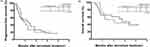Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Significance of Physical Status and Liver Function Reserve for Outcome of Patients with Advanced Hepatocellular Carcinoma Receiving Lenvatinib Treatment
Authors Chan KM, Lai Y, Hung HC, Lee JC, Cheng CH, Wang YC, Wu TH , Lee CF, Wu TJ, Chou HS, Wang CT, Chai PM, Lien HY, Lee WC
Received 18 October 2022
Accepted for publication 11 February 2023
Published 18 February 2023 Volume 2023:10 Pages 281—290
DOI https://doi.org/10.2147/JHC.S393964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mohamed Shaker
Kun-Ming Chan,1 Yin Lai,1 Hao-Chien Hung,1 Jin-Chiao Lee,1 Chih-Hsien Cheng,1 Yu-Chao Wang,1 Tsung-Han Wu,1 Chen-Fang Lee,1 Ting-Jung Wu,1 Hong-Shiue Chou,1 Ching-Ting Wang,2 Pei-Mei Chai,2 Hsin-Yi Lien,3 Wei-Chen Lee1
1Department of General Surgery and Chang Gung Transplantation Institute, Chang Gung Memorial Hospital at Linkou, Chang Gung University College of Medicine, Taoyuan, Taiwan; 2Department of Nursing, Chang Gung Memorial Hospital at Linkou, Chang Gung University College of Medicine, Taoyuan, Taiwan; 3Graduate School of Education, Ming Chuan University, Taoyuan, Taiwan
Correspondence: Kun-Ming Chan, Department of General Surgery and Chang Gung Transplantation Institute, Chang Gung Memorial Hospital at Linkou, Chang Gung University College of Medicine, No. 5 Fu-Hsing Street, Gui-Shan District, Taoyuan City, 33305, Taiwan, Tel +886-3-3281200 Ext 3366, Fax +886-3-3285818, Email [email protected]
Background: Tyrosine kinase inhibitors (TKIs) remain the primary therapeutic option for patients with advanced-stage hepatocellular carcinoma (HCC). However, the selection of a suitable TKI is an issue in real-world clinical practice. Thus, this study aimed to identify patients most likely to benefit from lenvatinib treatment.
Methods: A retrospective review of 143 patients with unresectable advanced-stage HCC treated with lenvatinib between January 2020 and December 2021 was performed. Outcomes related to lenvatinib treatment were measured, and the clinical parameters affecting prognosis were analyzed.
Results: Overall, the median time of progression-free survival (PFS) and overall survival (OS) were 7.1 months and 17.7 months, respectively. Prognostic analyses found that Child-Pugh score > 5 (hazard ratio [HR] = 2.43, 95% confidence interval [CI] = 1.55– 3.80, p = 0.001) was a significant factor affecting the PFS of HCC after lenvatinib treatment. Child-Pugh score > 5 (HR = 2.12, 95% CI = 1.20– 3.74, p = 0.009), body weight ≥ 60 kg (HR = 0.54, 95% CI = 0.32– 0.90, p = 0.020), and additional trans-arterial chemoembolization (TACE) treatment (HR = 0.38, 95% CI = 0.21– 0.70, p = 0.003) were significant prognostic factors for OS. However, early α-fetoprotein reduction was not significantly correlated with patient outcomes. Additionally, patients with pre-treatment neutrophil-lymphocyte ratio > 4.07 showed a significant worse PFS and OS than other patients.
Conclusion: The outcome of patients with advanced-stage HCC remains poor. However, the host condition, including good physical status and better functional liver preservation, largely affected the outcome of patients receiving lenvatinib treatment. Moreover, additional locoregional therapy for intrahepatic HCC, other than TKI treatment, can be considered in certain patients to achieve a favorable outcome.
Keywords: hepatocellular carcinoma, advanced-stage, tyrosine kinase inhibitor, lenvatinib, outcome
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy and a leading cause of cancer-related deaths worldwide. The golden rule for long-term survival of patients with HCC is early detection followed by curative treatment. However, the majority of patients with HCC are detected at late stages and are ineligible for curative treatment, requiring a complex combination of therapeutic modalities.1,2 Generally, patients presenting with advanced-stage HCC, specifically stage C based on the Barcelona Clinic Liver Cancer (BCLC) staging classification, should be evaluated for appropriate systemic therapy. It is well known that sorafenib was the first regimen to be an efficacious therapeutic option for patients with advanced HCC.3,4 Subsequently, there have been major advances in the systemic treatment for advanced HCC in terms of numerous distinguished studies during the last decade.5–10 Nonetheless, the decision to select appropriate therapeutic regimens to achieve the greatest benefit for individualized patient remains a dilemma.
The combination of atezolizumab with bevacizumab has been shown to provide superior therapeutic efficacy and is considered the preferred regimen for the treatment of advanced-stage HCC.7 Nonetheless, all patients might not be eligible for this combination therapy. Accordingly, oral tyrosine kinase inhibitors (TKIs), including sorafenib and lenvatinib, could also be considered the first-line treatment for advanced-stage HCC if the combination of atezolizumab and bevacizumab is not feasible. However, selecting either sorafenib or lenvatinib remains an issue in real-world clinical practice. Although lenvatinib showed non-inferiority in terms of overall survival compared with sorafenib, identifying patients who are most likely to benefit from this therapy is crucial for improving the overall outcome of patients with advanced HCC. Therefore, this study aimed to assess the efficacy of lenvatinib for advanced HCC in clinical practice. In addition, prognostic factors associated with patient outcomes and beneficial effects of lenvatinib were also examined to facilitate decision-making in clinical practice for improving long-term outcomes of patients with advanced-stage HCC.
Materials and Methods
Lenvatinib Prescription
The Taiwan National Health Insurance (NHI) program is a globally well-known public health program that covers the entire national population, in which many chemotherapeutic regimens are offered and reimbursed by the program for numerous malignancies. Currently, only sorafenib and lenvatinib are covered by this program as first-line therapies for advanced-stage HCC, and the combination of atezolizumab with bevacizumab regimen is not included because of financial concerns despite its superior efficacy for these patients. However, patients with advanced-stage HCC are free to select combination regimens as long as they are covered by their own payment.
Lenvatinib has been fully covered and reimbursed by the NHI since January 2020 for patients with advanced-stage HCC under certain indications. Based on the BCLC treatment algorithm for HCC, patients with clinical stage C HCC that is characterized by macroscopic vascular invasion and/or extrahepatic metastasis ineligible for complete surgical resection were proposed for lenvatinib prescription. In addition, patients should have preserved liver function and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. All patients who met the aforementioned conditions were approved by the NHI for lenvatinib prescription after reviewing the patients’ clinical data. The application of lenvatinib has to be renewed every two months or when the patient runs out of medication. Patients who had no clinical evidence of disease progression were eligible for another term of lenvatinib usage. Lenvatinib dosage was prescribed depending on body weight as follows: 12 mg/day for body weight ≥60 kg or 8 mg/day for body weight <60 kg as recommended in the initial trial study.9
Patients
The evaluation and diagnosis of HCC generally follows the guidelines proposed by the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) in our institute.11,12 Moreover, every patient with HCC was fully reviewed and discussed by the institute’s liver cancer committee, which comprises members from the liver surgery, oncology, hepatology, radiology, and interventional radiology departments for the consensus of clinical staging and optimal treatment.
All patients who received any treatment for primary HCC at Chang Gung Memorial Hospital Linkou Medical Center, Taiwan, between January 2020 and December 2021, were retrospectively reviewed. With the approval of the Institutional Review Board, 143 patients with BCLC stage C HCC who had received lenvatinib treatment were included in the study for further analysis. The requirement of written informed consent was waived due to the retrospective nature of the study. All the data was anonymized or maintained with confidentiality, and the study was performed in accordance with the ethical standards of the Declaration of Helsinki.
Follow-Up and Statistical Analysis
After the initiation of lenvatinib treatment, all patients were regularly followed up for HCC surveillance and survival until death or the end of the present study. During the follow-up period, clinical assessments included lenvatinib-related toxicities, serum biochemical laboratory tests, and radiologic imaging examinations. Tumor status was assessed in accordance with the modified Response Evaluation Criteria in Solid Tumors (mRECIST) by triphasic computed tomography (CT) and/or magnetic resonance imaging (MRI).13
The outcomes were measured in terms of HCC progression-free survival (PFS) and the patient’s overall survival (OS). PFS was defined as the period between the starting date of lenvatinib treatment and the date of detection of HCC progression according to the mRECIST criteria. OS was measured from the date of lenvatinib treatment to the date of death from any cause. Survival curves were reconstructed using the Kaplan-Meier method and compared using Log rank tests. Categorical variables were analyzed using the chi-square or Fisher’s exact test, and the independent t-test was used for continuous data, as appropriate. An optimal cut-off value of continuous data was determined by receiver operating characteristic (ROC) curve analysis or as literature citation. Specifically, an ROC analysis was unable to identify an optimal cut-off value for AFP, and it was set at 400 ng/mL for risk stratification as previous studies.14,15 The potential prognostic factors for PFS and OS were identified using the Cox regression proportional hazards model of univariate and multivariate analyses. In order to cover more potential risk factors that might affect the outcome of patients, the univariate analysis was set at a p value less than 0.1 for identifying potential risk factors. Statistical significance of multivariate analysis was set at p < 0.05. All statistical analyses were performed using SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient Characteristics
Of the 143 patients, there were 108 (75.5%) men and 35 (24.5%) women with a median age of 63.7 years, ranging from 14.7 to 90.5 years at the time of lenvatinib prescription. The clinical characteristics of the patients are summarized in Table 1. Hepatitis B virus (62.9%) remained the major etiology in this cohort, and 7 of them had detectable virus were simultaneously treated by antiviral therapy. None of HCV patient had detectable virus in this study. The majority of patients (95.8%) had well-preserved liver function (Child-Pugh class A) at the time of initial lenvatinib treatment. Major vascular invasion, including major portal vein tumor thrombosis and hepatic vein involvement were noted in 64 (44.8%) patients. In addition, extrahepatic metastasis and tumor burden greater than 50% of liver parenchyma were detected in 81 (56.6%) patients and 35 (24.5%) patients, respectively. Overall, 70 (49.0%) patients died of HCC and 73 (51%) patients were still alive under regular follow up at the end of this study. Of these, 108 (75.5%) patients experienced disease progression and were ineligible for continuation of lenvatinib treatment according to the program regulations. The median time of lenvatinib therapy was 4.4 months, ranging 0.4 to 20.5 months for these patients. The remaining 35 patients were still alive and under lenvatinib treatment, and the median time of lenvatinib therapy was 15.0 months, ranging from 5.5 to 26.7 months.
 |
Table 1 Clinical Characteristics of Patients Receiving Lenvatinib Treatment for Advanced-Stage Hepatocellular Carcinoma |
Prognostic Factors Affecting Outcomes
The median follow-up time in this cohort was 11.1 months, ranging from 0.6 to 28.0 months. During the follow-up period, the median PFS was 7.1 months, and the PFS rates at 1- and 2-year were 29.4% and 16.9%, respectively. (Figure 1A) The median time of OS was 17.7 months, and the overall 1- and 2-year OS rates were 60.3% and 47.3%, respectively. (Figure 1B) The prognostic factors for outcomes in terms of PFS and OS were analyzed according to the clinical features. The univariate analysis identified four potential risk factors for PFS, and further multivariate regression analysis resulted in a Child-Pugh score > 5 (hazard ratio [HR] = 2.43, 95% confidence interval [CI] = 1.55–3.80, p = 0.001) as the only significant prognostic factor affecting HCC disease progression after lenvatinib treatment. (Table 2) With regard to OS, five potential risk factors, including sex, tumor burden, body weight, Child-Pugh score, and additional Trans-arterial Chemoembolization (TACE) treatment, were identified by univariate analysis. However, multivariate regression analysis of the aforementioned factors showed that Child-Pugh score > 5 (HR = 2.12, 95% CI = 1.20–3.74, p = 0.009), body weight ≥ 60 kg (HR = 0.54, 95% CI = 0.32–0.90, p = 0.020), and additional TACE treatment (HR = 0.38, 95% CI = 0.21–0.70, p = 0.003) were independent prognostic factors of OS in these patients. (Table 3)
 |
Table 2 Univariate and Multivariate Analyses of Prognostic Factor Affecting Progression-Free Survival of Patients with Hepatocellular Carcinoma Under Lenvatinib Treatment |
 |
Table 3 Univariate and Multivariate Analyses of Prognostic Factor Affecting Overall Survival of Patients with Hepatocellular Carcinoma Under Lenvatinib Treatment |
Influence of Specific Biomarkers
Alpha-fetoprotein (AFP) has been a crucial biomarker of HCC, and thus the therapeutic response based on AFP variation after lenvatinib treatment was also analyzed. Overall, 97 patients (67.8%) had serum AFP greater than a baseline value of 10 ng/mL, of which 54 patients had AFP >10% reduction within the first 4 weeks of treatment. However, the outcome analysis with (n=54) or without (n=43) AFP > 10% reduction showed no significant difference in PFS and OS of these patients. (Figure 2) Additionally, 46 patients had AFP > 400 ng/mL and 31 of them had an AFP > 10% reduction within the first 4 weeks of lenvatinib treatment. Among these patients, the PFS and OS related to whether the AFP was > 10% reduction or not was also not significantly different. (Figure 3)
Additionally, systemic inflammatory index has also been considered as a predictor of outcomes for many malignancies, as well as HCC. Therefore, this study analyzed two inflammatory indices including neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) to explore their prognostic value for patients with advanced HCC treated with lenvatinib. The ROC curve analysis had identified the optimal cut-off point of NLR was 4.07, in which the estimated percentage of lymphocytes was 18%. Patients with pretreatment NLR > 4.07 (n = 55) showed a significantly worse PFS (p = 0.014) and OS (p = 0.010) than those with NLR ≤ 4.07 (n = 88). (Figure 4) The optimal cut-off point for PLR was 121 based on ROC curve analysis. Nonetheless, no significant differences were observed in both PFS (p = 0.497) and OS (p = 0.821) for patients with lenvatinib treatment based on the PLR. (Figure 5)
Discussion
Systemic therapy for HCC has evolved remarkably in the past decade. With the advancement of oncology, numerous molecular targeted agents, anti-angiogenesis monoclonal antibodies, and immunotherapeutic regimens are receiving more attention as effective treatments for advanced HCC. However, the selection of an optimal therapy and providing beneficial effects for patients with advanced HCC remains a dilemma. Currently, there are no effective indicators for selecting specific therapeutic regimens for individualized advanced HCC treatment. This study showed that certain groups of patients with advanced HCC, such as those with Child-Pugh Score = 5, body weight ≥ 60 kg, and additional TACE, could achieve favorable outcomes from lenvatinib treatment. Moreover, the results could provide important scientific information for decision-making in clinical practice for patients with advanced HCC.
Lenvatinib was the first regimen to show a treatment effect that was non-inferior to sorafenib on the overall survival of patients with advanced HCC in a global trial.9 Moreover, lenvatinib also showed a clinically meaningful improvement in all secondary efficacy endpoints, including progression-free survival, time to progression, objective response, and quality-of-life assessments.9,16 Based on these promising results, lenvatinib has been listed as the first-line treatment option for advanced HCC in the BCLC algorithm as well as our NHI. The real-world experience from this study showed a median PFS time of 7.1 months that is similar to the initial REFLECT study. However, the study showed that the overall survival was better than that of the original REFLECT study. A possible explanation might be related to continuous improvements in care for advanced HCC and multimodal therapies, including locoregional treatments such as TACE, even after disease progression following lenvatinib treatment.
Currently, there are no suitable indicators for selecting patients with advanced HCC to gain the greatest therapeutic benefit from tyrosine kinase inhibitors or lenvatinib. Several studies have identified numerous prognostic factors that affect the outcomes of patients who receive lenvatinib treatment for advanced HCC.17–20 However, along with the rapid improvement of multidisciplinary treatment for patients with advanced HCC, the identification of prognostic factors remains informative in clinical scenarios. The study identified that the host factor regarding liver function status at Child-Pugh score 5 was the key factor affecting both the therapeutic response and survival of patients with advanced HCC. Theoretically, better liver function preservation might translate to better tolerability and therapeutic effect of lenvatinib treatment. In this context, lenvatinib should be recommended for HCC patients with good liver function preservation to achieve the greatest benefit.
Additionally, the study also showed that a body weight ≥ 60 kg and additional locoregional therapy could generate a favorable outcome from lenvatinib treatment. Based on preliminary Phase II and pharmacological studies, the dosage of lenvatinib should be adjusted according to body weight at a cut-off value of 60 kg to achieve a balance between drug tolerability and therapeutic effect.16,21 Although the original lenvatinib study showed similar outcomes in terms of body weight and dosage adjustment, patients with body weight ≥ 60 kg had a better outcome than the others. As such, patients with a lower body weight might have a weakened physical or nutritional status, leading to unfavorable survival in a real-world clinical setting.
The concept of managing advanced HCC has evolved towards multidisciplinary treatment. Unimodal treatment is rarely used as the sole treatment for advanced HCC in clinical practice. As shown in this study, patients treated with lenvatinib and TACE had a favorable overall survival. As such, patients who had undergone additional locoregional therapy were naturally in a better clinical condition, reflected by good drug tolerability as well as a longer survival period. Meanwhile, TACE may induce tumor necrosis, which could also impede tumor progression and possibly diminish the tumor burden in certain circumstances. Therefore, primary intrahepatic HCC should also be properly treated with TACE in addition to systemic treatment in order to gain a better outcome for patients with advanced HCC.
Additionally, AFP has been shown to be a good predictor of therapeutic response and outcome.22–25 Nonetheless, AFP response had no significant role in predicting the therapeutic effect of lenvatinib in patients with HCC in this study. Generally, the main reason might be related to the wide range of variation in serum AFP levels and the small number of patients in this study. In addition, host immunity has been an important issue in assessing the outcomes of malignancy. Systemic inflammatory indices including NLR and PLR have been reported as a predictor of outcomes in many malignancies, including HCC.26–31 In line with a previous study, NLR but not PLR was a significant factor affecting the outcomes of patients with advanced HCC treated by TKI.32 However, the heterogeneous clinical presentation of patients from this study perhaps could not well elucidate this observation. The molecular mechanisms through the NLR and PLR associated with HCC remain mostly unknown, and further studies to clarify the detail mechanism are required in the future. The optimal cut-off point of NLR was estimated at 4.0, reflected by approximately 18% lymphocytes among white blood cell differentiation counts in this study. Therefore, HCC patients with lower than 18% lymphocytes might indicate worse host immunity, resulting in poor therapeutic response to TKI and survival of patients.
Conclusion
The major limitation of this study was a retrospective study from a single center. Although generalizations cannot be easily made, several observations may be helpful in managing patients with advanced HCC. Additionally, accumulated data have shown that an aggressive attitude through multidisciplinary treatment can effectively provide benefits to patients with advanced HCC. Therefore, HCC patients who have a good physical status and functional liver preservation representing a better host condition should be considered for additional locoregional therapy such as TACE other than TKI treatment to achieve a favorable outcome.
Abbreviations
HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; TKI, tyrosine kinase inhibitor; NHI, National Health Insurance; mRECIST, modified Response Evaluation Criteria in Solid Tumors; CT, computed tomography; MRI, magnetic resonance imaging; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; TACE, Trans-arterial Chemoembolization; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; AFP, alpha-fetoprotein.
Data Sharing Statement
All data were included in this study.
Ethical Approval and Informed Consent Statement
The study was approved by the Institutional Review Board of the Chang Gung Memorial Hospital (IRB No: 201800970B0). The requirement of written informed consent was waived due to the retrospective nature of the study. All the data was anonymized or maintained with confidentiality, and the study was performed in accordance with the ethical standards of the Declaration of Helsinki.
Acknowledgments
The authors would like to thank all members of the institute’s liver cancer committee for their supports in review and discussion of every patient in this study for the consensus of clinical staging and optimal treatment.
Funding
This work was supported by grants from the Taiwan Ministry of Health and Welfare (MOHW111-TDU-B-221-014009) to W.-C. Lee and K.-M. Chan.
Disclosure
All authors have no conflict of interest to disclosure.
References
1. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
2. Dhir M, Melin AA, Douaiher J, et al. A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann Surg. 2016;263(6):1112–1125. doi:10.1097/SLA.0000000000001556
3. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
5. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
6. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
7. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
8. Kelley RK, Yau T, Cheng AL, et al. VP10-2021: cabozantinib (C) plus atezolizumab (A) versus sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): results from the randomized phase III COSMIC-312 trial. Ann Oncol. 2022;33(1):114–116. doi:10.1016/j.annonc.2021.10.008
9. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
10. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
11. Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the study of the liver. J Hepatol. 2001;35(3):421–430. doi:10.1016/S0168-8278(01)00130-1
12. Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi:10.1002/hep.20933
13. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
14. Huang JF, Wu SM, Wu TH, et al. Liver resection for complicated hepatocellular carcinoma: challenges but opportunity for long-term survivals. J Surg Oncol. 2012;106(8):959–965. doi:10.1002/jso.23172
15. Yang SL, Liu LP, Yang S, et al. Preoperative serum alpha-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2016;103(6):716–724. doi:10.1002/bjs.10093
16. Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52(4):512–519. doi:10.1007/s00535-016-1263-4
17. Burgio V, Iavarone M, Di Costanzo GG, et al. Real-life clinical data of lenvatinib versus sorafenib for unresectable hepatocellular carcinoma in Italy. Cancer Manag Res. 2021;13:9379–9389. doi:10.2147/CMAR.S330195
18. Hiraoka A, Kumada T, Kariyama K, et al. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: multicenter analysis. Cancer Med. 2019;8(1):137–146. doi:10.1002/cam4.1909
19. Rapposelli IG, Tada T, Shimose S, et al. Adverse events as potential predictive factors of activity in patients with advanced hepatocellular carcinoma treated with lenvatinib. Liver Int. 2021;41(12):2997–3008. doi:10.1111/liv.15014
20. Rimini M, Shimose S, Lonardi S, et al. Lenvatinib versus Sorafenib as first-line treatment in hepatocellular carcinoma: a multi-institutional matched case-control study. Hepatol Res. 2021;51(12):1229–1241. doi:10.1111/hepr.13718
21. Tamai T, Hayato S, Hojo S, et al. Dose finding of lenvatinib in subjects with advanced hepatocellular carcinoma based on population pharmacokinetic and exposure-response analyses. J Clin Pharmacol. 2017;57(9):1138–1147. doi:10.1002/jcph.917
22. Lee PC, Chao Y, Chen MH, et al. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers. 2020;12:1.
23. Shao YY, Liu TH, Hsu C, et al. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019;39(11):2184–2189. doi:10.1111/liv.14210
24. Teng W, Lin CC, Ho MM, et al. Alpha-fetoprotein response at different time-points is associated with efficacy of nivolumab monotherapy for unresectable hepatocellular carcinoma. Am J Cancer Res. 2021;11(5):2319–2330.
25. Lee JC, Hung HC, Wang YC, et al. Risk score model for microvascular invasion in hepatocellular carcinoma: the role of tumor burden and alpha-fetoprotein. Cancers. 2021;13:17.
26. Hung HC, Lee JC, Cheng CH, et al. Impact of neutrophil to lymphocyte ratio on survival for hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci. 2017;24(10):559–569. doi:10.1002/jhbp.498
27. Hung HC, Lee JC, Wang YC, et al. Response prediction in immune checkpoint inhibitor immunotherapy for advanced hepatocellular carcinoma. Cancers. 2021;13:7. doi:10.3390/cancers13071607
28. Jung MR, Park YK, Jeong O, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104(5):504–510. doi:10.1002/jso.21986
29. Mazaki J, Katsumata K, Kasahara K, et al. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis. BMC Cancer. 2020;20(1):922. doi:10.1186/s12885-020-07429-5
30. Zheng J, Cai J, Li H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem. 2017;44(3):967–981. doi:10.1159/000485396
31. Nakano M, Kuromatsu R, Niizeki T, et al. Immunological inflammatory biomarkers as prognostic predictors for advanced hepatocellular carcinoma. ESMO Open. 2021;6(1):100020. doi:10.1016/j.esmoop.2020.100020
32. Casadei Gardini A, Scarpi E, Faloppi L, et al. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget. 2016;7(41):67142–67149. doi:10.18632/oncotarget.11565
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.