Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Population Sensitive to Lenvatinib Plus Anti-PD-1 for Unresectable Hepatocellular Carcinoma Infected with Hepatitis B Virus
Authors Chang X , Yu S , Pang J , Zhang W , Kong H , Huang J , Zhang G , Zhang H, Gu Y , Chen Y , Yang B , Liu J , Zeng Z
Received 9 March 2023
Accepted for publication 1 June 2023
Published 6 June 2023 Volume 2023:10 Pages 847—861
DOI https://doi.org/10.2147/JHC.S411748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Xiujuan Chang,1,* Shumin Yu,2,* Jianzhi Pang,1 Wei Zhang,1 Huifang Kong,1 Jiagan Huang,1 Guojie Zhang,1 Huixin Zhang,1 Yueyue Gu,1,3 Yan Chen,1 Bin Yang,1 Jingping Liu,4 Zhen Zeng1– 3
1Department of Liver Disease Medicine, The Fifth Medical Center of Chinese PLA General Hospital, Beijing, 100039, People’s Republic of China; 2 302 Clinical Medical School Peking University, Beijing, 100039, People’s Republic of China; 3The Fifth School of Clinical Medicine, Anhui Medical University, Hefei, Anhui Province, 230032, People’s Republic of China; 4Oncology Department, Electric Power Hospital of Beijing, Beijing, 100073, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhen Zeng, Department of Liver Disease Medicine, The Fifth Medical Center of Chinese PLA General Hospital, No. 100 West 4th Ring Middle Road, Beijing, 100039, People’s Republic of China, Tel +86 15010540233, Email [email protected]
Background: We explore the dose–efficacy relationship of lenvatinib plus anti-PD-1 in patients with unresectable hepatocellular carcinoma (u-HCC) infected with hepatitis B virus (HBV) in real-world practice. Furthermore, we identify the population sensitive to lenvatinib plus anti-PD-1 treatments.
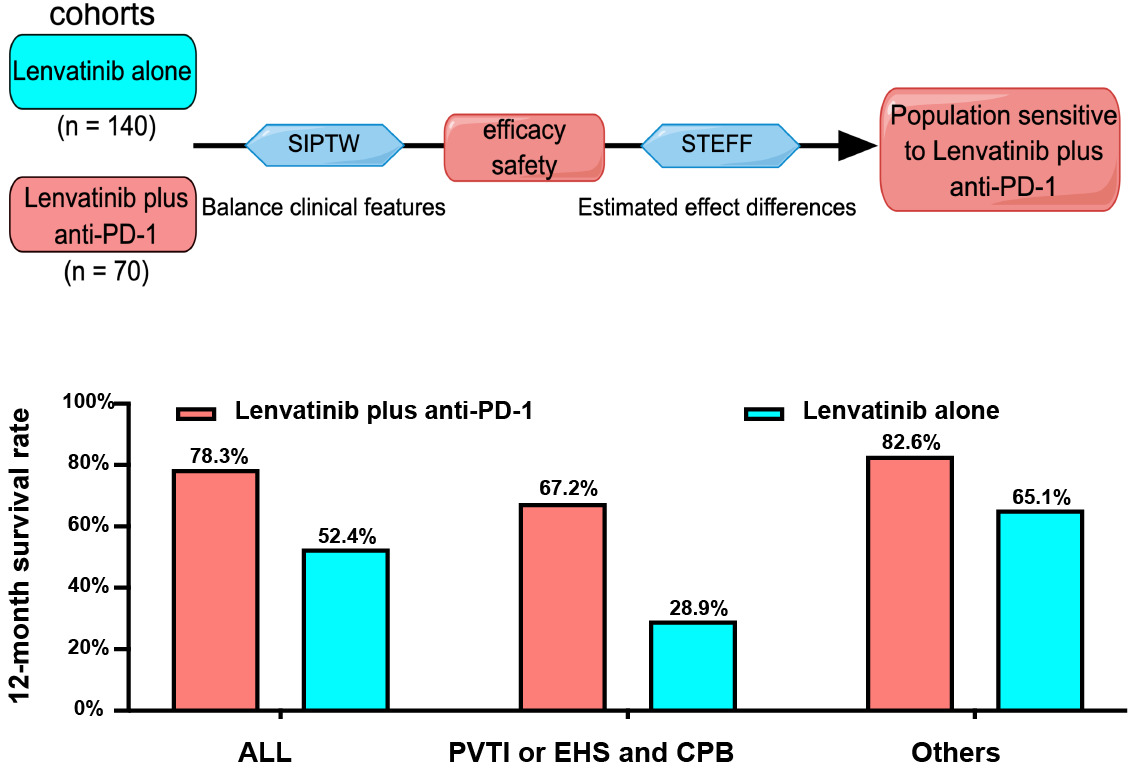
Methods: This retrospective study included 70 patients treated with lenvatinib plus at least 3 cycles of anti-PD-1 and 140 with lenvatinib alone. Stabilized inverse probability of treatment weighting (SIPTW) was used to balance clinical features between the two groups. The overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and adverse events (AEs) were analyzed. Subpopulation treatment effect pattern plot (STEPP) estimated treatment-effect differences between the two groups.
Results: The median age was 54 years, and 189 (90%) cases were male. A total of 180 (85%) patients were infected with HBV. A slowly increasing 12-month survival rate was with the cycles of anti-PD-1, and 5 cycles and more of anti-PD-1 appeared the most beneficial and stable survival rate. The lenvatinib plus at least 3 cycles anti-PD-1 group had better OS (21.4 vs 14 months, p = 0.041), PFS (8.0 vs 6.3 months, p = 0.015) than the lenvatinib alone group in unadjusted cohorts, and the SIPTW adjusted cohorts had confirmed it. For patients with portal vein trunk invasion (PVTI) or extrahepatic spread (EHS) combined with Child-Pugh class B (CPB), lenvatinib plus anti-PD-1 made the 12-month survival rate increase by 38%, while, in the other population, it did only 18%. The two groups had similar AEs (p ≥ 0.05).
Conclusion: The lenvatinib combined with at least 3 cycles of anti-PD-1 was efficacy and safe for u-HCC patients infected with HBV. Especially, patients with PVTI or EHS combined with CPB may benefit most from the combination therapy.
Keywords: hepatocellular carcinoma, real-world study, anti-PD-1, lenvatinib
Graphical Abstract:
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death globally.1 For most patients, the opportunity for complete resection is lost at the first diagnosis due to the lack of early symptoms. Therefore, about half of the HCC patients receive systemic therapies that are the standard for unresectable hepatocellular carcinoma (u-HCC) according to official guidelines.2–5
Notably, the systemic therapies of immune checkpoint inhibitors (ICI) with anti-angiogenic or tyrosine kinase inhibitors (TKIs) have been found promising for u-HCC. The combination therapy with ICI is superior to the monotherapy of TKIs in clinical trials and real-world studies.6–8 The clinical trials, atezolizumab plus bevacizumab (IMbrave150), apatinib plus camrelizumab (RESCUE), and lenvatinib plus pembrolizumab (the Phase IIb KEYNOTE-524) showed improved survival rates and controllable tolerability in advanced HCC patients.6,9,10 Lenvatinib with pembrolizumab has been recommended for advanced HCC in the clinical guideline, the diagnosis and treatment of HCC, from the Chinese Society of Clinical Oncology (CSCO).10
Recently, the LEAP-002 trial released a negative result about lenvatinib plus pembrolizumab versus lenvatinib alone in the European Society for Medical Oncology (ESMO). The subgroup analysis showed that the patients infected with hepatitis B virus (HBV) may benefit from lenvatinib plus pembrolizumab treatments. Besides, the real-world study in 378 u-HCC patients, 90% infected with HBV, showed that the lenvatinib plus anti-PD-1 had long survival and considerable objective response rate (ORR) and disease control rate (DCR).11
In addition, just as the duration of lenvatinib is a key factor in the efficacy, the number of cycles of anti-PD-1 may be important for the efficacy and safety of combination therapy of lenvatinib and anti-PD-1. However, few studies mentioned the number of cycles for anti-PD-1. Therefore, the dose–efficacy relationship of anti-PD-1 was unknown.
Although the systemic therapy of the targeted drug combined with ICI is a promising therapy for advanced HCC, only less than half of patients would benefit from it. So far, there are no indicators to identify the population sensitive to combination therapy. Subpopulation treatment effect pattern plot (STEPP) methodology was commonly used to explore the treatment-effect heterogeneity along continuous variations of biomarker expression.12,13 Specifically, STEPP analysis can estimate the absolute effect difference between two treatment groups’ survival curves at a specified time point, and then identify the sensitive population.14 For example, STEPP was used in hormone receptor-positive breast cancer to compare letrozole with tamoxifen, and it showed patients with higher Ki-67 levels may benefit most from letrozole, not tamoxifen.15
In the real-world study, we explored the dose–efficacy relationship of lenvatinib plus anti-PD-1 and identified the population sensitive to lenvatinib plus anti-PD-1 treatment. It will help physicians to individualize lenvatinib combined with anti-PD-1 decision-making for u-HCC patients infected with HBV.
Materials and Methods
Study Design and Patients
This study collected data from consecutive hospital u-HCC patients treated with lenvatinib with or without anti-PD-1. A total of 210 eligible patients came from the Fifth Medical Center of Chinese PLA General Hospital and Electric Power Hospital of Beijing from October 2018 to February 2021. They were diagnosed as HCC by the international guidelines.2,3 Of these, 70 patients had received lenvatinib plus at least 3 cycles anti-PD-1, and 140 patients with lenvatinib alone (Figure S1).
The eligibility criteria were as follows: 1) age >18 years and u-HCC patients, including intermediate-stage HCC and advanced HCC (BCLC staging system),16 2) treatment with lenvatinib more than 1 month with or without at least 3 cycles anti-PD-1 treatment, 3) patients assessed by multi-phase dynamic contrast-enhanced magnetic resonance imaging (MRI) or computed tomography (CT) study within 1 month before initiation of lenvatinib with or without anti-PD-1, and tumor response evaluated every 2 months after treatments, and at least once, 4) at least one measurable target lesion, 5) patients with Child-Pugh class A (CPA), Child-Pugh class B (CPB) ≤7 and with ECOG performance status (ECOG-PS) ≤2. Patients with incomplete medical information, failed follow-up, autoimmune disease, severe coagulation dysfunction (platelet count <50×109/L, prothrombin activity <40%), other malignancies, and HIV were excluded.
This study was approved by the ethics committee of the Fifth Medical Center of Chinese PLA General Hospital and Electric Power Hospital of Beijing, approval number [KY-2021-12-33-1]. Written informed consent was obtained from patients. The study adhered to the Declaration of Helsinki.
Data Collection and Definition of Variables
Clinical parameters were collected from electronic medical records and images as follows: clinical features within 1 month before initiation of treatments (age, sex, weight, etiology, antivirus therapy, prothrombin time (PT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), total bilirubin (TBIL), platelet count (PLT), α-fetoprotein (AFP), ECOG performance status (PST), Child-Pugh (CP), ALBI, BCLC stage, etc.); tumor characteristics from images (tumor size, number, intrahepatic tumor occupation, extrahepatic spread (EHS), and portal vein trunk invasion (PVTI)); treatments (the dose of initiation of lenvatinib, relative dose intensity for initial 8 weeks, modification or interruption of lenvatinib and anti-PD-1, duration with lenvatinib and cycles of anti-PD-1, discontinuation, and subsequent anticancer medicine).
HBV infected was defined as patients with positive HBsAg for more than six months. HCV infected was defined as patients with hepatitis C antibody positive. Patients with detectable HBV DNA received nucleotide analogs. Here, none of the patients had detectable HCV RNA. Cirrhosis was diagnosed when ultrasonography, MRI, or CT found a blunted, nodular liver edge with splenomegaly (length of spleen >12 cm) and platelet counts of <100×109/L. According to the 1999 World Health Organization criteria, diabetes was diagnosed by endocrinologists.
The follow-up ended in March 2022. Overall survival (OS) was defined as the time from baseline to the date of patients’ last follow-up or death and was collected through the phone. Progression-free survival (PFS) was defined as the time from the start of treatment to the date of disease progression or death.
Tumor response was independently evaluated blindly by one independent radiologist and one hepatologist through CT/MRI images according to RECIST (Response Evaluation Criteria in Solid Tumor) v1.1.17 Relative dose intensity for the initial 8 weeks of lenvatinib therapy (8W-RDI) was defined as the ratio of actual dose to recommended dose in the first 8 weeks.18,19
Adverse events (AEs) were assessed based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0.20 Treatment-related AEs were collected from the start of treatments to 3 months after the last dose, or the start of new anticancer agents, whichever came up first.
Treatment Regiments
Lenvatinib was from Eisai (Tokyo, Japan), and anti-PD-1 was from sintilimab (IBI308, Innovent Biologics [Suzhou] Co. Ltd.) and tislelizumab (BGB-A317, BeiGene). The dosing schedule of lenvatinib and anti-PD-1 was as per the drug instructions. Lenvatinib was administered as per the patient’s weight: 8mg/day for <60kg, 12mg/day for ≥60kg, and 200mg for weighting ≥50kg or 3mg/kg for <50kg every 21 days of anti-PD-1, except for patients with Child-Pugh class B, in which, 8mg/day of lenvatinib was administered for all patients.
The dose reduction and interruption of lenvatinib were based on the drug instructions. The dose reduction of anti-PD-1, from 200mg to 3mg/kg, was for patients with weight loss from ≥50kg to <50kg during treatments, and the dose interruption of anti-PD-1 was up to ≤12 weeks. In case of progressive disease or uncontrollable serious AEs, lenvatinib with or without anti-PD-1 was discontinued. When patients developed PD, the subsequent anticancer treatments were based on physicians’ suggestions and patients’ choices.
Statistical Analysis
Categorical variables were shown as percentages, testing with Chi-square, and continuous variables were displayed as the median with interquartile range (IQR), testing with Mann–Whitney U-test. The median follow-up was calculated by reverse Kaplan–Meier (KM-PF).21 Standardized mean difference (SMD) was defined as the difference between two groups divided by the standard deviation. SMD < 0.1, 0.1~0.3, 0.3~0.5, > 0.5 indicate negligible, mild, moderate, and large differences, respectively.22,23 Stability inverse probability of treatment weight (SIPTW) was used to balance the bias between the two groups, weighting clinical features using appropriate math of propensity score. OS and PFS were calculated using Kaplan–Meier analysis and compared by the Log rank test. Variables with p-value <0.10 in the SIPTW-weighted univariate analysis were entered into the SIPTW-weighted multivariate Cox regression analysis. Nonparametric sliding-window subpopulation treatment effect pattern plot (STEPP) methodology was used to estimate effect differences between the two groups at a 12-month survival rate.22,23 In addition, power was calculated by PASS (software: release 11.0). All statistical analyses were conducted by R (4.1.2) using the packages of “survival”, “survminer”, “tidyverse”, “stepp”, “ggplot2” and “MatchIt”. P < 0.05 (two-sided) denotes statistical significance.
Results
Patients Characteristics
In the unadjusted cohort, some of the clinical features had slight differences between the two groups. Mild differences (0.1 < SMD < 0.3) were observed on sex, PT, AFP, ALB, liver cirrhosis, BCLC stage, macrovascular invasion, 8W-RDI, and previous targeted therapy. Moderate differences (SMD of 0.303) were shown on ALT. SIPTW was used to balance the variable bias between the two groups. After SIPTW adjustment, the weighted cohort showed balanced clinical features between the two groups, all clinical features with SMD < 0.1 indicating negligible differences (Table 1).
 |
Table 1 Clinical Features of the Lenvatinib Alone Group (Monotherapy) and the Lenvatinib Plus Anti-PD-1 Group (Combination Therapy) Before and After SIPTW Adjustment |
The median age was 54 (IQR, 47–62) years, and 189 (90%) cases were male. The etiologies included HBV-infected (n = 180, 85.7%), chronic hepatitis C virus (HCV) infected (n = 8, 3.8%), and others (n = 22, 10.5%). Among 180 patients with HBV infected, 170 (94.4%) patients had received nucleotide analogs (NAs), 10 patients with HBV and 8 patients with HCV had an undetectable viral load. A total of 166 (79%) patients were classified at BCLC stage C and 44 (21%) were at stage B. 135 (64.3%) patients with CPA and 75 (35.7%) with CPB. Eighty-seven (41.1%) patients showed EHS. Forty-three (20.5%) patients had PVTI and 79 (37.6%) with portal vein branch invasion. For previous treatments, 39 (18.6%) received targeted therapies, 127 (60.5%) with TACE, 144 (68.6%) with radiotherapy, and 32 (15.2%) with surgery. Sixty-four (30.5%) patients had diabetes. A total of 137 (65.2%) patients did not meet REFLECT criteria for PVTI (n = 43, 20.5%), intrahepatic tumor occupation ≥50% (n = 50, 23.8%), previous targeted therapy (n = 39, 18.6%) and CPB (n = 75, 35.7%) (Table 1).
Efficacy
The respective median follow-up of the combination and monotherapy groups was 21.1 (95% CI: 15.6–32.4) and 22.9 (95% CI: 20.6–30.8) months (P = 0.439), respectively. At the end of the follow-up, 33 (47.1%) and 87 (62.1%) patients died in the combination and monotherapy groups, respectively. The respective median OS of the combination and monotherapy groups was 21.4 (95% CI: 16.4–31.4) and 14.0 (95% CI: 11.2–17.5) months (HR: 0.65, 95% CI: 0.44–0.97) (Figure 1A). After SIPTW adjustment, the combination group still showed a superior survival over the monotherapy group (Combination vs Monotherapy: 22.3 vs 14.2 months, HR = 0.58, 95% CI: 0.38–0.89, p = 0.014, Figure 1B).
At the end of follow-up, 52 (74.3%) and 119 (85%) patients developed PD or death in combination and monotherapy groups, respectively. The respective median PFS was 8.03 (95% CI: 6.97–12.1) and 6.33 (95% CI: 5.03–7.6) months in the combination and monotherapy groups (HR = 0.67, 95% CI: 0.47–0.92, p= 0.015) (Figure 1C). After SIPTW adjustment, the combination group still had better PFS than the monotherapy group (Figure 1D).
Among patients with PD, 30 (57.7%) patients accepted subsequent anticancer treatments in the combination group and 60 (50.4%) did in the monotherapy group (P = 0.381). The subsequent anticancer drugs mainly were regorafenib or anti-PD-1. In addition, 8 patients received TACE after PD, including 4 and 4 patients in combination and monotherapy groups, and there were no statistical differences between them (P = 0.45).
Interestingly, the STEPP analysis showed a slowly increasing 12-month survival rate with the cycles of anti-PD-1, and 5 cycles and more of anti-PD-1 appeared the most beneficial and stable survival rate (Figure 2A). This suggested that patients with 5 cycles and more of anti-PD-1 may benefit most from the combination therapy.
We defined lenvatinib plus 4 cycles of anti-PD-1 as the combination therapy group. Eleven patients with lenvatinib plus 3 cycles of anti-PD-1 were excluded from the combination group and then entered into the monotherapy group. Then, the respective median OS was 24.2 (95% CI:21.4-NA) and 13.6 (95% CI: 11.2–16.4) months in the combination and monotherapy groups (HR: 0.44, 95% CI: 0.28–0.70) (Figure 2B).
The best tumor responses are shown in Table 2. As per RECIST v1.1, the combination group had superior DCR and ORR over the monotherapy group (DCR: 80% vs 63.6%, p = 0.023; ORR: 25.7% vs 12.1%, p = 0.013). The SIPTW adjustment cohort confirmed it (Combination vs Monotherapy: DCR:78.1% vs 62.4%, p = 0.046; ORR: 24.5% vs 12.5%, p = 0.043). Nobody achieved CR in the two groups (Table 2).
Prognostic Factors of Overall Survival and Progression-Free Survival for u-HCC
The associated factors with OS were analyzed by the SIPTW-weighted univariate and multivariate Cox regression analyses. Collinearity between variables did not exist. The results showed that duration of treatments (≥7.6 vs <7.6 months: HR 0.28, 95% CI 0.18–0.43, p < 0.001), combination therapy (yes vs no: HR 0.44, 95% CI 0.27–0.72, p = 0.001) and EHS (yes vs no: HR 1.69, 95% CI 1.01–2.85, p = 0.046) were independent factors of OS (Table S1). Also, the duration of treatments (≥7.6 vs <7.6 months: HR 0.33, 95% CI 0.23–0.49, p < 0.001), the combination therapy (yes vs no: HR 0.65, 95% CI 0.46–0.92, p = 0.014), and the previous radiotherapy (yes vs no: HR 0.67, 95% CI 0.47–0.96, p = 0.027) were independent protective factors of PFS. 8W-RDI showed no relevance to the OS and PFS (Table S2). The results suggested that the duration of treatments and lenvatinib combined with anti-PD-1, not 8W-RDI, were key to the OS and PFS for u-HCC patients.
After the factors associated with treatments were removed, multivariate Cox regression analysis showed that PVTI (Trunk invasion vs No vascular invasion: HR 2.18, 95% CI 1.21–3.92, p = 0.009), EHS (yes vs no: HR 2.07, 95% CI 1.33–3.22, p = 0.001) and Child-Pugh class (CPB vs CPA: HR 1.68, 95% CI 1.10–2.55, p = 0.015) were independent risk factors of OS (Table 3). Patients with CPB have a poorer OS than that with CPA (11.5 vs 21.4 months, P < 0.001). However, the combination therapy group (Lenvatinib plus 3 cycles anti-PD-1) have a slightly longer overall survival compared with the monotherapy group in patients with CPA (the combination therapy vs Monotherapy: 22.3 vs 18.3 months, P = 0.16) or CPB 7 scores (the combination therapy vs Monotherapy: 13.2 vs 11.2 months, P = 0.16) (Figure S2A and B), but not reach statistically significant. Moreover, the combination therapy group (Lenvatinib plus 4 cycles anti-PD-1) has an evident longer overall survival than the monotherapy group in patients with CPA (the combination therapy vs Monotherapy: 24.4 vs 16.7 months, P = 0.014) or CPB 7 scores (the combination therapy vs Monotherapy: 21.3 vs 11.2 months, P = 0.014) (Figure S2C and D). Therefore, although patients with CPB have a poor prognosis, they may benefit from the combination therapy.
 |
Table 3 The Independent Factors of Over Survival by SIPTW-Weighted Cox Regression Analysis |
The Population Sensitive to Lenvatinib Plus Anti-PD-1
The features associated with OS were integrated into a composite risk score according to the coefficients of the variables from the above multivariate Cox regression analysis. The composite risk score was calculated as follows:
Composite risk score = portal vein trunk invasion (No vascular invasion = 0, existence = 1) * 0.83 + extrahepatic spread (nonexistence = 0, existence = 1) * 0.74+ Child-Pugh (class A = 0, class B = 1) * 0.54 + portal vein branch invasion (No vascular invasion = 0, existence = 1) * 0.34.
Across the composite risk score, the STEPP analysis revealed that the 12-month survival rate of all patients was 61% (120 of 210 patients died), increasing from 27.8% in the highest quartile of the composite risk score to 76% in the lowest quartile (Figure 3A and B). The median composite risk score was 0.74. For patients with a composite risk score >0.74, the combination therapy made the 12-month survival rate increase by 38%. On the contrary, the combination therapy made the 12-month survival rate increase by only 18% (Figure 3C and D).
For the convenience of application, the composite risk score was simplified as three factors, PVTI, EHS, and CPB. Meanwhile, portal vein branch invasion was removed for no statistical significance. Patients with PVTI or EHS combined with CPB had a lower OS than the other population (11.2 vs 22.3 months) (Figure 4A). However, for patients with PVTI or EHS combined with CPB, the combination therapy made the 12-month survival rate increased by 38%, while, in the other population, it did only 18% (Figure 4B and C).
Safety Profile
The respective median duration of the combination therapy and monotherapy was 8.5 and 6.6 months. The respective median lenvatinib 8W-RDI was 83.3% and 91.7% in the combination and monotherapy groups. Grade 3 and more treatment-related AEs with a frequency ≥1% were presented in Table S3 and any grade treatment-related AEs with frequent ≥10% in Table S4. Treatment-related deaths or serious AEs were not observed. Serious immune-related adverse events (irAE) were not observed: myocarditis, pneumonia, and myositis. Any grade treatment-related AEs of the combination group were comparable to that of the monotherapy group (any grade: 70 (100%) vs 135 (96.4%), p = 0.26; Grade 3 and more AEs: 42 (60.0%) vs 69 (49.3%), p = 0.143). The most common AEs included hypertension (n = 88, 42%), fatigue (n = 87, 41%), decreased appetite (n = 81, 39%), diarrhea (n = 75, 36%), hand-foot syndrome (n = 73, 35%) and proteinuria (n = 67, 32%). The combination group had similar adverse events leading to interruption or dose reduction/discontinuation to the monotherapy group (Table S4).
Discussion
This study showed that a slowly increasing 12-month survival rate was with the cycles of anti-PD-1, and 5 cycles and more of anti-PD-1 appeared the most beneficial and stable survival rate. The lenvatinib plus at least 3 cycles anti-PD-1 group had better OS (21.4 vs 14 months), PFS (8.0 vs 6.3 months), ORR (25.7% vs 12.1%), and DCR (80% vs 63.6%) than the monotherapy group. For patients with PVTI or EHS combined with CPB, lenvatinib plus anti-PD-1 made the 12-month survival rate increase by 38%, while, in the other population, it did only 18%. The two groups showed a similar toxicity profile.
The median OS and PFS of the lenvatinib plus pembrolizumab group were 22 and 9.3 months in the KEYNOTE-524 trial (phase Ib).10 However, ESMO released a negative result (lenvatinib plus pembrolizumab vs lenvatinib) in the LEAP-002 trial (phase III). The reason may be that only 48% of patients enrolled were infected with HBV in the LEAP-002 trial. A meta-analysis of systemic therapies of Phase III RCTs (2002–2020) suggests that immunotherapies may be more effective in viral-related HCC than non-viral-related HCC.24 Interestingly, the subgroup analysis of the LEAP-002 trial also showed consistent results with this study in the population with HBV, extrahepatic spread, or macrovascular portal vein invasion/extrahepatic spread: the lenvatinib plus pembrolizumab had a superior OS over the lenvatinib alone. Besides, in a real-world study, Yang reported that lenvatinib plus anti-PD-1 had long survival and considerable ORRs and DCRs in 378 u-HCC patients, and 90% infected with HBV.11 Some real-world studies reported consistent results: lenvatinib plus ICI had superior efficacy over lenvatinib alone.25–27
Furthermore, we explored the dose–efficacy relationship between lenvatinib and anti-PD-1. The SIPTW-weighted multivariate Cox regression analysis indicated that the duration of lenvatinib, not 8W-RDI of lenvatinib, can prolong the OS and PFS for u-HCC patients. A study also reported that the duration of lenvatinib was a protective factor of OS.28 In addition, the STEPP analysis suggested that a slowly increasing 12-month survival rate was with the cycles of anti-PD-1, and patients with 5 cycles and more of anti-PD-1 may benefit most from the combination therapy, which was one very important finding.
Although lenvatinib plus anti-PD-1 may be a promising therapy for u-HCC, only less than half of patients would benefit from it. Therefore, individualized therapy decisions should weigh benefits against high costs and adverse effects. However, there were no indicators to clarify who was sensitive to Lenvatinib plus anti-PD-1 treatments. In this study, the other important finding was that patients with PVTI or EHS combined with CPB would benefit most from the lenvatinib plus anti-PD-1 treatments. Similarly, a real-world study also reported that lenvatinib plus anti-PD-1 can improve OS for HCC patients with invasion in Vp4.26 Although patients with PVTI or EHS combined with CPB had a poor prognosis, they may benefit most from the combination therapy. This finding may help physicians to individualize the choice of lenvatinib plus anti-PD-1.
The mechanism of synergy between TKIs and ICI is being increasingly characterized. Vascular abnormalities are a hallmark of most solid tumors and stem from elevated levels of VEGF and angiopoietin 2 (ANG2). Lenvatinib targets these molecules and then normalizes the abnormal tumor vasculature, to increase the infiltration of immune effector cells into tumors. It promotes the efficacy of anti-PD-1, which depends on the increment and activity of immune effector cells in the tumor microenvironment (TME). Vascular normalization and immune responses may be reciprocally regulated.29 For example, lenvatinib plus anti-PD-1 can increase the percentage of early-activated CD8+T cells.30 Therefore, it may be the reason that patients with metastasizes or vascular invasion would benefit more from the lenvatinib plus anti-PD-1.
In addition, this study showed that the two groups had similar AEs and interruption and/or modification of treatment. A real-world study reported a similar result for the interruption and/or modification of treatment.31
The common limitations were bias from the retrospective design. However, information from electronic medical records and overall survival were objective. Besides, we performed rigorous statistical analyses to balance the clinical features between two groups by the SIPTW and adjust possible prognostic factors by the SIPTW-weighted multivariate Cox regression analysis. Although SIPTW could not deal with unmeasured confounding variables, such as the economic background of patients, the results still provided robust information. In addition, this study included two kinds of anti-PD-1, sintilimab and tislelizumab, which are more affordable than pembrolizumab. Although both are common anti-PD-1, there may be slight differences between them. Therefore, the multicentre randomized controlled trial (RCT) needs to be inspired to further verify the efficacy and safety of lenvatinib plus at least 3 cycles of anti-PD-1 in the dominant population.
Conclusions
The lenvatinib plus at least 3 cycles of anti-PD-1 treatment was efficacy and safe for u-HCC patients infected with HBV in real-world studies. Especially, patients with PVTI or EHS combined with CPB may benefit most from the combination therapy.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study protocol was reviewed and approved by the institution review board of the Fifth Medical Center of Chinese PLA General Hospital and Electric Power Hospital of Beijing, approval number [KY-2021-12-33-1]. Written informed consent was obtained from patients. The study was performed by the ethics standards of the institutional research committee and the recent Declaration of Helsinki.
Consent for Publication
All subjects gave written informed consent.
Acknowledgments
We appreciate the State Key Projects Specialized on Infectious Disease, Chinese Ministry of Science and Technology (2018ZX10302205-001) and National Science Foundation of China (81970525), and Beijing Natural Science Foundation (7212101) for funding support. We are very grateful to Professor Jingfeng Bi for statistical guidance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the State Key Projects Specialized on Infectious Disease, Chinese Ministry of Science and Technology (2018ZX10302205-001), and Beijing Natural Science Foundation (7212101).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
3. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
4. Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31(3):334–351. doi:10.1016/j.annonc.2019.12.001
5. Vogel A, Martinelli E, Vogel A. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann Oncol. 2021;32(6):801–805. doi:10.1016/j.annonc.2021.02.014
6. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
7. Kelley RK, Sangro B, Harris W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39(27):2991–3001. doi:10.1200/jco.20.03555
8. D’Alessio A, Fulgenzi CAM, Nishida N, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology. 2022;76:1000–1012. doi:10.1002/hep.32468
9. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, Phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi:10.1158/1078-0432.Ccr-20-2571
10. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/jco.20.00808
11. Yang X, Chen B, Wang Y, et al. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol Int. 2023:1–11. doi:10.1007/s12072-022-10480-y
12. Bonetti M, Gelber RD. Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics. 2004;5(3):465–481. doi:10.1093/biostatistics/5.3.465
13. Bonetti M, Zahrieh D, Cole BF, Gelber RD. A small sample study of the STEPP approach to assessing treatment-covariate interactions in survival data. Stat Med. 2009;28(8):1255–1268. doi:10.1002/sim.3524
14. Crivellari D, Sun Z, Coates AS, et al. Letrozole compared with tamoxifen for elderly patients with endocrine-responsive early breast cancer: the BIG 1-98 trial. J Clin Oncol. 2008;26(12):1972–1979. doi:10.1200/jco.2007.14.0459
15. Lazar AA, Cole BF, Bonetti M, Gelber RD. Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: subpopulation treatment effect pattern plot. J Clin Oncol. 2010;28(29):4539–4544. doi:10.1200/jco.2009.27.9182
16. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi:10.1055/s-2007-1007122
17. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
18. Goh MJ, Oh JH, Park Y, et al. Efficacy and safety of lenvatinib therapy for unresectable hepatocellular carcinoma in a real-world practice in Korea. Liver Cancer. 2021;10(1):52–62. doi:10.1159/000512239
19. Sasaki R, Fukushima M, Haraguchi M, et al. Response to lenvatinib is associated with optimal relativedose intensity in hepatocellular carcinoma: experience in clinical settings. Cancers (Basel). 2019;11(11). doi:10.3390/cancers11111769
20. National Cancer Institute. Division of cancer treatment and diagnosis. Cancer therapy evaluation program. Adverse events/ CTCAE. Available from: https://ctepcancergov/protocolDevelopment/electronic_applications/ctchtm#ctc_50.
21. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi:10.1016/0197-2456(96)00075-x
22. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi:10.1002/sim.3697
23. Casadei-Gardini A, Scartozzi M, Tada T, et al. Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: an inverse probability of treatment weighting analysis. Liver Int. 2021;41(6):1389–1397. doi:10.1111/liv.14817
24. Haber PK, Puigvehí M, Castet F, et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002–2020). Gastroenterology. 2021;161(3):879–898. doi:10.1053/j.gastro.2021.06.008
25. Chen K, Wei W, Liu L, et al. Lenvatinib with or without immune checkpoint inhibitors for patients with unresectable hepatocellular carcinoma in real-world clinical practice. Cancer Immunol Immunother. 2021;71:1063–1074. doi:10.1007/s00262-021-03060-w
26. Sun X, Zhang Q, Mei J, Yang Z, Chen M, Liang T. Real-world efficiency of lenvatinib plus PD-1 blockades in advanced hepatocellular carcinoma: an exploration for expanded indications. BMC Cancer. 2022;22(1):293. doi:10.1186/s12885-022-09405-7
27. Huang C, Zhu XD, Shen YH, et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. Biomark Res. 2021;9(1):19. doi:10.1186/s40364-021-00274-z
28. Kirino S, Tsuchiya K, Kurosaki M, et al. Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS One. 2020;15(4):e0231828. doi:10.1371/journal.pone.0231828
29. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi:10.1038/nrclinonc.2018.29
30. Kato Y, Tabata K, Kimura T, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14(2):e0212513. doi:10.1371/journal.pone.0212513
31. Cheon J, Chon HJ, Bang Y, et al. Real-world efficacy and safety of lenvatinib in Korean patients with advanced hepatocellular carcinoma: a multicenter retrospective analysis. Liver Cancer. 2020;9(5):613–624. doi:10.1159/000508901
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





