Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Application and Resistance Mechanisms of Lenvatinib in Patients with Advanced Hepatocellular Carcinoma
Authors Tao M, Han J, Shi J, Liao H, Wen K , Wang W , Mui S , Li H, Yan Y, Xiao Z
Received 9 March 2023
Accepted for publication 1 June 2023
Published 10 July 2023 Volume 2023:10 Pages 1069—1083
DOI https://doi.org/10.2147/JHC.S411806
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmed Kaseb
Meng Tao,1,2 Jing Han,3 Juanyi Shi,1,2 Hao Liao,1,2 Kai Wen,1,2 Weidong Wang,1,2 Sintim Mui,1,2 Huoming Li,1,2 Yongcong Yan,1,2 Zhiyu Xiao1,2
1Department of Hepatobiliary Surgery, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, 510120, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, 510120, People’s Republic of China; 3Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, 450008, People’s Republic of China
Correspondence: Yongcong Yan; Zhiyu Xiao, Department of Hepatobiliary Surgery, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Yanjiang West Road #107, Guangzhou, 510120, People’s Republic of China, Tel +86-15800039176 ; +86-13682283695, Fax +86 20 34071073, Email [email protected]; [email protected]
Abstract: Lenvatinib, a multitargeted tyrosine kinase inhibitor (TKI), is one of the preferred targeted drugs for the treatment of advanced hepatocellular carcinoma (aHCC). Since the REFLECT study showed that lenvatinib was noninferior to sorafenib in overall survival (OS), lenvatinib monotherapy has been widely used for aHCC. Moreover, lenvatinib combination therapy, especially lenvatinib combined with immune checkpoint inhibitors (ICIs), has shown more encouraging clinical results. However, drug development and comprehensive treatment have not significantly improved the prognosis, and lenvatinib resistance is often encountered in treatment. The underlying molecular mechanism of lenvatinib resistance is still unclear, and studies to solve drug resistance are ongoing. The molecular mechanisms of lenvatinib resistance in patients with aHCC include the regulation of signaling pathways, the regulation of noncoding RNAs, the impact of the immune microenvironment, tumor stem cell activation and other mechanisms. This review aims to (1) summarize the progress of lenvatinib in treating aHCC, (2) delineate the known lenvatinib resistance mechanisms of current therapy, and (3) describe the development of therapeutic methods intended to overcome these resistance mechanisms.
Keywords: lenvatinib, application, drug resistance, hepatocellular carcinoma
Introduction
Approximately 40% of HCCs can be diagnosed by imaging examination at an early stage and well treated by hepatectomy, radiofrequency ablation, liver transplantation or transcatheter arterial chemoembolization for better survival. Nonetheless, at least 50% of patients who receive a diagnosis at an advanced or intermediate stage miss the chance for a cure.1 Although patients with advanced-stage [Barcelona Clinic Liver Cancer (BCLC)-C] or intermediate-stage (BCLC-B) disease can receive locoregional therapies, systemic therapies are the optimal choice for those who do not benefit from the previous treatment.2–4 Since 2007, sorafenib, a multitarget tyrosine kinase inhibitor with antiangiogenic and antiproliferation effects, has been applied to treat advanced hepatocellular carcinoma (aHCC).5 In addition, compared with sorafenib, lenvatinib showed statistically significant, clinically significant improvements for all secondary efficacy endpoints across subgroups, including progression-free survival (PFS), time to progression (TTP), and objective response rate (ORR), as well as in quality-of-life assessments, in a randomized Phase 3 noninferiority trial.6 Lenvatinib has become a first-line alternative to comprehensive treatment for unresectable liver cancer patients. Lenvatinib monotherapy and combination therapy have achieved positive clinical therapeutic effects, which have been verified by many clinical trials. Of note, combination with immune checkpoint inhibitors (ICIs) is currently a vital part of HCC treatment; for example, atezolizumab plus bevacizumab has become the recommended first-line therapy for selected patients.7–9 Lenvatinib has shown efficacy in inducing tumor responses in approximately 25% of patients with aHCC.6,10 Acquired resistance to treatment is unavoidable and a major problem that has not been resolved. Therefore, more research is needed to elucidate the underlying causes and mechanisms of lenvatinib resistance. Several potential resistance mechanisms have been revealed, such as changes in the tumor immune microenvironment, and novel therapeutic strategies have achieved some success in solving lenvatinib resistance.11–13 In this review, we aim to summarize various types of research concentrated on the application of lenvatinib, including network meta-analyses, systematic reviews, prospective studies, retrospective studies, and so on. In addition, resistance mechanisms to lenvatinib in HCC patients and relevant solutions are also systematically summarized to provide references for future lenvatinib-centered therapy.
Structure and Pharmacological Mechanism of Lenvatinib
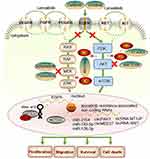
Several pathways, such as growth factor receptor-related pathways, [including vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor (PDGFR), epidermal growth factor receptor (EGFR), insulin-like growth factor receptor (IGFR) and transforming growth factor alpha (TGFα)], important cell differentiation pathways (including Wnt/-β-catenin, Hippo-YAP, JAK/STAT, Notch, and Hedgehog) and/or their cytosolic intermediate pathways (including PI3K-AKT-mTOR and RAF/ERK/MAPK), are frequently altered in HCC patients.14 Lenvatinib, a multitargeted receptor kinase inhibitor, chemical structure consists of a pyridine and quinoline ring system with several substituents, blocks the activities of FGFR 1, 2, 3 and 4, PDGFR α, VEGFR 1, 2 and 3, RET, and KIT. Lenvatinib binds to receptors, ultimately inhibiting the PLCγ, Ras-Raf-ERK and PI3K-AKT pathways in the process. It also inhibits tumor angiogenesis, which results in nutrient deprivation and hypoxia, slowing tumor growth or causing tumor cells to die (Figure 1).15–18 Lenvatinib can prevent the phosphorylation of RET and limit the proto oncogene c-KIT to reduce cell proliferation.19–21 Lenvatinib can also control the tumor immune microenvironment by increasing the fraction of activated CD8 T cells while decreasing the proportion of myeloid-derived suppressor cells (MDSCs) and other negative-regulatory immune cells.8,22,23 Lenvatinib is taken orally and is metabolized by the liver. Its peak plasma concentration is reached within 1–4 hours after administration, and it has a half-life of approximately 28 hours.
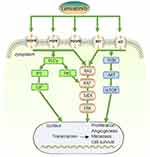 |
Figure 1 Schematic representation of the known pharmacological mechanisms of lenvatinib. |
Application of Lenvatinib in HCC
Evidence for Lenvatinib Monotherapy for HCC Patient
Today, lenvatinib monotherapy is frequently employed to treat aHCC. Real-world data on the efficacy and safety of lenvatinib are becoming increasingly available, and several studies have been published providing comprehensive results on the use of lenvatinib for the treatment of aHCC. Researchers examined the effectiveness and safety of lenvatinib and sorafenib in a Phase III, international, randomized, noninferior trial in patients with unresectable hepatocellular carcinoma (uHCC). Lenvatinib had a median survival time of 13.6 months, which was noninferior to that of sorafenib (12.3 months). Lenvatinib considerably outperformed sorafenib in terms of the ORR, PFS, and TTP. Its tolerance was acceptable, and the most common adverse events (AEs) caused by treatment included hypertension, diarrhea, loss of appetite and weight loss.6 BCLC stage, portal neoplastic thrombosis, and Child‒Pugh classification were identified as important predictive variables for PFS in a multivariate analysis according to a Chinese real-world study of lenvatinib monotherapy in 2020.24 The median overall survival (mOS) for lenvatinib-treated patients in the multicenter cohort study ELEVATOR in Germany and Austria was 12.8 months. The outcomes align with those noted in REFLECT study.25 Another multicenter study in Japan registered 343 uHCC patients receiving lenvatinib treatment. The median observation period was 10.5 months. The median progression-free survival (mPFS) was 8.8 months, and the mOS was 21.0 months for Child‒Pugh A (n=276) patients, while the mOS was 9.0 months for Child‒Pugh B (n = 67) patients. The ORR and disease control rate (DCR), as measured by the modified Response Evaluation Criteria in Solid Tumors (mRECIST), were 42.1% and 82.1%, respectively.26
These results support the clinical effectiveness of lenvatinib as a first-line therapy for uHCC. Although some comparative studies have shown better efficacy than lenvatinib, such as ICIs versus lenvatinib and Hepatic artery infusion chemotherapy (HAIC) versus lenvatinib, considering economic factors and efficacy, lenvatinib monotherapy is still a better choice for HCC.27–29
Evidence of ICIs Combined with Lenvatinib in the Treatment of HCC Patients
ICIs have ushered in a new age of aHCC treatment in recent years. Combining ICIs with TKIs can cause immune cells to infiltrate “cold” tumors, turning them into “hot” tumors and increasing response rates.30 Lenvatinib possesses immunomodulatory properties that allow it to exert antitumor effects and boost the synergistic effects of anti-PD-1 antibodies. As a first-line therapy for aHCC, TKIs and anti-PD-1 monoclonal antibodies are currently being investigated.31
We summarize the representative research on lenvatinib and ICIs in Table 1. Lenvatinib plus pembrolizumab (an anti-PD-1 antibody) was tested in a preliminary phase Ib study for its effectiveness and safety in treating uHCC patients in 2020. A total of 104 patients were enrolled in this trial. In the combined treatment group, PFS was 8.6–9.3 months, OS was 22 months, the ORR was 36–46%, and adverse reactions could be alleviated after symptomatic treatment.32 For the treatment of solid tumors, a systematic review compared the efficacy and safety of palbociclib and lenvatinib in combination with their respective monotherapies. Pembrolizumab plus lenvatinib prolonged the mPFS (9.3 months) and mOS (22.0 months) in HCC.33 Next, we discuss some retrospective controlled studies. In a retrospective analysis, the effectiveness and side effects of combining lenvatinib with the anti-PD-1 antibody nivolumab were assessed. The combined treatment group outperformed the lenvatinib group in terms of PFS (7.5 vs 4.8 months, p = 0.05) and OS (22.9 vs 10.3 months, p = 0.01).34 Another multicenter retrospective cohort study compared the effects of lenvatinib monotherapy with lenvatinib in combination with carrelizumab (an anti-PD-1 antibody) on uHCC patients. Similarly, this combination therapy prolonged OS and PFS, and the safety of the two groups was comparable.35 Another retrospective analysis comparing the lenvatinib group to the group that also received camrelizumab came to a similar outcome.36 The 6-month OS and PFS rates of lenvatinib combined with nivolumab in the treatment of aHCC patients were 62.5% and 43.8%, respectively, and the 12-month OS and PFS were 52.1% and 30.0%, respectively, according to a subgroup analysis of a retrospective study. The 6-month and 12-month OS rates of patients with aHCC treated with lenvatinib combined with pembrolizumab were 51.3% and 51.3%, respectively. The 6-month and 12-month PFS rates were 49.2% and 49.2%, respectively.37
 |
Table 1 Relevant Studies on the Combination of Immune Checkpoint Inhibitors and Lenvatinib for HCC |
Successful progress and many breakthrough achievements have been made in the field of immunotherapy for aHCC. Atezolizumab combined with bevacizumab, as shown by IMbrave150, led to improved OS and PFS compared with sorafenib.7,38 Atezolizumab combined with bevacizumab showed superiority to lenvatinib in terms of the prognosis and conversion rate as first-line therapy.39–41 A variety of drug combination studies are in full progress, including single-arm studies, controlled studies, prospective studies, and retrospective studies on the combination of lenvatinib with pembrolizumab, camrelizumab, nivolumab or other drugs. Combining lenvatinib and ICIs has shown efficacy in multiple cancers, but the mechanism of disease-specific and drug-specific benefits is unclear. The enhanced therapeutic effect of ICIs can be obtained by changing the tumor immune microenvironment with lenvatinib, and ICIs may overcome the lenvatinib resistance pathway. Therefore, the combination of lenvatinib and ICIs has better prospects in the future. In the meantime, additional research with a larger sample size and a longer follow-up is needed to validate these findings.
Evidence for Multiple Treatment Methods for HCC Patients
Transarterial chemoembolization (TACE) with sorafenib was found to be superior to TACE in patients with uHCC, according to the TACTICS trial.42 The combination of lenvatinib and TACE is now ongoing to evaluate the synergistic effect.43 HAIC is a well-known and widespread treatment for aHCC patients, especially in East Asia. Compared with TACE, HAIC has systemic effects in addition to local effects.44 HAIC can shrink huge tumors and create opportunities for surgery. Lenvatinib plus TACE studies have achieved similar conclusions to the TACTICS trial. Hypoxia inducible factor 1 (HIF-1), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) expression is upregulated after TACE and tumor tissue necrosis occurs to promote tumor recurrence or growth. The inhibition of angiogenic factors by lenvatinib can improve the therapeutic effect of TACE.45–47 However, there are few studies on lenvatinib combined with chemotherapy for aHCC, and more evidence is needed to prove its feasibility.
These high evidence level clinical studies, such as TACTICS, IMbrave150, SILIUS, and LAUNCH, confirm that combination therapy, such as lenvatinib plus pembrolizumab combination therapy, can improve the prognosis of aHCC.32,38,42,48,49 Recent preclinical and clinical studies have suggested that lenvatinib and ICIs, when used in combination with other therapies, such as HAIC or TACE, might elicit synergistic antitumor effects. In recent years, multiple-treatment methods for aHCC have been widely reported, especially in China. Due to the high similarity between the experimental content and results, we listed some studies in Table 2 to provide references. We believe that lenvatinib combined with a PD-1 inhibitor plus TACE or HAIC may have a synergistic antitumor effect based on the research outcomes of dual-drug combinations or single-drug comparisons with triple-drug combinations. However, another study showed the opposite result. This study demonstrated that triple therapy (lenvatinib plus PD-1 antibody plus TACE or HAIC) is comparable to lenvatinib plus PD-1 antibody or lenvatinib plus TACE or HAIC.50 Thus, high-quality randomized controlled trials are needed to confirm the effectiveness of multiple treatments.
 |
Table 2 Relevant Studies on Multiple Treatments for HCC |
Other Ongoing Combined Therapies
For aHCC, the dual or triple scheme of lenvatinib in combination with ICIs, TACE or HAIC has been proven to have achieved ideal results. A number of clinical trials have been developed to assess the effectiveness and safety of combining various treatments with lenvatinib (clinicaltrials.gov). Here, we summarize some clinical studies about postoperative treatments, borderline resectable HCC, treatments before transplant and lenvatinib resistance in Table 3. The publication of the final results of these studies is still awaited. The mechanism of this research was clarified in a prospective clinical study of lenvatinib combined with gefitinib for the treatment of lenvatinib-resistant HCC.11 Neoadjuvant PD-1 antibody in combination with TKIs has demonstrated promising efficacy and tolerable mortality rates in transplant recipients, according to several studies.63
 |
Table 3 Other Ongoing Combined Therapies for HCC |
Resistance Mechanisms to Lenvatinib and Therapeutic Strategies to Overcome Lenvatinib Resistance
Lenvatinib resistance remains a major cause of aHCC targeted therapy failure. Cancer heterogeneity, including the relationship between subpopulations within and between tumors, may have significant implications for cancer drug therapy, particularly in aHCC, which is known for its significant heterogeneity.64 There are two reasons for drug resistance: natural resistance and adaptive regulation in the course of medication. Numerous studies have investigated the mechanisms and preventative measures of lenvatinib resistance in HCC considering the crucial role it plays in comprehensive treatment.65 Drug combination therapy may be a potential solution for some drug resistance mechanisms, but further exploration is required.
Regulation in Cell Signaling Pathways
In recent years, the exploration of pathways downstream of VEGFR, FGFR and PDGFR has been ongoing. In Table 4, some drug regimens to overcome lenvatinib resistance are shown. Lenvatinib medication that inhibits FGFR causes feedback stimulation of the EGFR-PAK2-ERK5 signaling axis, which can be stopped by EGFR inhibition. This promising finding was validated in vivo and in vitro. Then, a clinical trial showed that patients with aHCC who did not respond to lenvatinib achieved a meaningful clinical response after combination with gefitinib therapy (NCT04642547), gefitinib is an EGFR inhibitor.11 Based on previous research according to Qin et al, HCC cells become resistant to lenvatinib by boosting the EGFR-STAT3-ABCB1 signaling axis and activating EGFR. ABCB1 has been demonstrated to be inhibited by EGFR inhibitors. HCC is evidently improved by the combination of erlotinib and lenvatinib both in vitro and in vivo.66 WD repeat domain 4 (WDR4) and methyltransferase-like 1 (METTL1), two essential elements of the tRNA m7G methyltransferase complex, were shown to be markedly increased in lenvatinib-resistant cells in a different investigation. Drug resistance is initiated by METTL1 promotion of EGFR pathway gene translation.67 In a separate study related to EGFR, it was found that m6A mRNA modification was significantly higher in lenvatinib-resistant cells from HCC compared to the parental cells. Among the m6A regulators, Methyltransferase-like 3 (METTL3) showed the most significant up-regulation. Overexpression of the EGFR prevented the growth arrest of drug-resistant cells induced by METTL3 knockdown during lenvatinib treatment. This study concludes that targeting METTL3 with specific inhibitors can enhance sensitivity to lenvatinib both in vitro and in vivo.68
 |
Table 4 Drug Resistance Pathways and Combination Therapy |
Wong et al identified phosphoglycerate dehydrogenase (PHGDH) as a crucial driver of sorafenib, regorafenib, and lenvatinib resistance utilizing genome-wide CRISPR/Cas9 library screening.74 Similarly, other researchers discovered that neurofibromin 1 (NF1), dual-specificity phosphatase 9 (DUSP9), and dual-specificity phosphatase 4 (DUSP4) are important drivers of lenvatinib resistance in HCC using genome-wide CRISPR/Cas9 library screening. Lenvatinib resistance was caused by DUSP4, DUSP9, and NF1 deficiencies. The mechanisms by which DUSP9 deficiency activates the MAPK/ERK signaling pathways, NF1 deficiency stimulates the PI3K/AKT and MAPK/ERK signaling pathways, and DUSP4 loss is accompanied by control of p-ERK and p-MEK levels were also clarified. Lenvatinib resistance can be overcome using a MEK inhibitor.69,72
Vascular endothelial VEGFR2 expression and its downstream RAS/MEK/ERK signaling were evidently upregulated in lenvatinib-resistant cell lines.70 Another study discovered that when FGFR1 is overexpressed, lenvatinib resistance in hepatocarcinomas is caused by the downstream activation of mTOR, AKT, and ERK signals.75 Breast cancer resistance protein (BCRP) and multidrug resistance protein 1 (MDR1) transporters were considerably upregulated when lenvatinib resistance was induced in HCC cells, which was followed by activation of the PI3K/Akt, MEK/ERK, and EGFR pathways.71 According to a study, lenvatinib resistance was controlled by integrin subunit beta 8 (ITGB8) via the stabilization of AKT by Heat Shock Protein 90 (HSP90) and an increase in AKT signaling.73 Li et al found that YRDC promotes lenvatinib resistance by regulating the Ras/Raf/MEK/ERK pathway, while YRDC belongs to the universal family with the sua5-yciO-yrdC domain.76
According to one study, cellular mesenchymal–epithelial transition factor (c-MET) and mucoprotein 15 (MUC15) can interact, inactivating the PI3K/AKT/SOX2 signaling pathway. The response of carcinoma cells to lenvatinib is governed by the MUC15/c-MET/PI3K/AKT/SOX2 axis, and MUC15 overexpression can overcome lenvatinib resistance.77 Takehara et al found in 2020 that long-term exposure to lenvatinib decreases the expression of fibroblast growth factor 19 (FGF19) in HCC cells but that re-expression of FGF19 can increase sensitivity to lenvatinib. ST6 Beta-Galactoside Alpha-2,6-Sialyltransferase 1 (ST6GAL1) is a tumor-derived secretory protein that is positively controlled by FGF19, according to proteomic and secretome studies.78 By promoting autolysosome formation, lysosomal-associated protein transmembrane 5 (LAPTM5) may increase intrinsic macroautophagic/autophagic flux to cause lenvatinib resistance.79 Maina et al showed that secreted glycoprotein ADAMTS-like 5 (ADAMTSL5) expression is increased in liver tumors and is closely related to lenvatinib resistance. Inhibition of ADAMTSL5 can offset lenvatinib resistance.80 During treatment, some proteins can be used as potential markers of liver cancer to observe the therapeutic effect, and there is also the possibility of these markers being used as drug targets. Some specific protein changes were found in lenvatinib-resistant cell lines. Examples include MUC15, ADAMTSL5, LAPTM5, and ST6GAL1. The discovery of special proteins is very important. They can be used as therapeutic sensitive markers and can be detected in patients’ serum.
Noncoding RNA Regulation
Through epigenetic connections, noncoding RNAs such as microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs) promote the formation and progression of HCC.81 In the emergence of lenvatinib resistance in HCC, noncoding RNAs also have a significant impact. A study found that HCC and liver cancer stem cells (CSCs) have higher levels of miR-3154. Hepatoma cell responses to lenvatinib therapy are regulated by miR-3154. It was further suggested by analysis of the patient cohort and patient-derived xenografts (PDXs) that miR-3154 may be predictive of the therapeutic efficacy of lenvatinib in HCC patients.82 It has been established that lenvatinib resistance results from c-Met overexpression. The goal of later research was to find putative upstream miRNAs that control c-Met. Researchers discovered that miR-183-5p.1 specifically targets the 3’-UTR of the unique protein MUC15 in liver tumor-initiating cells (T-ICs) and that the MUC15/c-MET/PI3K/AKT/SOX2 axis controls the responses of hepatoma cells to lenvatinib treatment.77 In lenvatinib-resistant cells, Sun et al demonstrated that miR-128-3p strongly inhibits the regulation of c-Met. Lenvatinib resistance is caused by miR-128-3p and c-Met, which control the progression of the ERK cell cycle and the AKT apoptotic pathway.83 According to a second study, HGF decreased lenvatinib’s antiproliferative, proapoptotic, and anti-invasive actions in HCC cells with high c-MET expression. To promote epithelial–mesenchymal transition (EMT) in hepatoma cells, HGF/c-MET activates the downstream PI3K/AKT and MAPK/ERK pathways, which eventually results in lenvatinib resistance.12 2022, Wang et al discovered that circPAK1 can be transported to sensitive cells by exosomes from lenvatinib-resistant cells to induce receptor cells to develop lenvatinib resistance.84 Other studies found that upregulated circMED27, lncRNA MT1JP, and lncRNA XIST promoted HCC resistance to lenvatinib.85–87
Tumor Immune Microenvironment
Cancer cells interact with the host immune system in the tumor immune microenvironment to either promote or inhibit the pathological development of cancer. The immune system has the ability to identify cancer cells and activate an immunological response to destroy them. Due to the highly dynamic, strong adaptive and highly heterogeneous tumor microenvironment (TME) in HCC, various types of TKI and ICI resistance have emerged.88 Some studies have found that the emergence of lenvatinib resistance in HCC is closely related to TME changes. A subset of patients with tumors characterized by Treg cell infiltration, minimal inflammatory signaling, and an activated VEGFR pathway have been identified as prospective responders beyond monotherapy based on the immune-related genetic profile of human HCC. Torrens L et al reported that lenvatinib combined with anti-PD1 exerts immunomodulatory effects by activating immune pathways, reducing Treg infiltration, and inhibiting TGFβ signaling.8 After lenvatinib treatment with or without anti-PD-1 antibody, single-cell RNA sequencing analysis of an animal experiment revealed decreased proportions of monocyte and macrophage populations and increased proportions of CD8 T-cell populations.89 Another study discovered that lenvatinib suppresses FGFR to improve antitumor immunity and anti-PD-1 antibody activity and that activation of the FGFR pathway reduces IFN signaling.90 According to a neutrophil study, neutrophils polarize to the N2 phenotype after entering the TME. The expression of PD-L1 was also increased. As a result, lenvatinib’s ability to combat tumor cells is compromised, and using lenvatinib in conjunction with celecoxib lowers the survival of lactic acid-stimulated PD-L1 neutrophils while increasing lenvatinib’s antitumor effects.91
Expansion of Cancer Stem Cells
A handful of particular cell types are responsible for driving tumor growth, as shown by the CSC paradigm. Through the expansion of CSCs, tumor reproliferation mediates the formation of drug resistance. Cholesterol production via sterol-regulatory element-binding protein 2 (SREBP2) is essential for promoting liver cancer stem cells. HCC cells treated with exogenous cholesterol can enhance tumor stemness and drug resistance. Simvastatin can not only inhibit the growth of liver cancer but also make liver cancer cells sensitive to sorafenib. It may have the same effect on lenvatinib resistance, which needs further verification.92 A study identified cancer cell subsets with stem cell-like properties in a mouse HCC model. Stem cell-like properties are essential for tumor maintenance and growth. Additional research has revealed that a viable therapeutic target for liver cancer is the Jak/Stat pathway.93 Another study explored the mechanism by which CD73 regulates cancer stem cells. After CD73 knockdown, tumor sphere formation decreased, lenvatinib resistance and stemness-related gene expression increased, while CD73 overexpression achieved the opposite effect.94 The expression of numerous stem factors is correlated with high levels of POSTN (especially CD133). Cilengitide in combination with lenvatinib is more effective than lenvatinib monotherapy in inhibiting the growth of some HCC cells that express high levels of POSTN.95 In a recent study, researchers investigated the expression of Frizzled-10 (FZD10) in hepatocellular CSCs using RNA sequencing. They found that FZD10 plays a crucial role in promoting the self-renewal, tumorigenicity, and metastasis of hepatic CSCs by activating the β-catenin and YAP1 signaling pathways. Furthermore, the FZD10-β-catenin/c-Jun axis was shown to transcriptionally activate the expression of METTL3, which in turn determines the response of hepatocellular carcinoma cells to treatment with lenvatinib. Thus, targeting the FZD10/β-catenin/c-Jun/MEK/ERK axis may be a promising strategy for developing novel therapies for hepatocellular carcinoma.96
Discussion
The treatment pattern of aHCC has undergone tremendous changes in the past decade. Many clinical trials have explored the application of lenvatinib, and combination therapy has recently produced an unprecedented paradigm shift and shown promising results.
In the early stage, lenvatinib monotherapy was confirmed to be not inferior to sorafenib and is currently widely used as a first-line treatment drug in different populations around the world.6 Hypertensive proteinuria, alimentary tract syndrome, and pain syndrome are the main AEs.17 For aHCC patients who are intolerant to sorafenib or who experience regorafenib failure, the application of lenvatinib may lead to improvement.97 After sorafenib-experienced failure, lenvatinib combined with ICI showed significantly better survival than monotherapy with lenvatinib.98 Furthermore, after the failure of TKI and ICI combination therapy, the retention of lenvatinib and ICI monotherapy conversion can bring survival benefits.99 Similarly, the combination of lenvatinib with pembrolizumab, camrelizumab, nivolumab and other ICIs has shown efficacy in aHCC. Combinations of immunotherapies and molecularly targeted therapies are becoming increasingly common as methods to improve immune system responses to neoantigens produced from HCC. If determinants of responsiveness are found, these measures may increase the bar for systemic HCC therapy by increasing median overall survival beyond 2 years. Triple therapy showed a high result. We think that lenvatinib paired with PD-1 antibody in combination with TACE or HAIC may have a synergistic antitumor impact based on the outcomes of dual-drug combinations or single-agent versus triple-drug combinations.
Although the emergence of lenvatinib has further improved the prognosis of patients with aHCC, and it is currently widely used, the prominent problem is drug resistance. There are currently a large number of studies addressing drug resistance, and some have conducted clinical trials, which is exciting news for HCC patients. For lenvatinib resistance, Figure 2 shows some combination therapies. In hepatocellular carcinoma, EGFR inhibition with lenvatinib shows synthetic lethality. In vitro EGFR-expressing HCC cell lines demonstrated effective antiproliferative effects when gefitinib and lenvatinib were combined. The clinical trial of lenvatinib combined with gefitinib is ongoing (NCT04642547), and preliminary progress has been made.11 Further research showed that the EGFR-STAT3-ABCB1 pathway was activated in HCC cells to gain resistance to lenvatinib and that erlotinib may also be utilized in tandem with lenvatinib as an EGFR inhibitor.66 Two other studies on the EGFR resistance pathway confirmed that METTL1 and METTL3 promote the translation of genes in the EGFR pathway to trigger resistance.67,68 Activation of the PI3K/AKT, MAPK/ERK, and other pathways has also been the subject of numerous investigations with encouraging outcomes, and these investigations have all been validated with MEK inhibitors and AKT inhibitors.69,73,80 Some studies have found specific small-molecule changes (mainly in proteins) in lenvatinib-resistant cell lines. These proteins or other molecules provide promising predictive markers and intervention targets for drug resistance. Mucoprotein (MUC15), secreted glycoprotein ADAMTS-like 5 (ADAMTSL5), lysosomal-associated protein transmembrane 5 (LAPTM5), ST6 beta-galactoside alpha-2,6-sialyltransferase 1 (ST6GAL1), METTL1 and METTL3 are a few examples. Lenvatinib resistance is significantly influenced by noncoding RNAs. Other factors that contribute to lenvatinib resistance are the activation of cancer stem cells, the effect on epithelial–mesenchymal transition (EMT), and the overexpression of c-Met.
Conclusion and Perspectives
In conclusion, in addition to the clinical studies mentioned above, there are numerous novel therapeutic combinations under way in the early stages of clinical development. These combination treatments can be broadly classified as multitarget tyrosine kinase inhibitors, EGFR-TKIs, chemotherapy (TACE, HAIC), and ICIs, with lenvatinib being the core medication. Additionally, to overcome lenvatinib resistance, many new therapeutic combinations are being explored in early clinical studies, such as EGFR and MEK inhibitors combined with lenvatinib, which still need to be further tested in humans to prove their feasibility. Further exploration of the efficacy and resistance mechanisms of lenvatinib combination therapy is necessary, and future research may involve investigating the role of non-coding RNAs and elucidating the mechanisms by which combination therapy impacts the immune microenvironment. Researchers have produced positive results in combating drug resistance and enhancing prognosis of patients, bringing more hope to patients.
Consent for Publication
All authors approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82103090); Project funded by China Postdoctoral Science Foundation (2021M703742); Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515012391); Science and Technology Program of Guangzhou (No.202201020311).
Disclosure
The authors declare that there are no competing interests in this work.
References
1. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
2. Decraecker M, Toulouse C, Blanc JF. Is there still a place for tyrosine kinase inhibitors for the treatment of hepatocellular carcinoma at the time of immunotherapies? A focus on lenvatinib. Cancers. 2021;13(24):6310. doi:10.3390/cancers13246310
3. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi:10.21037/hbsn-20-480
4. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
5. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
7. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
8. Torrens L, Montironi C, Puigvehí M, et al. Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatology. 2021;74(5):2652–2669. doi:10.1002/hep.32023
9. Liu XF, Zhu XD, Feng LH, et al. Physical activity improves outcomes of combined lenvatinib plus anti-PD-1 therapy in unresectable hepatocellular carcinoma: a retrospective study and mouse model. Exp Hematol Oncol. 2022;11(1):20. doi:10.1186/s40164-022-00275-0
10. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
11. Jin H, Shi Y, Lv Y, et al. EGFR activation limits the response of liver cancer to lenvatinib. Natur. 2021;595(7869):730–734. doi:10.1038/s41586-021-03741-7
12. Fu R, Jiang S, Li J, Chen H, Zhang X. Activation of the HGF/c-MET axis promotes lenvatinib resistance in hepatocellular carcinoma cells with high c-MET expression. Med Oncol. 2020;37(4):24. doi:10.1007/s12032-020-01350-4
13. Wei CY, Zhu MX, Zhang PF, et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J Hepatol. 2022;77(1):163–176. doi:10.1016/j.jhep.2022.02.019
14. Garcia-Lezana T, Lopez-Canovas JL, Villanueva A. Signaling pathways in hepatocellular carcinoma. Adv Cancer Res. 2021;149:63–101.
15. Chen CY, Wu SM, Lin YH, et al. Induction of nuclear protein-1 by thyroid hormone enhances platelet-derived growth factor A mediated angiogenesis in liver cancer. Theranostics. 2019;9(8):2361–2379. doi:10.7150/thno.29628
16. Wang Y, Liu D, Zhang T, Xia L. FGF/FGFR signaling in hepatocellular carcinoma: from carcinogenesis to recent therapeutic intervention. Cancers. 2021;13(6):1.
17. Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a Review in Hepatocellular Carcinoma. Drugs. 2019;79(6):665–674. doi:10.1007/s40265-019-01116-x
18. Hatanaka T, Naganuma A, Kakizaki S. Lenvatinib for Hepatocellular Carcinoma: a Literature Review. Pharmaceuticals. 2021;14(1). doi:10.3390/ph14010036
19. Zhao Y, Zhang YN, Wang KT, Chen L. Lenvatinib for hepatocellular carcinoma: from preclinical mechanisms to anti-cancer therapy. Biochim Biophys Acta Rev Cancer. 2020;1874(1):188391. doi:10.1016/j.bbcan.2020.188391
20. Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16(4–5):441–467. doi:10.1016/j.cytogfr.2005.05.010
21. Lorusso L, Pieruzzi L, Biagini A, et al. Lenvatinib and other tyrosine kinase inhibitors for the treatment of radioiodine refractory, advanced, and progressive thyroid cancer. Onco Targets Ther. 2016;9:6467–6477. doi:10.2147/OTT.S84625
22. Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. doi:10.1084/jem.20140559
23. Donne R, Lujambio A. The liver cancer immune microenvironment: therapeutic implications for hepatocellular carcinoma. Hepatology. 2023;77(5):1773–1796. doi:10.1002/hep.32740
24. Wang DX, Yang X, Lin JZ, et al. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: a retrospective, real-world study conducted in China. World J Gastroenterol. 2020;26(30):4465–4478. doi:10.3748/wjg.v26.i30.4465
25. Welland S, Leyh C, Finkelmeier F, et al. Real-world data for lenvatinib in hepatocellular carcinoma (ELEVATOR): a retrospective multicenter study. Liver Cancer. 2022;11(3):219–232. doi:10.1159/000521746
26. Tsuchiya K, Kurosaki M, Sakamoto A, et al. The real-world data in Japanese patients with unresectable hepatocellular carcinoma treated with lenvatinib from a nationwide multicenter study. Cancers. 2021;13(11):2608. doi:10.3390/cancers13112608
27. Lee J, Han JW, Sung PS, et al. Comparative analysis of lenvatinib and hepatic arterial infusion chemotherapy in unresectable hepatocellular carcinoma: a multi-center, propensity score study. J Clin Med. 2021;10(18):1.
28. Zhao M, Pan X, Yin Y, et al. Cost-effectiveness analysis of five systemic treatments for unresectable hepatocellular carcinoma in China: an economic evaluation based on network meta-analysis. Front Public Health. 2022;10:869960. doi:10.3389/fpubh.2022.869960
29. Zhou T, Wang X, Cao Y, et al. Cost-effectiveness analysis of sintilimab plus bevacizumab biosimilar compared with lenvatinib as the first-line treatment of unresectable or metastatic hepatocellular carcinoma. BMC Health Serv Res. 2022;22(1):1367. doi:10.1186/s12913-022-08661-4
30. Gerard CL, Delyon J, Wicky A, Homicsko K, Cuendet MA, Michielin O. Turning tumors from cold to inflamed to improve immunotherapy response. Cancer Treat Rev. 2021;101:102227. doi:10.1016/j.ctrv.2021.102227
31. Rizzo A, Dadduzio V, Ricci AD, et al. Lenvatinib plus pembrolizumab: the next frontier for the treatment of hepatocellular carcinoma? Expert Opin Investig Drugs. 2022;31(4):371–378. doi:10.1080/13543784.2021.1948532
32. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
33. Mo DC, Luo PH, Huang SX, Wang HL, Huang JF. Safety and efficacy of pembrolizumab plus lenvatinib versus pembrolizumab and lenvatinib monotherapies in cancers: a systematic review. Int Immunopharmacol. 2021;91:107281. doi:10.1016/j.intimp.2020.107281
34. Wu WC, Lin TY, Chen MH, et al. Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest New Drugs. 2022;40(4):789–797. doi:10.1007/s10637-022-01248-0
35. Li Q, Cao M, Yuan G, et al. Lenvatinib plus camrelizumab vs. lenvatinib monotherapy as first-line treatment for unresectable hepatocellular carcinoma: a multicenter retrospective cohort study. Front Oncol. 2022;12:809709. doi:10.3389/fonc.2022.809709
36. Wei F, Huang Q, He J, Luo L, Zeng Y. Lenvatinib plus camrelizumab versus lenvatinib monotherapy as post-progression treatment for advanced hepatocellular carcinoma: a short-term prognostic study. Cancer Manag Res. 2021;13:4233–4240. doi:10.2147/CMAR.S304820
37. Huang X, Xu L, Ma T, et al. Lenvatinib plus immune checkpoint inhibitors improve survival in advanced hepatocellular carcinoma: a retrospective study. Front Oncol. 2021;11:751159. doi:10.3389/fonc.2021.751159
38. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
39. Kim BK, Cheon J, Kim H, et al. Atezolizumab/bevacizumab vs. lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a real-world, multi-center study. Cancers. 2022;14(7):1.
40. Hiraoka A, Kumada T, Tada T, et al. Does first-line treatment have prognostic impact for unresectable HCC?-Atezolizumab plus bevacizumab versus lenvatinib. Cancer Med. 2022;2022:1.
41. Niizeki T, Tokunaga T, Takami Y, et al. Comparison of efficacy and safety of atezolizumab plus bevacizumab and lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a propensity score matching analysis. Target Oncol. 2022;17(6):643–653. doi:10.1007/s11523-022-00921-x
42. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
43. Tsurusaki M, Murakami T. Surgical and locoregional therapy of HCC: TACE. Liver Cancer. 2015;4(3):165–175. doi:10.1159/000367739
44. Chen CT, Liu TH, Shao YY, Liu KL, Liang PC, Lin ZZ. Revisiting hepatic artery infusion chemotherapy in the treatment of advanced hepatocellular carcinoma. Int J Mol Sci. 2021;22(23):12880. doi:10.3390/ijms222312880
45. Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15(3):663–675. doi:10.1007/s12072-021-10184-9
46. Endo K, Kuroda H, Abe T, et al. Two hepatectomy cases for initially unresectable hepatocellular carcinoma after achieving a radiological complete response to sequential therapy with lenvatinib and transcatheter arterial chemoembolization. Hepatol Res. 2021;51(10):1082–1086. doi:10.1111/hepr.13665
47. Sun L, Xu X, Meng F, et al. Lenvatinib plus transarterial chemoembolization with or without immune checkpoint inhibitors for unresectable hepatocellular carcinoma: a review. Front Oncol. 2022;12:980214. doi:10.3389/fonc.2022.980214
48. Kudo M, Ueshima K, Yokosuka O, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):424–432. doi:10.1016/S2468-1253(18)30078-5
49. Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J Clin Oncol. 2022;2022:Jco2200392.
50. Zhu Y, Sun P, Wang K, et al. Efficacy and safety of lenvatinib monotreatment and lenvatinib-based combination therapy for patients with unresectable hepatocellular carcinoma: a retrospective, real-world study in China. Cancer Cell Int. 2021;21(1):503. doi:10.1186/s12935-021-02200-7
51. He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. doi:10.1177/17588359211002720
52. Qu S, Zhang X, Wu Y, et al. Efficacy and safety of TACE combined with lenvatinib plus PD-1 inhibitors compared with TACE alone for unresectable hepatocellular carcinoma patients: a prospective cohort study. Front Oncol. 2022;12:874473. doi:10.3389/fonc.2022.874473
53. Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi:10.3389/fimmu.2022.848387
54. Liu Y, Qiao Y, Zhou M, et al. Efficacy and safety of hepatic arterial infusion chemotherapy combined with lenvatinib and sequential ablation in the treatment of advanced hepatocellular carcinoma. Cancer Med. 2022;2022:1.
55. Chen S, Wu Z, Shi F, et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. 2022;148(8):2115–2125. doi:10.1007/s00432-021-03767-4
56. Chen S, Xu B, Wu Z, et al. Pembrolizumab plus lenvatinib with or without hepatic arterial infusion chemotherapy in selected populations of patients with treatment-naive unresectable hepatocellular carcinoma exhibiting PD-L1 staining: a multicenter retrospective study. BMC Cancer. 2021;21(1):1126. doi:10.1186/s12885-021-08858-6
57. Lai Z, He M, Bu X, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, Phase II trial. Eur J Cancer. 2022;174:68–77. doi:10.1016/j.ejca.2022.07.005
58. Mei J, Tang YH, Wei W, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. 2021;11:618206. doi:10.3389/fonc.2021.618206
59. Qu WF, Ding ZB, Qu XD, et al. Conversion therapy for initially unresectable hepatocellular carcinoma using a combination of toripalimab, lenvatinib plus TACE: real-world study. BJS Open. 2022;6(5). doi:10.1093/bjsopen/zrac114
60. Guo P, Pi X, Gao F, et al. Transarterial chemoembolization plus lenvatinib with or without programmed death-1 inhibitors for patients with unresectable hepatocellular carcinoma: a propensity score matching study. Front Oncol. 2022;12:945915. doi:10.3389/fonc.2022.945915
61. Xiang YJ, Wang K, Yu HM, et al. Transarterial chemoembolization plus a PD-1 inhibitor with or without lenvatinib for intermediate-stage hepatocellular carcinoma. Hepatol Res. 2022;52(8):721–729. doi:10.1111/hepr.13773
62. Li X, Fu Z, Chen X, et al. Efficacy and safety of lenvatinib combined with PD-1 inhibitors plus TACE for unresectable hepatocellular carcinoma patients in China real-world. Front Oncol. 2022;12:950266. doi:10.3389/fonc.2022.950266
63. Qiao ZY, Zhang ZJ, Lv ZC, et al. Neoadjuvant programmed cell death 1 (PD-1) inhibitor treatment in patients with hepatocellular carcinoma before liver transplant: a cohort study and literature review. Front Immunol. 2021;12:653437. doi:10.3389/fimmu.2021.653437
64. Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5(1):146. doi:10.1038/s41392-020-00264-x
65. Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013;108(3):479–485. doi:10.1038/bjc.2012.581
66. Hu B, Zou T, Qin W, et al. Inhibition of EGFR overcomes acquired lenvatinib resistance driven by STAT3-ABCB1 signaling in hepatocellular carcinoma. Cancer Res. 2022;82(20):3845–3857. doi:10.1158/0008-5472.CAN-21-4140
67. Huang M, Long J, Yao Z, et al. METTL1-mediated m7G tRNA modification promotes lenvatinib resistance in hepatocellular carcinoma. Cancer Res. 2023;83(1):89–102. doi:10.1158/0008-5472.CAN-22-0963
68. Wang L, Yang Q, Zhou Q, et al. METTL3-m(6)A-EGFR-axis drives lenvatinib resistance in hepatocellular carcinoma. Cancer Lett. 2023;559:216122. doi:10.1016/j.canlet.2023.216122
69. Lu Y, Shen H, Huang W, et al. Genome-scale CRISPR-Cas9 knockout screening in hepatocellular carcinoma with lenvatinib resistance. Cell Death Discov. 2021;7(1):359. doi:10.1038/s41420-021-00747-y
70. Zhao Z, Zhang D, Wu F, et al. Sophoridine suppresses lenvatinib-resistant hepatocellular carcinoma growth by inhibiting RAS/MEK/ERK axis via decreasing VEGFR2 expression. J Cell Mol Med. 2021;25(1):549–560. doi:10.1111/jcmm.16108
71. Sun D, Liu J, Wang Y, Dong J. Co-administration of MDR1 and BCRP or EGFR/PI3K inhibitors overcomes lenvatinib resistance in hepatocellular carcinoma. Front Oncol. 2022;12:944537. doi:10.3389/fonc.2022.944537
72. Huang S, Ma Z, Zhou Q, et al. Genome-wide CRISPR/Cas9 library screening identified that DUSP4 deficiency induces lenvatinib resistance in hepatocellular carcinoma. Int J Biol Sci. 2022;18(11):4357–4371. doi:10.7150/ijbs.69969
73. Hou W, Bridgeman B, Malnassy G, et al. Integrin subunit beta 8 contributes to lenvatinib resistance in HCC. Hepatol Commun. 2022;6(7):1786–1802. doi:10.1002/hep4.1928
74. Wei L, Lee D, Law CT, et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat Commun. 2019;10(1):4681. doi:10.1038/s41467-019-12606-7
75. Zhao Z, Song J, Zhang D, Wu F, Tu J, Ji J. Oxysophocarpine suppresses FGFR1-overexpressed hepatocellular carcinoma growth and sensitizes the therapeutic effect of lenvatinib. Life Sci. 2021;264:118642. doi:10.1016/j.lfs.2020.118642
76. Guo J, Zhu P, Ye Z, et al. YRDC mediates the resistance of lenvatinib in hepatocarcinoma cells via modulating the translation of KRAS. Front Pharmacol. 2021;12:744578. doi:10.3389/fphar.2021.744578
77. Han T, Zheng H, Zhang J, et al. Downregulation of MUC15 by miR-183-5p.1 promotes liver tumor-initiating cells properties and tumorigenesis via regulating c-MET/PI3K/AKT/SOX2 axis. Cell Death Dis. 2022;13(3):200. doi:10.1038/s41419-022-04652-9
78. Myojin Y, Kodama T, Maesaka K, et al. ST6GAL1 is a novel serum biomarker for lenvatinib-susceptible FGF19-driven hepatocellular carcinoma. Clin Cancer Res. 2021;27(4):1150–1161. doi:10.1158/1078-0432.CCR-20-3382
79. Pan J, Zhang M, Dong L, et al. Genome-Scale CRISPR screen identifies LAPTM5 driving lenvatinib resistance in hepatocellular carcinoma. Autophagy. 2022;2022:1–15.
80. Arechederra M, Bazai SK, Abdouni A, et al. ADAMTSL5 is an epigenetically activated gene underlying tumorigenesis and drug resistance in hepatocellular carcinoma. J Hepatol. 2021;74(4):893–906. doi:10.1016/j.jhep.2020.11.008
81. Han TS, Hur K, Cho HS, Ban HS. Epigenetic associations between lncRNA/circRNA and miRNA in hepatocellular carcinoma. Cancers. 2020;12(9):2622. doi:10.3390/cancers12092622
82. Wei Y, Wei L, Han T, Ding S. miR-3154 promotes hepatocellular carcinoma progression via suppressing HNF4α. Carcinogenesis. 2022;43(10):1002–1014. doi:10.1093/carcin/bgac067
83. Xu X, Jiang W, Han P, Zhang J, Tong L, Sun X. MicroRNA-128-3p mediates lenvatinib resistance of hepatocellular carcinoma cells by downregulating c-Met. J Hepatocell Carcinoma. 2022;9:113–126. doi:10.2147/JHC.S349369
84. Hao X, Zhang Y, Shi X, et al. CircPAK1 promotes the progression of hepatocellular carcinoma via modulation of YAP nucleus localization by interacting with 14-3-3ζ. J Exp Clin Cancer Res. 2022;41(1):281. doi:10.1186/s13046-022-02494-z
85. Zhang P, Sun H, Wen P, Wang Y, Cui Y, Wu J. circRNA circMED27 acts as a prognostic factor and mediator to promote lenvatinib resistance of hepatocellular carcinoma. Mol Ther Nucleic Acids. 2022;27:293–303. doi:10.1016/j.omtn.2021.12.001
86. Yu T, Yu J, Lu L, et al. MT1JP-mediated miR-24-3p/BCL2L2 axis promotes Lenvatinib resistance in hepatocellular carcinoma cells by inhibiting apoptosis. Cell Oncol. 2021;44(4):821–834. doi:10.1007/s13402-021-00605-0
87. Duan A, Li H, Yu W, Zhang Y, Yin L. Long noncoding RNA XIST promotes resistance to lenvatinib in hepatocellular carcinoma cells via epigenetic inhibition of NOD2. J Oncol. 2022;2022:4537343. doi:10.1155/2022/4537343
88. Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37(1):110. doi:10.1186/s13046-018-0777-4
89. Kato Y, Tabata K, Kimura T, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14(2):e0212513. doi:10.1371/journal.pone.0212513
90. Adachi Y, Kamiyama H, Ichikawa K, et al. Inhibition of FGFR reactivates IFNγ signaling in tumor cells to enhance the combined antitumor activity of lenvatinib with anti-PD-1 antibodies. Cancer Res. 2022;82(2):292–306. doi:10.1158/0008-5472.CAN-20-2426
91. Deng H, Kan A, Lyu N, et al. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer. 2021;9(6):e002305. doi:10.1136/jitc-2020-002305
92. Mok EHK, Leung CON, Zhou L, et al. Caspase-3-induced activation of SREBP2 drives drug resistance via promotion of cholesterol biosynthesis in hepatocellular carcinoma. Cancer Res. 2022;82(17):3102–3115. doi:10.1158/0008-5472.CAN-21-2934
93. Toh TB, Lim JJ, Hooi L, Rashid M, Chow EK. Targeting Jak/Stat pathway as a therapeutic strategy against SP/CD44+ tumorigenic cells in Akt/β-catenin-driven hepatocellular carcinoma. J Hepatol. 2020;72(1):104–118. doi:10.1016/j.jhep.2019.08.035
94. Ma XL, Hu B, Tang WG, et al. CD73 sustained cancer-stem-cell traits by promoting SOX9 expression and stability in hepatocellular carcinoma. J Hematol Oncol. 2020;13(1):11. doi:10.1186/s13045-020-0845-z
95. Chen G, Wang Y, Zhao X, et al. A positive feedback loop between Periostin and TGFβ1 induces and maintains the stemness of hepatocellular carcinoma cells via AP-2α activation. J Exp Clin Cancer Res. 2021;40(1):218. doi:10.1186/s13046-021-02011-8
96. Wang J, Yu H, Dong W, et al. N6-methyladenosine-mediated up-regulation of FZD10 regulates liver cancer stem cells’ properties and lenvatinib resistance through WNT/β-catenin and hippo signaling pathways. Gastroenterology. 2023;164(6):990–1005. doi:10.1053/j.gastro.2023.01.041
97. Hiraoka A, Kumada T, Kariyama K, et al. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: multicenter analysis. Cancer Med. 2019;8(1):137–146. doi:10.1002/cam4.1909
98. Lin PT, Teng W, Jeng WJ, Lin CY, Lin SM, Sheen IS. Combining immune checkpoint inhibitor with lenvatinib prolongs survival than lenvatinib alone in sorafenib-experienced hepatocellular carcinoma patients. Eur J Gastroenterol Hepatol. 2022;34(2):213–219. doi:10.1097/MEG.0000000000001956
99. Guan R, Yu C, Li S, Mei J, Wei W, Guo R. A preliminary study on drug switching strategy for second-line therapy after combination treatment of tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma. Front Pharmacol. 2022;13:998534. doi:10.3389/fphar.2022.998534
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.