10.3389/fmed.2022.904078
58. Cao LL, Guan PP, Zhang SQ, et al. Downregulating expression of OPTN elevates neuroinflammation via AIM2 inflammasome- and RIPK1-activating mechanisms in APP/PS1 transgenic mice. J Neuroinflammation. 2021;18(1):281. doi:10.1186/s12974-021-02327-4
59. Slowicka K, Vereecke L, van Loo G. Cellular functions of optineurin in health and disease. Trends Immunol. 2016;37(9):621–633. doi:10.1016/j.it.2016.07.002
60. Sarfarazi M, Rezaie T. Optineurin in primary open angle glaucoma. Ophthalmol Clin North Am. 2003;16(4):529–541. doi:10.1016/s0896-1549(03)00061-0
61. Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol. 2009;10(11):1215–1221. doi:10.1038/ni.1800
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.