Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Lenvatinib Induces Immunogenic Cell Death and Triggers Toll-Like Receptor-3/4 Ligands in Hepatocellular Carcinoma
Authors Zhou C , Yang ZF, Sun BY , Yi Y , Wang Z, Zhou J, Fan J, Gan W, Ren N, Qiu SJ
Received 18 December 2022
Accepted for publication 20 April 2023
Published 24 April 2023 Volume 2023:10 Pages 697—712
DOI https://doi.org/10.2147/JHC.S401639
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Cheng Zhou,1,* Zhang-Fu Yang,1,* Bao-Ye Sun,1,* Yong Yi,1 Zheng Wang,1 Jian Zhou,1 Jia Fan,1 Wei Gan,1 Ning Ren,1,2 Shuang-Jian Qiu1
1Department of Liver Surgery and Transplantation & Key Laboratory of Carcinogenesis and Cancer Invasion (Ministry of Education), Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Institute of Fudan Minhang Academic Health System & Key Laboratory of Whole-Period Monitoring and Precise Intervention of Digestive Cancer, Minhang Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shuang-Jian Qiu, Liver Cancer Institute and Zhongshan Hospital, Fudan University, Shanghai, 200030, People’s Republic of China, Tel +86 13916625289, Email [email protected]
Purpose: Immunogenic cell death (ICD) is a cell death modality that plays a vital role in anticancer therapy. In this study, we investigated whether lenvatinib induces ICD in hepatocellular carcinoma and how it affects cancer cell behavior.
Patients and Methods: Hepatoma cells were treated with 0.5 μM lenvatinib for two weeks, and damage-associated molecular patterns were assessed using the expression of calreticulin, high mobility group box 1, and ATP secretion. Transcriptome sequencing was performed to investigate the effects of lenvatinib on hepatocellular carcinoma. Additionally, CU CPT 4A and TAK-242 were used to inhibit TLR3 and TLR4 expressions, respectively. Flow cytometry was used to assess PD-L1 expression. Kaplan–Meier and Cox regression models were applied for prognosis assessment.
Results: After treatment with lenvatinib, there was a significant increase in ICD-associated damage-associated molecular patterns, such as calreticulin on the cell membrane, extracellular ATP, and high mobility group box 1, in hepatoma cells. Following treatment with lenvatinib, there was a significant increase in the downstream immunogenic cell death receptors, including TLR3 and TLR4. Furthermore, lenvatinib increased the expression of PD-L1, which was later inhibited by TLR4. Interestingly, inhibiting TLR3 in MHCC-97H and Huh7 cells strengthened their proliferative capacity. Moreover, TLR3 inhibition was identified as an independent risk factor for overall survival and recurrence-free survival in patients with hepatocellular carcinoma.
Conclusion: Our study revealed that lenvatinib induced ICD in hepatocellular carcinoma and upregulated PD-L1 expression through TLR4 while promoting cell apoptosis through TLR3. Antibodies against PD-1/PD-L1 can enhance the efficacy of lenvatinib in the management of hepatocellular carcinoma.
Keywords: hepatocellular carcinoma, immunogenic cell death, lenvatinib, toll-like receptor
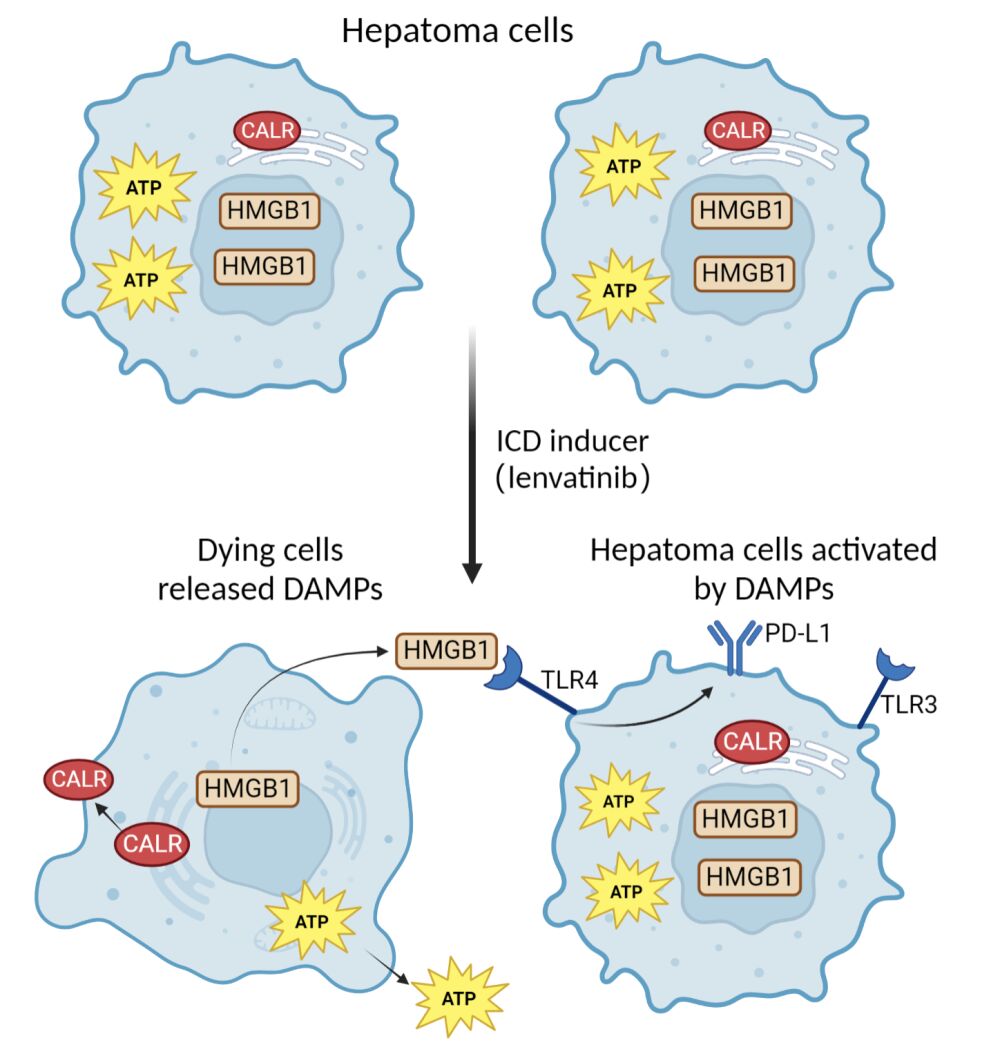
Graphical Abstract:
Introduction
Hepatocellular carcinoma (HCC) ranks sixth in cancer incidence and fourth in cancer-related deaths.1 Unfortunately, many patients with HCC are diagnosed at a moderate-to-advanced stage, where treatment is limited to local or systemic therapies. The current first-line systemic treatments for HCC, such as sorafenib, lenvatinib, donafenib, and anti-PD1 antibodies, have limited efficacy, extending patients’ median survival by less than six months.2–5 Given these shortcomings, there is an urgent need to explore new medication regimens that can effectively treat advanced HCC and improve patient outcomes. Lenvatinib is an emerging multiple kinase inhibitor targeting VEGFR1-3, FGFR1-4, PDGFRα, RET, and c-Kit,6 which is an alternative first-line treatment option for advanced HCC. Lenvatinib has been shown to suppress tumor growth by inhibiting angiogenesis and tumor proliferation and is characterized by immunomodulatory activity compared to other treatment regimens.7,8 Earlier research has indicated that that lenvatinib treatment increases the CD8+ T cell population7 and decreases the Treg cell population in HCC.9 Furthermore, it has been shown to decrease immune checkpoints like Tim-3 and PD-1 expression while increasing the expression of IFNG and GZMB in CD8+ T cells and natural killer cells.8 In addition, lenvatinib augments the expression of natural cytotoxicity receptors by tumor-infiltrating NK cells and the expression of cytotoxic cytokines in tumor tissues.10 To explore the immunomodulatory mechanism and improve the efficacy of lenvatinib, we hypothesized that lenvatinib could serve as an inducer of immunogenic cell death (ICD) in HCC.
ICD is a unique cell death modality triggered by selected anticancer therapies,11,12 stimulating the innate and adaptive immune system against cell death-associated antigens and subsequently inducing tumor cell immunogenicity. Various anticancer treatments, such as chemotherapy, phototherapy, and radiotherapy, are known to induce ICD by releasing damage-associated molecular patterns (DAMPs) from dying cells. The released DAMPs activate innate immune responses and establish immunological memory. ICD-characterized DAMPs include exposure to endoplasmic reticulum chaperones, secretion of ATP, high mobility group box 1 (HMGB1), activation of the cancer cell-intrinsic type I IFN response, and the consequent secretion of the chemokine ligand 10.13 DAMPs-triggered immune responses transform immune profile from “cold” to “hot” triggering synergies with immune checkpoint inhibitors and ultimately benefiting long-term tumor management.14 This is because ICD induction stimulates an effective antitumor immune response. However, it is important to note that not all anticancer therapies are capable of inducing ICD. For instance, while radiofrequency ablation,15 radiotherapy,16 FOLFOX4 regimen17 and doxorubicin18 have been shown to induce in HCC, small-molecule agents have yet to be detected.
In this study, we investigated the induction of ICD by lenvatinib and its effects on the behavior of HCC cancer cells. Our findings suggest that lenvatinib promoted ICD in HCC cells and increased PD-L1 expression via TLR4. PD-1 or PD-L1 antibodies can enhance the efficacy of lenvatinib in HCC treatment.
Methods
Cell Culture
Hepatoma cell lines, including Huh7, PLC, Hep1-6, and MHCC-97H, were obtained from the liver cancer institution in Zhongshan Hospital and maintained in DMEM (hyClone) containing 10% FBS (hyClone) at 37°C with 5% CO2. The use of all cell lines was approved by the Institutional Ethics Committee of Zhongshan Hospital. Agents including lenvatinib (0.5 μM, Selleck), CU CPT 4a (27 μM, TOCRIS),19 Poly I:C (10 μg/mL, Selleck)20 and TAK-242 (10 μM, Selleck)21 were solubilized in DMSO.
Patients
This study was approved by The Institutional Ethics Committee of Zhongshan Hospital, Fudan University and was conducted in accordance with the Helsinki Declaration (WMA Declaration of Helsinki, 2013). All the patients involved were pathologically diagnosed with HCC at Zhongshan Hospital. Written informed consent was obtained from all the patients before the surgery or pharmacological treatment. 27 patients who had received lenvatinib or lenvatinib plus anti-PD-1 antibodies and 343 patients who had not received any treatment before the surgery were collected were involved in this study. Only patients who met the following inclusion criteria were enrolled: (I) >18 years and ≤75 years of age; (II) diagnosed by cytologic/histologic evidence or by noninvasive diagnostic measurements recommended by the EASL; (III) preserved liver function was classified as Child-Pugh class A without any ascites but patients were intolerant to complete resection. Patients were excluded if they met any one of the following criteria: (I) a history of other malignancies; (II) liver functional status of Child-Pugh B/C; (III) cardiac, pulmonary, cerebral, or renal dysfunction; (IV) with extrahepatic metastasis or macroscopic vascular invasion.
The dose of lenvatinib was either 12 mg/day (weight; ≥ 60 kg) or 8 mg/day (weight; < 60 kg). The anti-PD-1 antibody administration protocol was applied according to the manufacturer’s instructions (1 time/21 days). Lenvatinib and the Anti-PD1 antibody were discontinued a week and a month before surgery, respectively.
HCC Xenograft Model
Animal experiments were approved by the Ethics Committee of the Zhongshan Hospital Biomedical Research Department and followed the Guideline for ethical review of animal welfare in China (GB/T 35892–2018). A total of 12 eight weeks old male BALB/c mice were obtained from Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China). Huh7 cells (1*10^6) were mixed with Matrigel (Corning) at 3:1 and injected into the armpit. Mice were randomized into lenvatinib-treated group and control group when the tumor grew up to 100mm3. Mice were fed up with lenvatinib (2mg/kg/day) or equivalent volume of saline by gastric gavage for four weeks in lenvatinib-treated group and control group, respectively. Neck breaking method was applied in mice to cause immediate death. All mice were raised in a specific pathogen-free environment in Zhongshan hospital.
RNA Extraction and Real-Time Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted using TRIzol (Thermo), phase-separated with chloroform, precipitated using isopropyl alcohol, washed with 75% ethanol, and redissolved in water. RNA reverse transcription reactions and RT-qPCR were conducted using Goldenstar™ RT6 cDNA Synthesis Mix and 2×TSINGKE® Master qPCR Mix from Tsingke Biotechnology. qRT-PCR primer pairs for TLR3, TLR4, and PIK3R1 were obtained from PrimerBank. Primer sequences for TLR3: (f)5′-CAAACACAAGCATTCGGAATCTG (r)5′-AGGAATCGTTACCAACCACATT; TLR4:(f)5′-AGACCTGTCCCTGAACCC (r)5′-CGATGGACTTCTAAACCAGCCA; pik3r1: (f) 5′-TGGACGGCGAAGTAAATT (r) 5′-AGTGTGACATTGAGGGAGTCG; gapdh: (f) 5′CTGGGCTACACTGAGCACC- (r) 5′-AAGTGGTCGTTGAGGGCAATG. RT-qPCR was performed using a 7500 Fast Real-Time PCR System. Fold change values were calculated using the 2−ΔΔCt method.
Protein Extraction and Western Blot
Cells or tissues were homogenized and lysed in RIPA buffer (Biyuntian) with protease inhibitors (Sigma). Proteins were separated by SDS-PAGE, electroblotted onto Trans-Blot Turbo Transfer Pack 0.2 µm PVDF membrane (BioRad), and incubated overnight with antibodies, followed by corresponding secondary antibodies (Abclone). The primary antibodies were used for Western blot were listed in Table 1. Secondary antibodies conjugated to horseradish peroxidase were then used as also listed in Table 1.
 |
Table 1 Antibodies Applied in Western Blot, Immunohistochemistry, Immunofluorescence and Flow Cytometry |
Immunofluorescence
The cells were cultured in six-well plates, fixed using 3% paraformaldehyde, and permeabilized using 0.5% Triton X-100 (Tx100). Fixed cells were incubated in 10% goat serum for 30 min and stained with primary antibodies overnight at 4°C. The samples were washed three times using PBST, incubated with fluorescent secondary antibodies for 1 h in a wet box, and stained using 0.1 ug/mL DAPI for 5 min in the dark.
Flow Cytometry
Flow cytometry was used to determine cell apoptosis rate and PD-L1 expression. Single-cell suspensions were resuspended in 2% BSA for flow cytometry, and the cells were subsequently incubated with antibodies for 30 min at 4°C. The antibodies used for flow cytometric analysis are listed in Table 1. Flow cytometric analysis was performed using a BD Accuri C6 Plus flow cytometer (BD Biosciences). Data analysis was conducted using FlowJo V10, and the relative protein expression was analyzed using the mean fluorescence intensity.
IHC
Tissues were fixed using 4% paraformaldehyde at 4°C overnight after washing with PBS. After dehydration, permeabilization, wax-dip, and embedding, 5 μM sections were cut. The slices were processed in the following order: dewaxing, hydration, antigen repair, and BSA blocking. Afterward, slices were incubated with primary antibodies overnight, and secondary antibodies were added for 1 h. Finally, the chromogenic substrate, tetramethylbenzidine, was added for color development. The antibodies used for IHC are listed in Table 1.
RNA Sequencing
Transcriptome sequencing was performed by Genergy Biotechnology Co. (Shanghai, China). RNA-Seq libraries were generated according to the manufacturer’s protocols using the NEB Next Ultra Directional RNA Library Prep Kit for Illumina (Illumina, CA, USA). All the samples were sequenced on the MGISEQ-2000 platform. 27.71Gb raw data was generated from 4 samples. The raw data were normalized. Clean reads were obtained from the raw data by discarding adapter and poly-N sequences and reads of low quality. Differential expression analysis was based on edgeR package in R software (v.4.1.2). A p value < 0.05 and a fold change > 2 were set as the threshold for significant differential expression.
Statistics
As appropriate, patients’ baseline characteristics were reported as mean (± standard deviation), median (range), or percentage. The Mann–Whitney U and Student’s t-tests were used in comparing continuous variables. The χ2 and Fisher exact tests were used to compare categorical variables. Overall survival was examined using the Kaplan–Meier and Log rank tests. Factors with a p-value ˂0.10 in univariate analyses were introduced into the multivariate Cox proportional hazards model to determine the independent impact on overall survival (OS). Hazard ratios (HR) and 95% confidence intervals (CIs) were estimated using a nonparametric Log rank test with the Cox proportional hazards model. All statistical analyses were performed using SPSS for Windows (version 24.0; IBM, NY). Correlation analyses were performed using Spearman’s rank correlation coefficient. Statistical significance was set at a two-tailed p-value ˂0.05. The graph abstract was created with biorender.
Results
Lenvatinib Induced ICD in Hepatoma Cells
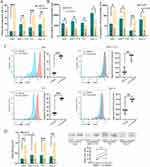
We investigated whether lenvatinib induces ICD in hepatoma cells by examining the presence of classical DAMPs in human hepatoma-derived cell lines, including Huh7, MHCC-97H, and PLC, as well as in mouse hepatoma-derived Hepa1-6 cells. In line with pharmacokinetic analyses, which revealed plateaued lenvatinib concentrations (8–12 mg once daily) at 1.23–2.11 ng/mL in HCC patients,22 we treated the cells with a lenvatinib concentration of 5 µM for two weeks to simulate the effects of lenvatinib on hepatoma tumor cells. Flow cytometry demonstrated that lenvatinib treatment resulted in a higher proportion of apoptotic cells in comparison to the control group (Figure 1A).
There was an increase in extracellular ATP in lenvatinib-treated hepatoma cells, whereas intracellular ATP levels decreased or remained constant (Figure 1B). Furthermore, we examined the surface expression of calreticulin on hepatoma cells using flow cytometry, which demonstrated a significant increase in calreticulin expression on the surface of all hepatoma cells following lenvatinib treatment (Figure 1C). In addition, ELISA and Western blot analyses showed higher HMGB1 levels in the culture medium of lenvatinib-treated hepatoma cells (Figure 1D). Summarily, these results demonstrated that ICD markers, including calreticulin expression, ATP release, and HMGB1 release, were significantly increased in hepatoma cells upon treatment with lenvatinib.
Transcriptome Sequencing Revealed the Increased Expression of TLR3 and TLR4 in Hepatoma Cells
To further understand the effects of lenvatinib treatment on hepatoma cells, we conducted transcriptome sequencing of both lenvatinib-treated and untreated Huh7 and MHCC-97H cells. Subsequently, we performed differential gene expression analysis on both cell types, identifying 48 genes that were significantly upregulated in both groups. To determine the biological context of these genes, we conducted GO and KEGG pathway analyses, which revealed enrichment in pathways such as the interferon signaling pathway and primary alcohol metabolic process, among others (Figure 2A). Notably activation of the interferon signaling pathway is one of the characteristics of ICD. The downstream receptors of DAMPs, including TLR3 and TLR4, exhibited 69-fold and 14-fold elevation in lenvatinib-treated Huh7 cells, respectively (Figure 2B). TLR4 expression exhibited a 4.2-fold increase in lenvatinib-treated MHCC-97H cells (Figure 2C). Based on the results of transcriptome sequencing analysis, we speculated that lenvatinib induces ICD in hepatoma cells and triggers TLR3 and TLR4 upregulation.
Lenvatinib Upregulated the Expression of TLR3 and TLR4
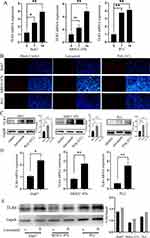
In support of our hypothesis, we quantified the expression of TLR3 and TLR4 in the hepatoma cells. Following two weeks of lenvatinib treatment, we observed a significant increase in mRNA expression of TLR3 in Huh7, PLC, and MHCC-97H cells (Figure 3A). Western blotting and immunofluorescence revealed enhanced TLR3 expression in lenvatinib-treated cells (Figures 3B and C). In addition, we observed an increase in mRNA and protein expression of TLR4 in Huh7, PLC, and MHCC-97H cells after lenvatinib treatment (Figures 3D and E).
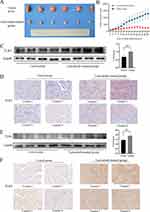
We examined the expression of TLR3 and TLR4 in vivo. Two groups of BALB/c mice with xenograft tumors were administered 5 mg/kg lenvatinib or saline. Mice treated with lenvatinib had significantly smaller tumor sizes (Figures 4A and B). Two tumors from each group were transferred to wild-type mice to evaluate their tumor-formation ability. No tumors transferred from lenvatinib-treated mice formed new tumors, indicating that the tumor formation ability was significantly decreased after lenvatinib treatment. In vivo, the expressions of TLR3 and TLR4 also increased in lenvatinib-treated mice (Figures 4C–F).
Lenvatinib Upregulated the Expression of PD-L1 by TLR4
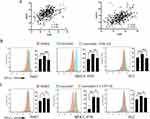
Previous studies reported the induction of ICD and the expression of toll-like receptors accompanied by alterations in the expression of immune checkpoints. Correlation analysis between TLR3/4 and common immune checkpoints in the TCGA database. We checked the gene expression level of TLR3/4 and a series of immune checkpoints including TIM3, CD274(PD-L1), OX40, CD27, CTLA4 and LAG3. Among all immune checkpoints, CD274 had the highest correlation with TLR3 and TLR4 (TLR3: r= 0.461, p< 0.001; TLR4: r= 0.503, p< 0.001; Figure 5A). Flow cytometry analysis of PD-L1 expression indicated an increase in lenvatinib-treated Huh7, MHCC-97H, and PLC cells (Figures 5B and C). We inhibited the expression of TLR4 using TAK-242 and observed that PD-L1 expression was decreased (Figure 5B); however, there was no significant change in PD-L1 expression after TLR3 inhibition (CU CPT 4A) (Figure 5C).
The Upregulation of TLR3 Promoted Cell Death in Hepatoma Cells
We investigate the effects of TLR3 and TLR4 inhibition on cell apoptosis and proliferation. We inhibited the expression of TLR3 and TLR4 using CU CPT 4A and TAK-242 in Huh7 and MHCC-97H cells, respectively. The corresponding proportion of apoptotic cells significantly decreased when TLR3 expression was inhibited (Figure 6A). Furthermore, the CCK8 assay indicated that TLR3 inhibition reinforced the proliferative capacity of MHCC-97H and Huh7 cells (Figures 6B and C).
Patients Who Had Received Lenvatinib-Treatment Had a Higher Positivity of TLR3 and TLR4
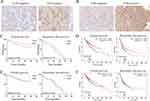
Most patients who received lenvatinib were in the moderate-to-advanced stages and had lost the chance to undergo surgery. Biopsy was also not recommended for these patients. However, the combination of lenvatinib and anti-PD-1 antibodies resulted in downstaging and subsequent eligibility for resection in some patients. Samples from 27 patients who had received lenvatinib or lenvatinib plus anti-PD-1 antibodies and 343 samples from patients who had not received any treatment before the surgery were collected. Among the 27 patients, 3 received lenvatinib, and 24 received lenvatinib plus anti-PD-1 antibodies. IHC was performed to assess TLR3 and TLR4 expression in these patients (Figures 7A and B), which revealed a higher positivity of TLR3 in patients treated with lenvatinib (70.4% vs 44.4%), and the same was observed in TLR4 (70.8% vs 14.2%). Among the 343 untreated patients, overall survival and recurrence-free survival were longer for patients with positive TLR3 than those with negative TLR3 (all p< 0.05, Figure 7C). TLR3 was also an independent risk factor for overall and recurrence-free survival in the multivariate analyses (Table 2). We also performed survival analysis using the TCGA database, and the results were consistent with IHC (Figure 7D). However, no significant association between TLR4 expression and overall survival was observed (Figures 7E and F). Correlation analysis revealed that patients with positive TLR3 and smaller tumor size had a higher proportion of the tumor capsule (Table 3).
 |
Table 2 Univariate and Multivariate Analysis of Overall Survival and Recurrence-Free Survival |
 |
Table 3 Clinic Baseline and Correlation Analysis with TLR3 or TLR4 |
Discussion
Through our study, we have found that lenvatinib has the ability to induce ICD in hepatoma cells and activate the TLR3/4 pathway in HCC, making it the only small-molecule agent with such properties for HCC treatment. We have also observed that upregulation of TLR4 enhances PD-L1 expression in hepatoma cells, while the upregulation of TLR3 promotes cell apoptosis. This is the first study to report on lenvatinib’s ability to induce ICD and activate TLR3/4 pathway in HCC cells.Previous studies have reported that Ienvatinib can increase the response rate to PD-1 treatment by upregulating the proportion of infiltrated CD8+ T cells7 and downregulating the proportion of Treg cells in HCC;9 however, these studies did not explore the reasons for this phenomenon. Lenvatinib is an angiogenesis inhibitor and a tumor suppressor in HCC.23 Angiogenesis inhibitors can improve the tumor microenvironment through vascular normalization.24 In this study, we observed that in addition to the suppression of angiogenesis, lenvatinib also has the ability to induce ICD in hepatoma cells. This additional contribution uncovers a new mechanism through which lenvatinib can remodel the tumor microenvironment and transformed “cold tumors”, which were insensitive to immunotherapy, into “hot tumors”.25,26 We also observed that lenvatinib promotes the expression of PD-L1 in HCC through TLR4. Lenvatinib plus anti-PD1 antibodies has emerged as a hotspot in HCC research. These results indicate synergy between lenvatinib and immune checkpoint therapy, especially PD-1/PD-L1 antibodies.
Additionally, we observed that lenvatinib upregulated the expression of PD-L1 in HCC, which is different from previous studies that have reported decreased expression of PD-L1 by lenvatinib. Previous studies have shown that lenvatinib can decrease the expression of PD-L1 in HCC by blocking the FGFR4-GSK3β axis.;27 however, FGFR inhibition with lenvatinib activated the IFNγ-pSTAT1-PDL1 axis and increased PD-L1 expression in renal cell carcinoma.28 In addition, lenvatinib inhibits PD-L1 expression in endothelial cells without affecting PD-L1 expression in cancer cells.8 One possible reason for this discrepancy is the variable drug concentration and timing. The drug concentrations and timings in the previous three studies were 10 µM for 24 h, 1 or 3 µM for 48 h, and 3 µM at any time. Such high concentrations are not achievable in clinical practice. Pharmacokinetic analyses of lenvatinib demonstrated that the highest plasma concentration (16 mg/day) in HCC patients was 344 ng/mL (<0.7µM), which was lower than the drug concentrations in the in vitro experiments. The most used dose of lenvatinib in patients with advanced HCC is 8 or 12 mg/day. Considering that the overall lenvatinib plasma concentrations were approximately 0.5 uM in the clinic (12 mg/day),22 we used the same concentration to treat cells for two weeks. A high dose of lenvatinib downregulates PD-L1 expression, whereas a low dose has the opposite effect. Besides, previous studies have found that PD-L1 is primarily expressed in macrophages in HCC29,30 and TLRs are also highly expressed in macrophages. In this study, we mainly focus on the regulation of TLR on PD-L1 expression in tumor cells, but tumor-associated macrophage also plays a critical role in the development of HCC.31,32
ICD-associated DAMPs activate antitumor immune responses by activating the innate immunity represented by TLR3 and TLR4.33,34 Interestingly, the expressions of TLR3 and TLR4 increased in lenvatinib-treated cells and played contrasting roles in tumors. High expression of TLR3 accelerates apoptosis of tumor cells, whereas TLR4 upregulates PD-L1 to enhance immune escape capacity. These results were consistent with previous studies. TLR3 mediates NK cell activation in HCC and directly induces apoptosis of liver cancer cells,35,36 whereas TLR4 promotes HCC initiation and development through the FGF21-IL17A37 or BCL6-PD138 axis. In addition to HCC, TLR4 activation induces PD‑L1 expression in non-small cell lung cancer39 and melanoma.40 Studies have indicated that TLR3 or TLR4 agonists cooperate with immune checkpoint blockade treatments in cancer.41–44 A combination of lenvatinib and immune checkpoints can resolve the deficiencies of monotherapies and improve the efficacy of drug therapy in advanced HCC.
This study had several limitations. First, we did not determine how ICD-associated DAMPs activated TLR3 or TLR4. Future studies can investigate the mechanisms underlying this interaction. Second, we were unable to access HCC samples from patients treated with only lenvatinib. Therefore, we collected tumor samples from patients receiving lenvatinib plus anti-PD1 antibodies. No studies have investigated the effect of anti-PD1 treatment on TLR3 or TLR4 expression. Third, the hypothesis that PD-L1 expression varies with lenvatinib concentration requires further validation using cell models, animal models and clinical trials.
In conclusion, our study provides new insights into the mechanisms of lenvatinib-induced remodeling of the tumor microenvironment in HCC. We found that lenvatinib induces ICD in HCC and upregulates PD-L1 expression via TLR4 while promoting cell apoptosis via TLR3. Remodeling of the immune microenvironment by lenvatinib is mediated in part by lenvatinib-induced ICD. The combination of lenvatinib and antibodies against PD-1 or PD-L1 may act synergistically in HCC treatment. PD-1 or PD-L1 antibodies can enhance the therapeutic effects of lenvatinib in HCC patients by targeting different aspects of the tumor microenvironment. Further studies are needed to validate these findings and explore the potential clinical implications of combining lenvatinib with immune checkpoint inhibitors in HCC treatment.
Abbreviations
ICD, immunogenic cell death; HCC, hepatocellular carcinoma; DAMPs, damage-associated molecular patterns; HMGB1, high mobility group box 1; HR, hazard ratios; CIs, confidence intervals; OS, overall survival; RFS, recurrence-free survival; TLR, toll-like receptor; IHC, immunohistochemistry.
Data Sharing Statement
The data used to support this research are included within this article and raw data are available from the corresponding authors.
Funding
This work was supported by National Natural Science Foundation of China (No. 82072672; No. 82072677), National Natural Science Youth Foundation of China (No. 82103417) and Beijing Igandan Foundation (GDXZ-08-22).
Disclosure
We declare no conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Kuczynski EA, Lee CR, Man S, Chen E, Kerbel RS. Effects of Sorafenib Dose on Acquired Reversible Resistance and Toxicity in Hepatocellular Carcinoma. Cancer Res. 2015;75:2510–2519. doi:10.1158/0008-5472.CAN-14-3687
3. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960–2970. doi:10.1200/JCO.20.00808
4. Qin S, Bi F, Gu S, et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: a Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol. 2021;39:3002–3011. doi:10.1200/JCO.21.00163
5. Qin S, Ren Z, Feng YH, et al. Atezolizumab plus Bevacizumab versus Sorafenib in the Chinese Subpopulation with Unresectable Hepatocellular Carcinoma: Phase 3 Randomized, Open-Label IMbrave150 Study. Liver Cancer. 2021;10:296–308. doi:10.1159/000513486
6. Hiraoka A, Kumada T, Fukunishi S, et al. Post-Progression Treatment Eligibility of Unresectable Hepatocellular Carcinoma Patients Treated with Lenvatinib. Liver Cancer. 2020;9:73–83. doi:10.1159/000503031
7. Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993–4002. doi:10.1111/cas.13806
8. Deng H, Kan A, Lyu N, et al. Dual Vascular Endothelial Growth Factor Receptor and Fibroblast Growth Factor Receptor Inhibition Elicits Antitumor Immunity and Enhances Programmed Cell Death-1 Checkpoint Blockade in Hepatocellular Carcinoma. Liver Cancer. 2020;9:338–357. doi:10.1159/000505695
9. Torrens L, Montironi C, Puigvehí M, et al. Immunomodulatory Effects of Lenvatinib Plus Anti-Programmed Cell Death Protein 1 in Mice and Rationale for Patient Enrichment in Hepatocellular Carcinoma. Hepatology. 2021;74:2652–2669. doi:10.1002/hep.32023
10. Zhang Q, Liu H, Wang H, et al. Lenvatinib promotes antitumor immunity by enhancing the tumor infiltration and activation of NK cells. Am J Cancer Res. 2019;9:1382–1395.
11. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi:10.1038/nri.2016.107
12. Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23:487–500. doi:10.1038/s41590-022-01132-2
13. Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi:10.1038/nm.3708
14. Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280:126–148. doi:10.1111/imr.12574
15. Leuchte K, Staib E, Thelen M, et al. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70:893–907. doi:10.1007/s00262-020-02734-1
16. Friedman D, Baird JR, Young KH, et al. Programmed cell death-1 blockade enhances response to stereotactic radiation in an orthotopic murine model of hepatocellular carcinoma. Hepatol Res. 2017;47:702–714. doi:10.1111/hepr.12789
17. Pinato DJ, Murray SM, Forner A, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9.
18. Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi:10.1084/jem.20050915
19. Zechendorf E, O’Riordan CE, Stiehler L, et al. Ribonuclease 1 attenuates septic cardiomyopathy and cardiac apoptosis in a murine model of polymicrobial sepsis. JCI Insight. 2020;5:e131571. doi:10.1172/jci.insight.131571
20. Cui J, Song Y, Li Y, et al. USP3 inhibits type I interferon signaling by deubiquitinating RIG-I-like receptors. Cell Res. 2014;24:400–416. doi:10.1038/cr.2013.170
21. Wagener BM, Hu PJ, Oh J-Y, et al. Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: a preclinical experimental study. PLoS Med. 2018;15:e1002522. doi:10.1371/journal.pmed.1002522
22. Ikeda M, Okusaka T, Mitsunaga S, et al. Safety and Pharmacokinetics of Lenvatinib in Patients with Advanced Hepatocellular Carcinoma. Clin Cancer Res. 2016;22(6):1385–1394. doi:10.1158/1078-0432.CCR-15-1354
23. Hoshi T, Watanabe Miyano S, Watanabe H, et al. Lenvatinib induces death of human hepatocellular carcinoma cells harboring an activated FGF signaling pathway through inhibition of FGFR-MAPK cascades. Biochem Biophys Res Commun. 2019;513:1–7. doi:10.1016/j.bbrc.2019.02.015
24. Du Four S, Maenhout SK, Niclou SP, Thielemans K, Neyns B, Aerts JL. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am J Cancer Res. 2016;6:2514–2531.
25. Passirani C, Vessières A, La Regina G, Link W, Silvestri R. Modulating undruggable targets to overcome cancer therapy resistance. Drug Resist Updat. 2022;60:100788. doi:10.1016/j.drup.2021.100788
26. Liu X, Xie X, Ren Y, et al. The role of necroptosis in disease and treatment. MedComm. 2021;2:730–755. doi:10.1002/mco2.108
27. Yi C, Chen L, Lin Z, et al. Lenvatinib Targets FGF Receptor 4 to Enhance Antitumor Immune Response of Anti-Programmed Cell Death-1 in HCC. Hepatology. 2021;74:2544–2560. doi:10.1002/hep.31921
28. Adachi Y, Kamiyama H, Ichikawa K, et al. Inhibition of FGFR Reactivates IFNγ Signaling in Tumor Cells to Enhance the Combined Antitumor Activity of Lenvatinib with Anti-PD-1 Antibodies. Cancer Res. 2022;82:292–306. doi:10.1158/0008-5472.CAN-20-2426
29. Lu LG, Zhou ZL, Wang XY, et al. PD-L1 blockade liberates intrinsic antitumourigenic properties of glycolytic macrophages in hepatocellular carcinoma. Gut. 2022;71(12):2551–2560. doi:10.1136/gutjnl-2021-326350
30. Wu Q, Zhou W, Yin S, et al. Blocking Triggering Receptor Expressed on Myeloid Cells-1-Positive Tumor-Associated Macrophages Induced by Hypoxia Reverses Immunosuppression and Anti-Programmed Cell Death Ligand 1 Resistance in Liver Cancer. Hepatology. 2019;70(1):198–214. doi:10.1002/hep.30593
31. Fu Y, Mackowiak B, Feng D, et al. MicroRNA-223 attenuates hepatocarcinogenesis by blocking hypoxia-driven angiogenesis and immunosuppression. Gut. 2023. doi:10.1136/gutjnl-2022-327924
32. Li D, Hu M, Liu Y, et al. CD24-p53 axis suppresses diethylnitrosamine-induced hepatocellular carcinogenesis by sustaining intrahepatic macrophages. Cell Discov. 2018;4:6. doi:10.1038/s41421-017-0007-9
33. Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020;80:1088–1101. doi:10.1158/0008-5472.CAN-19-2080
34. Klein JC, Wild CA, Lang S, Brandau S. Differential immunomodulatory activity of tumor cell death induced by cancer therapeutic toll-like receptor ligands. Cancer Immunol Immunother. 2016;65:689–700. doi:10.1007/s00262-016-1828-3
35. Chew V, Tow C, Huang C, et al. Toll-like receptor 3 expressing tumor parenchyma and infiltrating natural killer cells in hepatocellular carcinoma patients. J Natl Cancer Inst. 2012;104:1796–1807. doi:10.1093/jnci/djs436
36. Bonnin M, Fares N, Testoni B, et al. Toll-like receptor 3 downregulation is an escape mechanism from apoptosis during hepatocarcinogenesis. J Hepatol. 2019;71:763–772. doi:10.1016/j.jhep.2019.05.031
37. Zheng Q, Martin RC, Shi X, et al. Lack of FGF21 promotes NASH-HCC transition via hepatocyte-TLR4-IL-17A signaling. Theranostics. 2020;10:9923–9936. doi:10.7150/thno.45988
38. Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi:10.1016/j.ccr.2012.02.007
39. Wen ZF, Liu H, Gao R, et al. Tumor cell-released autophagosomes (TRAPs) promote immunosuppression through induction of M2-like macrophages with increased expression of PD-L1. J Immunother Cancer. 2018;6:151. doi:10.1186/s40425-018-0452-5
40. Fleming V, Hu X, Weller C, et al. Melanoma Extracellular Vesicles Generate Immunosuppressive Myeloid Cells by Upregulating PD-L1 via TLR4 Signaling. Cancer Res. 2019;79:4715–4728. doi:10.1158/0008-5472.CAN-19-0053
41. Jang GY, Kim YS, Lee SE, et al. Improvement of DC-based vaccines using adjuvant TLR4-binding 60S acidic ribosomal protein P2 and immune checkpoint inhibitors. Cancer Immunol Immunother. 2021;70:1075–1088. doi:10.1007/s00262-020-02759-6
42. Farias A, Soto A, Puttur F, et al. A TLR4 agonist improves immune checkpoint blockade treatment by increasing the ratio of effector to regulatory cells within the tumor microenvironment. Sci Rep. 2021;11:15406. doi:10.1038/s41598-021-94837-7
43. Seya T, Takeda Y, Matsumoto M. A Toll-like receptor 3 (TLR3) agonist ARNAX for therapeutic immunotherapy. Adv Drug Deliv Rev. 2019;147:37–43. doi:10.1016/j.addr.2019.07.008
44. Takeda Y, Kataoka K, Yamagishi J, Ogawa S, Seya T, Matsumoto M. A TLR3-Specific Adjuvant Relieves Innate Resistance to PD-L1 Blockade without Cytokine Toxicity in Tumor Vaccine Immunotherapy. Cell Rep. 2017;19:1874–1887. doi:10.1016/j.celrep.2017.05.015
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.