Back to Journals » Journal of Pain Research » Volume 16
Painful Peripheral Neuropathies of the Lower Limbs and/or Lower Extremities Treated with Spinal Cord Stimulation: A Systematic Review with Narrative Synthesis
Authors Burkey AR, Chen J, Argoff CE, Edgar DR, Petersen EA
Received 18 January 2023
Accepted for publication 21 April 2023
Published 18 May 2023 Volume 2023:16 Pages 1607—1636
DOI https://doi.org/10.2147/JPR.S403715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Andrea Tinnirello
Adam R Burkey,1 Jeffrey Chen,2 Charles E Argoff,3 Deborah R Edgar,4 Erika A Petersen5
1Anesis Spine and Pain Care, Renton, WA, USA; 2UCSD Department of Anesthesiology Center for Pain, University of California San Diego Medical Center, La Jolla, CA, USA; 3Department of Neurology, Albany Medical Center, Albany, NY, USA; 4Commexus Ltd, Dunblane, Perthshire, UK; 5Department of Neurosurgery, University of Arkansas for Medical Sciences, Little Rock, AR, USA
Correspondence: Erika A Petersen, Department of Neurosurgery, University of Arkansas for Medical Sciences, 4301 W Markham St, Slot 507, Little Rock, AR, 72205, USA, Tel +1-501-686-5270, Email [email protected]
Introduction: Painful peripheral neuropathy (PPN) is a debilitating condition with varied etiologies. Spinal cord stimulation (SCS) is increasingly used when conservative treatments fail to provide adequate pain relief. Few published reviews have examined SCS outcomes in all forms of PPN.
Methods: We conducted a systematic review of SCS in PPN. The PubMed database was searched up to February 7th, 2022, for peer-reviewed studies of SCS that enrolled PPN patients with pain symptoms in their lower limbs and/or lower extremities. We assessed the quality of randomized controlled trial (RCT) evidence using the Cochrane risk of bias tool. Data were tabulated and presented narratively.
Results: Twenty eligible studies documented SCS treatment in PPN patients, including 10 kHz SCS, traditional low-frequency SCS (t-SCS), dorsal root ganglion stimulation (DRGS), and burst SCS. In total, 451 patients received a permanent implant (10 kHz SCS, n=267; t-SCS, n=147; DRGS, n=25; burst SCS, n=12). Approximately 88% of implanted patients had painful diabetic neuropathy (PDN). Overall, we found clinically meaningful pain relief (≥ 30%) with all SCS modalities. Among the studies, RCTs supported the use of 10 kHz SCS and t-SCS to treat PDN, with 10 kHz SCS providing a higher reduction in pain (76%) than t-SCS (38– 55%). Pain relief with 10 kHz SCS and DRGS in other PPN etiologies ranged from 42– 81%. In addition, 66– 71% of PDN patients and 38% of nondiabetic PPN patients experienced neurological improvement with 10 kHz SCS.
Conclusion: Our review found clinically meaningful pain relief in PPN patients after SCS treatment. RCT evidence supported the use of 10 kHz SCS and t-SCS in the diabetic neuropathy subpopulation, with more robust pain relief evident with 10 kHz SCS. Outcomes in other PPN etiologies were also promising for 10 kHz SCS. In addition, a majority of PDN patients experienced neurological improvement with 10 kHz SCS, as did a notable subset of nondiabetic PPN patients.
Keywords: painful diabetic neuropathy, peripheral neuropathy, spinal cord stimulation, 10 kHz SCS, diabetes, neuropathic pain, systematic review
Introduction
Neuropathies are common neurological diseases, with an estimated prevalence of between 1% and 12% in all age groups and higher rates in older people.1 Etiologies are diverse; nevertheless, the unifying feature is damage to the peripheral nervous system, resulting in either motor, sensory or autonomic dysfunction.1 Common causes of peripheral neuropathies include Carpal tunnel syndrome, Bells’ palsy, diabetes mellitus, toxic exposure (eg, oncologic therapy), and hereditary polyneuropathy (eg, Charcot–Marie–Tooth disease).2 Sensory and/or motor nerves are the most often affected, with common neurological symptoms including burning pain, numbness, paresthesia, muscle weakness and atrophy, and gait abnormalities.1,3,4
Neuropathic pain is prevalent in peripheral neuropathy, affecting up to two-thirds of patients and severely impairing quality of life.4 Currently, oral pharmacotherapy is the mainstay of treatment, with gabapentinoids, serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants being the main recommended medications for symptom relief.5,6 However, these drugs generally have limited efficacy, and tolerability is often poor due to their systemic or centrally mediated effects.6–9 Other localized agents are also used in drug-refractory patients, including an 8% capsaicin patch recently approved by the FDA for the management of painful diabetic neuropathy (PDN).10 However, overall, many patients are left with unrelieved pain.6–11 Furthermore, the unmet need for efficacious treatments in patients with painful peripheral neuropathy (PPN) is set to increase, with the rising prevalence of diabetes a major contributing factor.
Spinal cord stimulation (SCS) is one of the current alternative treatment options for PPN patients who fail pharmacotherapy. During traditional low-frequency SCS (t-SCS), paresthesia is elicited over the painful area by applying electrical pulses to the dorsal column at a frequency between 40 Hz and 60 Hz. While the therapy is used to treat various neuropathic pain conditions, its efficacy is generally limited to approximately 50% of treated patients,12–15 and pain relief may diminish over time in those with initial treatment response.16–24
In an effort to overcome the limitations associated with t-SCS, several novel stimulation modalities and neural targets have been developed over the last decade, including high-frequency SCS at 10 kHz (10 kHz SCS), burst SCS (characterized by trains of 500 Hz pulses), and dorsal root ganglion stimulation (DRGS). In a randomized controlled trial (RCT), 10 kHz SCS showed superior pain relief over t-SCS in patients with chronic back and leg pain.14,25 In other RCTs, burst SCS demonstrated superiority over t-SCS in subjects with trunk and/or limb pain,26 and DRGS was superior to t-SCS in individuals with CRPS 1 or causalgia.27 During 10 kHz SCS, pain relief is provided without paresthesia, which may be a more comfortable therapy experience.14,25 Burst SCS and DRGS produce paresthesia symptoms in only a subset of patients,26,28 with both modalities requiring paresthesia mapping at implantation. In those who experience paresthesia with DRGS, the footprint is smaller and less intense than during t-SCS.29
Reviews of SCS in PPN have focused on the subpopulation with diabetes.30–37 However, growing evidence suggests that the therapy may provide meaningful pain relief in other PPN etiologies. Therefore, we conducted a comprehensive and systematic review of SCS across all PPN indications to explore and summarize the current state of the evidence. In addition, given the prevalence of sensorimotor symptoms in PPN patients, we also sought to highlight any evidence of neurological change after SCS.
Methods
This study was reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Supplementary Material).38
Eligibility
Articles were eligible for inclusion if they reported treatment outcomes from a prospective or retrospective study of SCS (or a related spinal stimulation technology) used to treat at least 3 human subjects with PPN of the lower limbs and/or lower extremities. Publications were excluded if (i) they were not peer-reviewed or had no full-text manuscript available (eg, conference proceedings), (ii) no original data were presented (eg, repeated data only, protocol or technical descriptions, commentaries, review articles), (iii) data were not reported separately for the population of interest, or (iv) data were presented for 2 or fewer human subjects (ie, case studies).
Search Strategy
We searched the PubMed electronic database from inception to February 7th, 2022, using a combination of MESH and free-text terms. The search strategy (Supplementary Material, Table S1) was designed to capture citations relating to the treatment of painful neuropathies with various SCS modalities, including dorsal column SCS, DRGS, spinal root stimulation, and nerve root stimulation.
Selection Process
A single reviewer (DRE) screened titles and abstracts to identify articles eligible for further review. Full-text manuscripts were subsequently obtained and assessed for compliance with the eligibility criteria.
Data Extraction and Outcomes
The same reviewer (DRE) extracted summary data from the eligible articles, with the primary outcomes of interest being pain intensity score and responder rate (proportion with ≥50% pain relief from baseline). Secondary outcomes of interest included neurological assessment outcomes, changes in function, and health-related quality of life (HR-QoL) improvement. Data were captured in an Excel spreadsheet to standardize group quantitative and qualitative outcomes. In addition, the reviewer retained relevant data from mixed population studies if the article reported results separately for a subgroup of interest.
Study Risk of Bias Assessment
A single reviewer (DRE) assessed the risk of bias for RCTs using the Cochrane Risk of Bias 2 (RoB 2) tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions.39 Several domains of potential bias were evaluated, including the randomization process, deviations from the intended intervention, missing outcome data, outcome measurements, and selective reporting. After a structured assessment, the reviewer graded each domain as low risk, high risk, or with some concerns. Nonrandomized studies were not assessed since they were considered inherently at risk of bias due to their observational nature.
Summary Measures
For each trial, we extracted or calculated (i) the percentage reduction in pain intensity from baseline and (ii) the proportion of patients who responded to treatment (ie, ≥50% pain relief from baseline). In addition, for a fair comparison across studies, we extracted or calculated the responder rate relative to the number of implanted patients with available data. We also retained the ITT results in RCTs if the authors reported between-group statistical significance. Data were grouped by indication in mixed indication studies if the subgroup comprised at least 3 patients.
Data Synthesis
Meta-analysis was not considered appropriate for the included studies due to the heterogeneous disease etiologies, interventions, and study methodologies. Therefore, we presented a description of the data in the text and a tabulated summary of pain measures and other outcomes. The narrative outline and tabulated summary grouped studies principally by etiology, SCS modality, and study type to help clinicians evaluate the various treatment options for specific patient groups. We also prepared a separate tabulated summary of neurological data to enable an overview by technology.
Results
Study Selection
The PubMed search strategy retrieved 2482 citations. Of these, 2434 were excluded based on title and abstract content. After a full-text assessment of the remaining 48 citations, 27 articles met the eligibility criteria (Figure 1),19,40–65 reporting the outcomes from 20 studies.
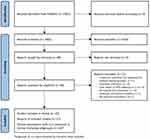 |
Figure 1 PRISMA flow diagram of study selection. Notes: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Creative Commons.75 Registration number: INPLASY202310004. DOI: 10.37766/inplasy2023.1.0004. |
Characteristics of Included Studies
Study characteristics (including extracted subgroups) are summarized in Table 1. The most common indication for SCS treatment was PDN, documented in approximately 88% of all implanted patients. The remaining patient populations had mixed PPN etiologies (predominantly nondiabetic).
 |
Table 1 Characteristics of Included Studies (Including Subgroups)a |
Fifteen of the 20 studies reported outcomes in the PDN indication.19,40–59 Of these, 4 studies evaluated treatment with 10 kHz SCS (including 1 RCT),40–44 9 with t-SCS (including 2 RCTs),19,45–57 1 with DRGS,58 and 1 with burst SCS.59 Among the 8 studies with mixed PPN populations,19,42,44,61–65 2 presented outcomes from 10 kHz SCS,44,60 3 from t-SCS,19,61,62 and 3 from DRGS.63–65
In total, 451 patients were implanted with a permanent SCS system. Of these, 10 kHz SCS systems were implanted in 267 (59%), t-SCS in 147 (33%), DRGS in 25 (6%), and burst SCS in 12 (3%). Follow-up duration for pain relief across the studies varied from the trial postoperative period to over 7 years. Most studies measured pain intensity using a visual analog scale (VAS; 0 to 10 cm/points or 0 to 100 mm/points) or numerical rating scale (NRS; 0 to 10 points).
Risk of Bias in Studies
A risk-of-bias assessment was completed for the included RCTs using the Cochrane Risk of Bias 2 (RoB 2) tool. Figure 2 summarizes the evaluation, with the full details available in Supplementary Material, Table S2.
 |
Figure 2 Risk of bias assessment using the Cochrane RoB-2 tool. Randomized controlled trials assessed: Peterson et al (2021),40 Slangen et al (2014),46 and de Vos et al (2014).51 |
We judged all 3 RCTs to have a low risk of bias for the randomization process (D1), deviations from the intended interventions (D2), and missing outcome data (D3). In the D3 domain, the reasons for the “low risk” categorizations varied. In the Slangen RCT, there was an imbalance in the levels of missing data between the groups; however, the levels of missing data were low, and the analysis classified missing subjects as nonresponders.46 The study by de Vos et al also used an ITT analysis approach, with a low and balanced level of missing data.45 Finally, in the Petersen RCT, the level of missing data was imbalanced between the groups but generally low.40 We also noted in this study that 8 individuals with a successful trial (ie, pain reduction ≥50%) dropped out of the study. Further examination of the individual patient data suggested that the missingness of the data was probably not attributable to the failure of pain relief since all were responders to therapy, leading to a low risk of bias judgment for D3.
In the D4 domain, the use of a patient-reported outcome (PRO) in an unblinded study (ie, the assessor was aware of the treatment allocation) led to a high risk of bias judgment for all 3 RCTs. Using such validated pain scales (eg, VAS and NRS) is standard in SCS studies. The need for an implanted system and induced paresthesia during t-SCS also means that SCS RCTs are typically open-label. During the unblinded RCTs, patient expectations could have influenced the pain-related PRO; for example, patients may have expected a benefit from SCS stimulation or have perceived conventional medical management (CMM) as inadequate prior to enrollment prompting selection bias. Participants in all 3 studies also knew they could cross to the stimulation treatment arm after 6 months if their pain relief was inadequate.
Some concern about potential bias arose in the fifth domain (D5) in the Slangen and de Vos studies since neither published a protocol nor a statistical analysis plan (SAP).45,46 In contrast, a protocol summary and SAP were available for the Petersen RCT.66,67 After examining these documents, we judged the study to be at low risk of selective data reporting.
The overall risk of bias was deemed high for each RCT due to the high risk of bias rating in D4 arising from the use of a PRO in the absence of patient blinding.
Outcomes in Painful Diabetic Neuropathy
For studies that met the search eligibility criteria, study outcomes are summarized in Table 2. Of these studies, 10 specifically included neurological outcomes, which are summarized in Table 3.
 |
Table 2 Summary of Outcomes |
 |
Table 3 Neurological Outcomes Summary |
High-Frequency 10 kHz SCS
Four studies included in the review reported outcomes from PDN patients treated with high-frequency 10 kHz SCS,40–44 including 1 RCT,40,41 1 prospective study,42 and 2 retrospective reviews.43,44
Randomized Controlled Trials
The largest of the 4 studies was an open-label, multicenter RCT conducted by Petersen et al.40 The study compared the safety and effectiveness of CMM with and without adjunctive 10 kHz SCS during a 6-month randomized phase. Participants had (i) PDN for a minimum of 12 months that was refractory to gabapentin or pregabalin and at least 1 other class of analgesic and (ii) a lower limb pain intensity of ≥5 cm on the VAS (0–10 cm scale). Of the 216 randomized individuals, 113 were allocated to 10 kHz SCS plus CMM and 103 to CMM alone. In the 10 kHz SCS group, 104 participants underwent a stimulation trial procedure, and 90 received permanent systems. Subjects could cross over to the other treatment arm at 6 months if pain relief was inadequate, the current treatment was dissatisfactory, and the investigator agreed it was appropriate.
Measurements of participants’ pain on the VAS showed that significantly more 10 kHz SCS than CMM subjects met the composite primary endpoint of ≥50% pain relief without neurological deterioration at 3 months (10 kHz SCS: 79%, 75 of 95; CMM: 5%, 5 of 94; p<0.001). At 6 months, the responder rate (≥50% pain relief) also favored the stimulation group (85% vs 5%; p<0.001). In addition, stimulation-treated subjects had improved HR-QoL on several scales (EQ-5D, EuroQol 5-Dimension Questionnaire; GAF, Global Assessment of Functioning; PSQ-3, Pain and Sleep Questionnaire 3-item index). Pain outcomes in all 4 components of the SF-MPQ-2 (Short Form-McGill Pain Questionnaire-2) questionnaire—ie, continuous pain, intermittent pain, neuropathic pain, and affective pain—also benefited from treatment, especially affective pain.
The investigators also evaluated neurological status at baseline and follow-up visits, including sensory, motor, and reflexes testing. After 6 months of treatment, significantly more individuals treated with 10 kHz SCS were assessed as having an improvement over baseline in at least 1 neurological function category without worsening in any other (62% vs 3%; p<0.001), with most improvements observed in sensory function. The investigators noted a meaningful neurological deficit at 6 months versus baseline in 19% of the CMM group (17 of 92) and 6% of the 10 kHz SCS group (5 of 84).
At the end of the 6-month randomized phase, no participants treated with 10 kHz SCS crossed over to the CMM alone arm. In contrast, 81% (77 of 95) of the CMM cohort crossed to the stimulation group, resulting in a further 64 permanent implantations.41 Both groups subsequently had significantly improved pain relief over their pre-implant value at the 12-month assessment, with at least 70% mean pain relief (p<0.001) and a responder rate exceeding 80%. In addition, the investigators noted neurological improvement (particularly in sensory function) at the 12-month follow-up in 68% (52 of 76) of those initially treated with 10 kHz SCS and in 62% (32 of 52) of the CMM-to-SCS crossover cohort.
Prospective Studies
A smaller, prospective cohort study by Galan et al evaluated the use of 10 kHz SCS to treat various types of peripheral polyneuropathy.60 Among the 26 subjects, 9 had PDN of the lower limbs with pain refractory to conservative treatment and a VAS pain score ≥5 cm (0–10 cm scale). Eight of these subjects received permanent systems after a successful trial. A published post hoc analysis of the PDN subgroup documented 74% pain relief at the 12-month assessment and a responder rate of 88% (6 of 7).42
The investigators also conducted sensory, motor, and reflexes testing throughout the study using the same battery of tests as the previous Petersen RCT. The 12-month tests indicated improvements over baseline in at least 1 neurological function category without worsening in any other in 71% (5 of 7) of the cohort. At the same time, the remaining participants maintained their neurological status (ie, none worsened in any category relative to baseline). All postimplantation neurological improvements were observed during the sensory and reflexes tests. In parallel, participants had improved mean disability (Pain Disability Index, PDI), functioning (GAF), and sleep (PSQ-3) scores at 12 months. In addition, pain outcomes assessed with the SF-MPQ-2 showed that all 4 pain descriptors benefited from treatment.
Retrospective Studies
Chen et al real-world, retrospective review assessed pain relief for 89 consecutive PDN patients treated with permanent 10 kHz SCS after achieving at least 50% pain relief during their trial phase.43 The investigators used data extracted from a commercial database, including patient-reported percentage pain relief (PRPPR). After a mean follow-up of 22 months, the average PRPPR was 61%, and 79% of the group (58 of 73) were responders (≥50% pain relief). In addition, over three-quarters of the participants reported general improvements in sleep and function.
Another retrospective case series by Sills examined 10 kHz SCS outcomes in patients with various etiologies of PPN, including 3 individuals with PDN of their lower limbs and/or extremities.44 All patients were implanted with a permanent system after a successful trial. After a mean follow-up of 33 months, the average NRS pain score among the PDN subgroup decreased by 74%, and 2 of the 3 individuals had at least 50% pain relief. In addition, all 3 patients had a general improvement in sensation, with anecdotal reports of improved function.
Traditional SCS (t-SCS)
Among the studies that documented the use of t-SCS in PDN subjects, 2 open-label, multicenter RCTs compared CMM (also known as best medical treatment) with and without adjunctive t-SCS.45,46 A further 5 prospective studies49–56 and 2 retrospective reviews19,57 also observed the outcomes of t-SCS treatment.
Randomized Controlled Trials
In the larger RCT by de Vos et al, 40 individuals were assigned to CMM with adjunctive t-SCS and 20 to CMM alone.45 Participants were refractory to conservative treatments and had an average VAS pain score ≥50 points (0–100 points scale), with upper limb neuropathic pain ≤20 points at rest. Subjects with inadequate improvement could cross over to the other treatment arm after 6 months. Of the 40 individuals assigned to t-SCS, 37 were converted to a permanent system after a successful trial; however, one individual dropped out early after choosing to enter another study.
In the ITT analysis with last observation carried forward (LOCF), subjects in the t-SCS group reported a 55% decrease in average pain score after 6 months of treatment compared with no pain relief in the CMM group (p<0.001). Furthermore, 60% of the stimulation-treated subjects (25 of 40) in this analysis had ≥50% pain relief compared with only 5% of the control group (1 of 20) (p<0.001). Relative to the number of implanted subjects with available data, the t-SCS responder rate was 69% (25 of 36). In addition, investigators observed significantly improved HR-QoL and pain characteristics among the t-SCS group on various scales (EQ-5D; MPQ-QoL, McGill Pain Questionnaire-Quality of Life; MPQ, McGill Pain Questionnaire pain quality). Neurological outcomes were not reported.
In the other RCT by Slangen et al, 22 individuals were assigned to CMM with adjunctive t-SCS and 14 to CMM alone.46 Participants were refractory or intolerant to conventional drug treatments and had an average NRS score ≥5 points (0–10 points scale), with minimal or no upper limb neuropathic pain. Seventeen of the t-SCS subjects had a successful trial phase and proceeded to permanent implantation. After 6 months of treatment, the ITT analysis found a 44% decrease in average daytime pain score among participants treated with t-SCS compared with no change in the control group (p<0.001), with corresponding reductions during the night of 38% and 12% (p<0.003). In addition, the responder rate (≥50% pain relief) during both day and night also favored t-SCS (day: 41% [9 of 22] vs 0% [0 of 14], p<0.001; night: 36% [8 of 22] vs 7%, [1 of 14], p<0.01). Our recalculation of the responder rate relative to the number of implanted subjects with available data yielded day and night time proportions of 56% (9 of 16) and 50% (8 of 16), respectively. However, we could not calculate an overall responder rate since the study report did not clarify if there was an overlap between day and night-time responders. The investigators noted significantly improved neuropathic pain characteristics (McGill NPS, McGill Neuropathic Pain Scale) in the t-SCS recipients compared to the CMM group. However, the change in HRQoL (EQ-5D) did not differ between the groups.
After 2 years of treatment, the subjects initially implanted with t-SCS had significantly reduced average pain from baseline during both the day (45%, p<0.001) and night (48%, p<0.001), with respective day and night responder rates of 53% (8 of 15) and 40% (6 of 15).48 Significant improvements were also observed in HR-QoL and neuropathic pain measures (modified BPI; MOS SF-36 PCS, Medical Outcomes Study Short Form-36 Physical Component Scale; MOS sleep scale; McGill NPS). The authors did not report any neurological outcomes.
In a later publication, the investigators reported 5-year follow-up data from all RCT participants that completed the 6-month follow-up (n=33)46 combined with participants from a pilot study by Pluijms et al (n=15).52 Among the 48 subjects, 40 had a successful trial phase and were converted to permanent systems. At the 5-year follow-up, data were available from 55% of the implanted cohort (22 of 40). These individuals had average pain relief of 36% (p≤.05) during the day and 31% during the night (p≤.05), with corresponding responder rates of 36% (8 of 22) and 32% (7 of 22). As per the earlier study publications, it was impossible to infer an overall responder rate.
Prospective Studies
In the earliest prospective observational evaluation of t-SCS in the PDN indication, Tesfaye et al analyzed outcomes in 10 subjects with severe PDN, unresponsive to conventional drugs.49 After a trial of both active and sham stimulation, 8 participants received a permanent system. Fourteen months later, the investigators reported significantly reduced background and peak pain VAS scores for stimulation on versus off (p=0.06 and p=0.03, respectively). Our examination of the 14-month data showed that 29% of participants (2 of 7) had ≥50% background pain relief from baseline, while 71% (5 of 7) had ≥50% peak pain relief from baseline. In a later report that analyzed data at 7.5 years postimplantation, the difference between pain scores for stimulation on versus off did not reach statistical significance (p=0.06). However, 2 of the 4 individuals had ≥50% background pain relief from baseline. Pain-related disability scores also markedly improved in all 4 subjects on the PDI.50
The investigators also conducted several neurological function tests at baseline, 3 months, and 6 months postimplantation, including (i) vibration perception-threshold (VPT) over the index fingers, great toes, and medial malleoli; (ii) sensory (median, ulnar, superficial peroneal, and sural) nerve conduction velocities; and (iii) motor (median, ulnar, peroneal, and tibial) nerve conduction velocities. The tests revealed no changes in any of these measures at either postimplantation assessment. In contrast, other outcomes measured at 6 months showed a significant improvement, including exercise threshold (vs baseline) and all 4 MPQ pain characteristics (on vs off).
Later studies by de Vos et al and Pluijms et al also examined t-SCS outcomes in treatment-refractory PDN subjects.51,52 In the de Vos study, 11 participants underwent a stimulation trial. Of these, 9 received a permanent system.51 After 30 months of treatment, the average VAS pain score decreased from baseline by 71%, and 75% of subjects (6 of 8) were responders to therapy (≥50% pain relief).
Among the 15 trialed participants in the Pluijms study, 11 met the criteria for trial success and received a permanent system.52 At 12 months postimplantation, median day and night NRS pain scores had significantly decreased by 60% (p<0.001) and 47% (p<0.01), respectively. Among the implanted subjects, 64% (7 of 11) responded to therapy (≥50% pain relief) during the day and 27% (3 of 11) during the night. The investigators also documented significant improvements in neuropathic pain characteristics (NPS, Neuropathic Pain Scale) and HR-QoL (SF-36 PCS; quality of sleep NRS). In addition, 36-month follow-up data published by Slangen et al demonstrated sustained daytime pain relief (62%), and 64% (7 of 11) of the implanted group were still responders. The authors also noted improved HR-QoL in 64% of the cohort (EQ-5D).53
During the Pluijms study, investigators also assessed small fiber nerve function at baseline and the end of the 2-week trial using contact heat evoked potentials (CHEPs).54 The test results showed no significant differences in upper limb N2 latency, P2 latency, or N2–P2 amplitude, except for a significantly shorter P2 latency on the dorsal forearm (p=0.002). In addition, the correlation between the CHEP measurements was high.
In another mixed indication prospective study, Denisova et al provided subgroup outcome data from 4 PDN subjects treated with t-SCS.55 After 12 months of stimulation, the investigators noted a reduction in VAS pain score of approximately 5 points (0–10 points scale).
Finally, Zhou and Bao (2021) evaluated t-SCS in individuals with diabetic foot syndrome (DFS).56 All 19 recruited subjects had foot lesions of grades 1 to 4 on the Meggitt–Wagner 0 to 5 classification system. While the authors did not confirm a neuropathy diagnosis, the vascular disease exclusion criterion implied a PDN etiology. After the activation of trial stimulation, the investigators noted a 74% decrease from baseline in the subjects’ average VAS pain score (p<0.05). They also observed a significant improvement in both sensory (right phoebra, left superficial peroneal, and right sural) and motor (common peroneal and tibial) nerve conduction velocities. In addition, after 6 months of permanent stimulation, the cohort’s HR-QoL significantly improved (Quality of Life-Liver Cancer v2.0).
Retrospective Studies
Two retrospective reviews by Kumar et al published t-SCS outcomes from predominantly PDN subgroups.19,57 In the earlier study, a group of 5 neuropathy patients comprised 4 with PDN and 1 with idiopathic neuropathy.57 After a successful trial phase, all 5 patients received a permanent t-SCS system and were followed for a minimum of 36 months. At their last follow-up, 4 of the 5 patients (80%) reported at least 50% pain relief (VAS), including 3 PDN patients.
The investigators subsequently published outcomes from 17 individuals with PDN in a review spanning 22 years, with a minimum follow-up of 6 months.19 The cohort may include PDN patients from the 1996 dataset. However, given the long period between the reports, we retained the 2006 cohort separately. Of the 17 patients, 14 (82%) were converted to a permanent system after a successful trial. According to a modified VAS, 12 implanted patients (86%) reported ≥50% pain relief at their last assessment.
Dorsal Root Ganglion Stimulation (DRGS)
One study in our review by Eldabe et al retrospectively documented the outcomes of DRGS in PDN patients.58 The cohort comprised 10 individuals refractory to drug treatment and/or previous SCS. Of these, 7 received a permanent implant after a successful trial or intraoperative test; however, 2 patients were explanted within a week because of poor efficacy or personal reasons. After 12 months of treatment, 4 patients with available data reported a 64% reduction in average VAS pain score (p<0.001), and 2 of the 4 (50%) experienced at least 50% pain relief.
Burst SCS
One prospective study in our review tested burst SCS in postlaminectomy syndrome and PDN patients over 2 weeks in a single-arm study.59 All participants had at least 6 months of prior treatment with t-SCS before being reprogrammed with burst SCS for the 2-week test period. Among the 12 PDN participants, burst SCS reduced the average VAS pain score from the mean t-SCS value by 43% (p<0.05). In total, 8 of the 12 subjects (67%) reported additional pain reduction from t-SCS during the burst SCS test. Compared with the preimplant average pain score, burst SCS provided 77% pain relief (p<0.001).
Outcomes in Other Painful Peripheral Neuropathies and Mixed Etiology Populations
High-Frequency 10 kHz SCS
Two studies in our review reported outcomes from mixed etiology PPN patients treated with high-frequency 10 kHz SCS, including 1 prospective cohort study60 and 1 retrospective case series.44
Prospective Studies
As described above, Galan et al evaluated 10 kHz SCS treatment in 26 subjects with various types of PPN.60 Among the cohort, 9 had PDN, while the remaining 17 had other forms of PPN, most commonly idiopathic in etiology and present in the lower limbs (94%, 16 of 17). Of the 17 participants with PPN, 10 had a successful trial and underwent permanent implantation. After 12 months of treatment, 56% (5 of 9) were responders (≥50% pain relief). The investigators also observed that almost half of the cohort (3 of 8) had improved over baseline in at least 1 neurological function category without worsening in any other. While improvements were predominantly in the sensory domain (75%, 3 of 4), 1 participant experienced increased motor function. None of the 3 neurological deficits recorded throughout the study (1 sensory, 2 motor) were stimulation-induced.
Retrospective Studies
In Sills’ retrospective case series (discussed previously), the nondiabetic PPN population comprised 2 individuals with iPN and 1 with CIDP, all of whom had bilateral lower extremity pain.44 Of the 3 patients, 2 received a permanent 10 kHz SCS system after reporting pain relief ≥50% during the trial phase, and 1 proceeded to permanent implantation after experiencing a 33% reduction in pain during the trial.44 After a mean follow-up of 26.3 months, patients’ average NRS pain score decreased by 42%. In addition, 1 patient (CIDP) had ≥50% pain relief, 1 individual (iPN) had improved sensation, and all patients reported generally improved function.
Traditional SCS (t-SCS)
Among the studies included in our review, 3 retrospective reviews evaluated the use of t-SCS to treat PPN.19,61,62 In the most recent study, Abd-Elsayed et al presented outcomes from 3 PPN patients with bilateral lower extremity pain treated with t-SCS.61 Among these were diagnoses of PDN, HIV-induced peripheral neuropathy, and chemotherapy-induced neuropathy (CIN). At the end of the stimulation trial, patients’ average VAS pain score was 85% lower than baseline, and all individuals reported at least 50% pain relief. Of the 3 patients, 2 proceeded to permanent implantation and continued to benefit at long-term follow-up. Both individuals also reported general improvements in their function and sleep. The remaining patient postponed permanent implantation for other health reasons.
In their long-spanning 2006 retrospective review (previously discussed), Kumar et al presented outcomes from a subgroup of 19 patients with lower extremity pain secondary to multiple sclerosis.19 Of these, 17 (89%) had a successful stimulation trial and were converted to a permanent system. At their last follow-up, 15 of the 17 implanted patients (88%) reported a reduction in pain score of at least 50%, according to a modified VAS.
In the last of the 3 retrospective reviews, Devulder et al included 3 patients with polyneuropathy treated with t-SCS.62 The authors reported good pain relief in 2 to 3 patients, while little relief was noted in 0 to 1.
Dorsal Root Ganglion Stimulation (DRGS)
Three studies in our review reported outcomes from mixed etiology PPN patients treated with DRGS, including 1 prospective cohort study63 and 2 retrospective reviews.64,65
Prospective Studies
In the only prospective evaluation, Koetsier et al recruited 9 individuals with painful polyneuropathy in the lower limbs.63 Subjects were refractory to conventional drug treatment and had a pain intensity of ≥5 points on the NRS (0–10 points scale). Etiologies included diabetes (n=3), iPN (n=3), CIN (n=1), and CIDP (n=1). Of the 9 participants, 8 had a successful stimulation trial, and 7 received a permanent system. At 6 months postimplantation, the subjects had significantly decreased median pain during the day (57%, p=0.031) and night (81%, p=0.036); this was also true for median peak pain (56%, p=0.035). The interference of pain with general functioning, measured using the BPI, also significantly decreased. However, the investigators observed no significant change in other HR-QoL and mood measures.
Retrospective Studies
In the most recent of the 2 retrospective reviews, Ho et al analyzed DRGS outcomes from 4 patients with pharmacologic-refractory axonal polyneuropathy, primarily in their feet.64 The etiology was hereditary in 1 individual, suspected as hereditary in another, and idiopathic in the remaining 2 patients. Three of the 4 patients had a successful trial phase and proceeded to permanent implantation. After 6 months of treatment, patients’ average VAS pain score had decreased by 74% (p=0.026), and all 3 patients had at least 50% pain relief.
In the final study included in our review, Falowski et al examined DRGS outcomes in 8 patients with peripheral neuropathy in their lower extremities.65 All individuals had failed conventional treatment before their DRGS trial and permanent implantation. Among the patients, 5 had sensory polyneuropathy, 2 had PDN, and 1 had chronic L5 radiculopathy. After 6 weeks of treatment, the investigators reported an 80% (p<0.001) reduction in the average VAS pain score across the group.
Discussion
Peripheral neuropathy represents a group of disorders with many and varied etiologies, with diabetes being a common cause. The painful symptoms associated with the condition are often inadequately treated. Alternative treatment options, such as SCS, are required to address the large and growing need for effective pain relief in this patient group.
Our literature search identified a substantial body of literature (20 studies) that presented evaluations of SCS to treat the painful lower limb and/or lower extremity symptoms associated with peripheral neuropathy. Of these, 3 were RCTs with 6-month randomized phases,40,45,46 and the remainder were observational studies. Among the studies, stimulation modalities included 10 kHz SCS, t-SCS, DRGS, and burst SCS.
Interpretation of Results
Pain Relief
Based on the minimum clinically important difference of at least a 2-point or a 30% reduction in pain,68 our analysis found (when available) clinically meaningful pain relief (range: 31–81%) at the last assessment (range: 0.5–90 months) after permanent implantation, regardless of stimulation modality.41–45,48,50,51,53,58,59,63–65 In addition, the responder rate (when available) was at least 50%19,41–45,50,51,53,57,58,60,63–65 in all but 2 studies.44,48 These are important findings, given the current and growing need for efficacious treatments in patients with drug-refractory PPN.
Fifteen studies in our review documented outcomes in PDN,19,40–59 including 1 RCT that evaluated 10 kHz SCS40 and 2 that investigated t-SCS.45,46 Results from all 3 RCTs supported the efficacy of SCS in this indication during 6-month randomized periods. However, 10 kHz SCS was associated with markedly higher pain relief than t-SCS (76% vs 38–55%). In addition, the proportion of responders to 10 kHz SCS at 6 months exceeded that of t-SCS (85% vs 50–69%). Extended RCT follow-up to 12 months also underscored this differential response to therapy (86% vs 38–56%).41,47 The more robust pain relief observed in the 10 kHz SCS RCT compared to the t-SCS studies is consistent with the results of an RCT that directly compared the 2 modalities in patients with chronic back and leg pain. In this study, 10 kHz SCS was found to provide statistically superior pain relief to t-SCS over 24 months.14,25
Study data in our review also suggested that pain relief from t-SCS may diminish over time in PDN patients.47 For example, in one study cohort, daytime pain relief decreased from 59% to 44% between 3 and 12 months of follow-up, with reduced pain relief persisting at 24 months (45%).47 A similar pattern of reduction occurred in nocturnal pain relief. Such diminishing pain relief (often referred to as therapy “tolerance” or “habituation”) has also been observed in other pain indications after t-SCS16–24 and is the most common reason for explantation.16 In contrast, 12-month RCT follow-up of PDN patients demonstrated sustained pain relief with 10 kHz SCS and replicated outcomes in crossover patients.41 In addition, multiple observational studies of at least 12 months duration support these results.42–44 Experience with 10 kHz SCS in other chronic pain etiologies also indicates that pain relief is durable after several years.25,69,70 Our review also showed promising outcomes in PDN patients with DRGS and burst SCS.58,59 However, the very small sample sizes in both studies (n<10), the lack of control groups, and the very short follow-up period in the burst SCS test (2 weeks) significantly limited the clinical significance of the findings.
During our review, we observed a recent interest in using newer SCS modalities to treat other forms of PPN, including idiopathic, chemotherapy-induced, radiation-induced, and hereditary types. Pain relief results from 10 kHz SCS and DRGS (when available) were promising in the mixed etiology populations, with a similar range of pain relief from baseline (42-81%)44,63–65 to PDN (61–77%).41–44,58 In another cohort with mixed etiology, burst stimulation resulted in additional pain relief after patients had been reprogrammed from t-SCS to burst stimulation for 2 weeks.59
While t-SCS remains a valuable option for treating PPN, the newer modalities may mitigate some of its limitations. For example, in contrast to t-SCS, 10 kHz SCS provides pain relief without paresthesia, which may be a more comfortable and tolerable option for patients. This factor may be especially useful in PPN patients with nerve damage that manifests as paresthesia. In addition, DRGS provides higher paresthesia concordance in the feet than t-SCS, with reduced stimulation sensation in nonpainful areas.27,71 The specificity of DRGS paresthesia coverage may be beneficial in PPN patients with predominant pain in the lower extremities and feet.
Neurological Outcomes
Among the 10 kHz SCS studies in our review, we observed that many patients experienced clinically meaningful neurological improvement, an important finding not yet observed with other modalities. In the RCT by Petersen et al,40,41 the results indicated potentially disease-modifying neurological improvements over 6 to 12 months in almost two-thirds of individuals (66%, 84 of 128), with most improvements observed in sensory function. The 12-month prospective study by Galan et al substantiated this outcome, with 71% (5 of 7) of PDN participants exhibiting neurological improvement in sensory and/or reflexes testing.42,60 Over a third of the remaining PPN subjects (37.5%, 3 of 8) also had improved neurological status, mainly in sensory function. In Sill’s retrospective evaluation of 10 kHz SCS in a mixed PPN group, 4 of the 5 patients with available data self-reported general sensory improvement after 10 kHz SCS, 3 of whom had PDN and 1 of whom had iPN.44
Only 2 other studies in our review provided neurological outcomes. In these studies, the investigators conducted sensory and motor nerve conduction velocity tests before and after t-SCS,49,56 with conflicting results. One study reported no change in PDN patients,49 while the other found significant improvements in a PDN (DFS) cohort.56 Unfortunately, the investigators did not measure clinical sensory or motor characteristics; such complementary data may have provided insight into whether nerve function changes were clinically meaningful.
Strengths and Limitations of the Review
This study is a comprehensive and systematic review of SCS that reports state-of-the-art research, The reviewer carried out the searches in a structured manner without time limitations to ensure the capture of both older and newer SCS modalities. In addition, the eligible studies were conducted in multiple countries and institutions and included several RCTs. Finally, while most reviews of SCS in this disease area limit their scope to the diabetic population, our study addressed the broader peripheral neuropathy indications.
As with all studies, our results should be interpreted with some caution, given the limitations of our review. Firstly, we restricted our search to the PubMed database, which means we may have missed some citations that are only indexed in other databases, including trials with negative findings that were not published. In addition, we noted substantial heterogeneity between studies in terms of study designs, interventions, and populations, complicating data synthesis and precluding meta-analysis. In addition, the articles presented responder rate data in various ways.72 To provide a more consistent overview of the data, we attempted to standardize responder rates relative to the number of implanted patients with available data, which may have resulted in minor inaccuracies in our presented ranges. In addition, our responder rate measure deviated from the study-defined primary endpoint (PE) in several trials. For example, the Slangen RCT had a composite PE in which patients could achieve treatment success if they met 1 of 4 criteria (day or night pain relief ≥50% or Patient Global Impression of Change pain or sleep score ≥6). The Petersen RCT also had a composite PE; however, this was narrower and comprised only a single pain threshold measure (≥50% pain relief) combined with no observed neurological deterioration. Our use of a different endpoint to the original study analyses may have introduced bias.
The studies included in our review also had shortcomings that limited the clinical significance of their findings. Principally, the RCTs did not include a sham control arm, making it impossible to blind participants or personnel to the treatment allocation. Consequently, we cannot rule out a meaningful placebo effect; this also applies to the remaining observational studies in our review, which have an inherent risk of bias due to their open-label design. In addition, some studies in our review may be particularly susceptible to a placebo effect due to their lack of a control group or short-term nature (eg, the 2-week burst SCS test59 and 6-week retrospective DRGS review65). However, in some studies, longer-term follow-up durations of at least 12 months may mitigate placebo-effect concerns if the attrition rate over time is low. The t-SCS PDN RCTs also had asymmetric allocation ratios that resulted in a low sample size for the control group in each study, creating uncertainty in the t-SCS treatment effect relative to control.72
We noted in our review that clinical neurological assessments have recently been introduced in 10 kHz SCS studies; this is a welcome step towards a more holistic approach to SCS therapy, especially in a patient population with sensorimotor issues. However, the tests used in the prospective 10 kHz SCS studies relied on clinical examination and judgment, factors that should be considered when interpreting their results.40,42,60 That said, independent neurologists developed the standardized neurological examinations in collaboration with the US Food and Drug Administration for a previous RCT.40
Interestingly, several prospective t-SCS studies incorporated quantitative neurological measurements, including sensory and motor nerve conduction velocities. However, despite statistically significant effects in one study, we gained little or no insight into the clinical meaning of the outcomes.56
Finally, it should be remembered that all studies in our review performed SCS evaluations in patients with lower limb and/or lower extremity pain. Therefore, our results cannot be generalized to patients with neuropathy symptoms in their upper limbs/extremities.
Implications for Practice, Policy, and Future Research
The results of our review suggest that SCS is a valuable treatment option in patients with lower limb and/or lower extremity pain symptoms associated with PPN. High-level evidence exists for 10 kHz SCS and t-SCS treatment in diabetic patients, supported by observational studies. Outcomes from pilot studies conducted in primarily nondiabetic PPN patients were also promising. In particular, the potential for beneficial neurological effects after 10 kHz SCS deserves attention, especially in patients with sensorimotor problems. Considering the known complications associated with all types of SCS,73 pain specialists should consider the therapy after the failure of conservative medical management.
Additional RCTs and long-term, real-world studies performed in the PDN indication will strengthen the evidence base for SCS in this large patient group. Given the absence of controlled studies in other forms of PPN, implementing RCTs in this field is also important, with a careful selection of indication for use based on the existing pilot study data. Additional controlled studies that compare waveforms will also help identify the most appropriate SCS modality for individual etiologies.
In any future study, it would be advantageous to incorporate a range of outcome measures to help provide a broad and patient-centered view of treatment benefits. These measures could include, for example, HR-QoL, function, and medication use. Analyzing neurological change would also be helpful; ideally, using objective, quantitative, and validated measures that characterize whether changes are clinically meaningful to the patient and/or their caregivers. We should also bear in mind that neurological benefits could occur in the context of limited or no analgesic effect, requiring a shift away from viewing SCS success only through the traditional lens of pain relief. In t-SCS studies, due consideration should be given to the challenge of assessing quantitative sensory changes in patients with overlapping stimulation- and disease-induced paresthesia. Future studies may also benefit from the inclusion of sensory profiling to determine how subgroups with various sensory characteristics respond to treatment.74
Conclusions
Our review found clinically meaningful pain relief after SCS treatment in patients with PPN of the lower limbs and/or lower extremities. High-level evidence supports the use of 10 kHz SCS and t-SCS in PDN patients, with more robust pain relief observed with 10 kHz SCS. The results in other PPN etiologies were also promising for 10 kHz SCS. Moreover, a majority of PDN patients experienced neurological improvement with 10 kHz SCS, as did a notable subset of nondiabetic PPN patients; this may be particularly beneficial in peripheral neuropathy patients with reduced sensation in their legs and/or feet. Additional RCTs and long-term, real-world studies will help confirm the effectiveness and durability of SCS in PPN patients.
Funding
Nevro Corp., Redwood City, CA, USA, funded this research.
Disclosure
ARB has received consulting fees from Nevro and research support from Nevro. JLC has received consulting fees from Vertos and research support from Nevro. CEA has received consulting fees from Nevro, Vertex, Amgen, Lundbeck, Collegium, Gene Pharma, Clexio Biosciences, Biohaven, Teva; personal fees from Averitas, Gruenenthal, Kowa, Neumentum, Tioga Research, Triss, Xgene; research support from Abbvie, Amgen, Lilly, Vertex and Teva. DRE received a fee from Nevro in her capacity as an independent medical writer. EAP has received consulting fees from Abbott Neuromodulation, Biotronik, Boston Scientific, Medtronic Neuromodulation, Nalu, Neuros Medical, Nevro, Presidio Medical, Saluda, and Vertos; research support from Mainstay, Medtronic Neuromodulation, Nalu, Neuros Medical, Nevro, ReNeuron, Saluda, and SPR; and stock options from neuro42 and SynerFus
References
1. Lehmann HC, Wunderlich G, Fink GR, Sommer C. Diagnosis of peripheral neuropathy. Neurol Res Pract. 2020;2(1):20.
2. Hanewinckel R, Ikram MA, Van Doorn PA. Peripheral neuropathies. Handb Clin Neurol. 2016;138:263–282.
3. Jensen TS, Karlsson P, Gylfadottir SS, et al. Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain. 2021;144(6):1632–1645.
4. Girach A, Julian TH, Varrassi G, Paladini A, Vadalouka A, Zis P. Quality of life in painful peripheral neuropathies: a systematic review. Pain Res Manag. 2019;2019:2091960.
5. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154.
6. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173.
7. Khdour MR. Treatment of diabetic peripheral neuropathy: a review. J Pharm Pharmacol. 2020;72(7):863–872.
8. Staudt MD, Prabhala T, Sheldon BL, et al. Current strategies for the management of painful diabetic neuropathy. J Diabetes Sci Technol. 2022;16(2):341–352.
9. Snyder MJ, Gibbs LM, Lindsay TJ. Treating painful diabetic peripheral neuropathy: an update. Am Fam Physician. 2016;94(3):227–234.
10. Arora V, Campbell JN, Chung MK. Fight fire with fire: neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol Ther. 2021;220:107743.
11. James CF, Tripathi S, Karampatou K, Gladston DV, Pappachan JM. Pharmacotherapy of painful diabetic neuropathy: a clinical update. Sisli Etfal Hastan Tip Bul. 2022;56(1):1–20.
12. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98–106; discussion 106–107.
13. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1–2):179–188.
14. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: the SENZA-RCT Randomized Controlled Trial. Anesthesiology. 2015;123(4):851–860.
15. Kemler MA, De Vet HC, Barendse GA, Van Den Wildenberg FA, Van Kleef M. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trial. Ann Neurol. 2004;55(1):13–18.
16. Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603–608; discussion 608–609.
17. Sears NC, Machado AG, Nagel SJ, et al. Long-term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation. 2011;14(4):312–318; discussion 318.
18. Kemler MA, de Vet HC, Barendse GA, van den Wildenberg FA, van Kleef M. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108(2):292–298.
19. Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58(3):481–496; discussion 481–496.
20. Alo KM, Redko V, Charnov J. Four year follow-up of dual electrode spinal cord stimulation for chronic pain. Neuromodulation. 2002;5(2):79–88.
21. Ohnmeiss DD, Rashbaum RF, Bogdanffy GM. Prospective outcome evaluation of spinal cord stimulation in patients with intractable leg pain. Spine. 1996;21(11):1344–1350; discussion 1351.
22. Burchiel KJ, Anderson VC, Brown FD, et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine. 1996;21(23):2786–2794.
23. Kupers RC, Van den Oever R, Van Houdenhove B, et al. Spinal cord stimulation in Belgium: a nation-wide survey on the incidence, indications and therapeutic efficacy by the health insurer. Pain. 1994;56(2):211–216.
24. North RB, Kidd DH, Zahurak M, James CS, Long DM. Spinal cord stimulation for chronic, intractable pain: experience over two decades. Neurosurgery. 1993;32(3):384–394; discussion 394–385.
25. Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79(5):667–677.
26. Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56–66.
27. Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669–681.
28. Mekhail N, Deer TR, Kramer J, et al. Paresthesia-free dorsal root ganglion stimulation: an accurate study sub-analysis. Neuromodulation. 2020;23(2):185–195.
29. Deer TR, Levy RM, Kramer J, et al. Comparison of paresthesia coverage of patient’s pain: dorsal root ganglion vs. spinal cord stimulation. An Accurate study sub-analysis. Neuromodulation. 2019;22(8):930–936.
30. Strand NH, Burkey AR. Neuromodulation in the treatment of painful diabetic neuropathy: a review of evidence for spinal cord stimulation. J Diabetes Sci Technol. 2022;16(2):332–340.
31. Raghu ALB, Parker T, Aziz TZ, et al. Invasive electrical neuromodulation for the treatment of painful diabetic neuropathy: systematic review and meta-analysis. Neuromodulation. 2021;24(1):13–21.
32. Henson JV, Varhabhatla NC, Bebic Z, et al. Spinal cord stimulation for painful diabetic peripheral neuropathy: a systematic review. Pain Ther. 2021;10(2):895–908.
33. Liampas A, Rekatsina M, Vadalouca A, Paladini A, Varrassi G, Zis P. Non-pharmacological management of painful peripheral neuropathies: a systematic review. Adv Ther. 2020;37(10):4096–4106.
34. Amato Nesbit S, Sharma R, Waldfogel JM, et al. Non-pharmacologic treatments for symptoms of diabetic peripheral neuropathy: a systematic review. Curr Med Res Opin. 2019;35(1):15–25.
35. Gupta M, Knezevic NN, Abd-Elsayed A, Ray M, Patel K, Chowdhury B. Treatment of painful diabetic neuropathy-A narrative review of pharmacological and interventional approaches. Biomedicines. 2021;9:5.
36. D’Souza RS, Langford B, Dombovy-Johnson M, Abd-Elsayed A. Neuromodulation interventions for the treatment of painful diabetic neuropathy: a systematic review. Curr Pain Headache Rep. 2022;26(5):365–377.
37. Pluijms WA, Slangen R, Joosten EA, et al. Electrical spinal cord stimulation in painful diabetic polyneuropathy, a systematic review on treatment efficacy and safety. Eur J Pain. 2011;15(8):783–788.
38. Christelis N, Simpson B, Russo M, et al. Persistent spinal pain syndrome: a proposal for failed back surgery syndrome and ICD-11. Pain Med. 2021;22(4):807–818.
39. Higgins JPT, Thomas J, Chandler J et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Available from: www.training.cochrane.org/handbook.
40. Petersen EA, Stauss TG, Scowcroft JA, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol. 2021;78(6):687–698.
41. Petersen EA, Stauss TG, Scowcroft JA, et al. Durability of high-frequency 10-kHz spinal cord stimulation for patients with painful diabetic neuropathy refractory to conventional treatments: 12-month results from a randomized controlled trial. Diabetes Care. 2022;45(1):e3–e6.
42. Galan V, Scowcroft J, Chang P, et al. 10-kHz spinal cord stimulation treatment for painful diabetic neuropathy: results from post-hoc analysis of the SENZA-PPN study. Pain Manag. 2020;10(5):291–300.
43. Chen JL, Hesseltine AW, Nashi SE, et al. A real-world analysis of high-frequency 10 kHz spinal cord stimulation for the treatment of painful diabetic peripheral neuropathy. J Diabetes Sci Technol. 2022;16(2):282–288.
44. Sills S. Treatment of painful polyneuropathies of diabetic and other origins with 10 kHz SCS: a case series. Postgrad Med. 2020;132(4):352–357.
45. de Vos CC, Meier K, Zaalberg PB, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 2014;155(11):2426–2431.
46. Slangen R, Schaper NC, Faber CG, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes Care. 2014;37(11):3016–3024.
47. van Beek M, Slangen R, Schaper NC, et al. Sustained treatment effect of spinal cord stimulation in painful diabetic peripheral neuropathy: 24-month follow-up of a prospective two-center randomized controlled trial. Diabetes Care. 2015;38(9):e132–134.
48. van Beek M, Geurts JW, Slangen R, et al. Severity of neuropathy is associated with long-term spinal cord stimulation outcome in painful diabetic peripheral neuropathy: five-year follow-up of a prospective two-center clinical trial. Diabetes Care. 2018;41(1):32–38.
49. Tesfaye S, Watt J, Benbow SJ, Pang KA, Miles J, MacFarlane IA. Electrical spinal-cord stimulation for painful diabetic peripheral neuropathy. Lancet. 1996;348(9043):1698–1701.
50. Daousi C, Benbow SJ, MacFarlane IA. Electrical spinal cord stimulation in the long-term treatment of chronic painful diabetic neuropathy. Diabetic Med. 2005;22(4):393–398.
51. de Vos CC, Rajan V, Steenbergen W, van der Aa HE, Buschman HP. Effect and safety of spinal cord stimulation for treatment of chronic pain caused by diabetic neuropathy. J Diabetes Complications. 2009;23(1):40–45.
52. Pluijms WA, Slangen R, Bakkers M, et al. Pain relief and quality-of-life improvement after spinal cord stimulation in painful diabetic polyneuropathy: a pilot study. Br J Anaesth. 2012;109(4):623–629.
53. Slangen R, Pluijms WA, Faber CG, Dirksen CD, Kessels AG, van Kleef M. Sustained effect of spinal cord stimulation on pain and quality of life in painful diabetic peripheral neuropathy. Br J Anaesth. 2013;111(6):1030–1031.
54. Pluijms WA, Slangen R, van Kleef M, Joosten EA, Reulen JP. Increased contact heat evoked potential stimulation latencies in responders to spinal cord stimulation for painful diabetic polyneuropathy. Neuromodulation. 2015;18(2):126–132; discussion 132.
55. Denisova NP, Rogov DY, Rzaev DA, Khabarova EA, Dmitriev AB. Spinal cord stimulation in the treatment of chronic pain syndromes. Zh Vopr Neirokhir Im N N Burdenko. 2016;80(2):47–52.
56. Zhou PB, Bao M. Clinical effect analysis of spinal cord electrical stimulator implantation for diabetic foot. Neuromodulation. 2021;2021:5.
57. Kumar K, Toth C, Nath RK. Spinal cord stimulation for chronic pain in peripheral neuropathy. Surg Neurol. 1996;46(4):363–369.
58. Eldabe S, Espinet A, Wahlstedt A, et al. Retrospective case series on the treatment of painful diabetic peripheral neuropathy with dorsal root ganglion stimulation. Neuromodulation. 2018;21(8):787–792.
59. de Vos CC, Bom MJ, Vanneste S, Lenders MW, de Ridder D. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation. 2014;17(2):152–159.
60. Galan V, Scowcroft J, Chang P, et al. Ten kHz spinal cord stimulation for the treatment of chronic peripheral polyneuropathy: 12-month results from prospective open-label pilot study. Pain Pract. 2021;21(8):898–906.
61. Abd-Elsayed A, Schiavoni N, Sachdeva H. Efficacy of spinal cord stimulators in treating peripheral neuropathy: a case series. J Clin Anesth. 2016;28:74–77.
62. Devulder J, De Colvenaer L, Rolly G, Caemaert J, Calliauw L, Martens F. Spinal cord stimulation in chronic pain therapy. Clin J Pain. 1990;6(1):51–56.
63. Koetsier E, van Kuijk SMJ, Melli G, et al. Dorsal root ganglion stimulation for the management of intractable painful polyneuropathy: a prospective pilot study. Neuromodulation. 2021;24(4):685–694.
64. Ho KWD, Rempe T, Jerath N, Antony A. Dorsal root ganglion stimulation as a potentially effective treatment for painful hereditary and idiopathic axonal polyneuropathy: a retrospective case series. Neuromodulation. 2020;23(2):234–238.
65. Falowski S, Pope JE, Early RA. US experience with stimulation of the dorsal root ganglia for the treatment of peripheral neuropathy in the lower extremities: a multicenter retrospective case series. Neuromodulation. 2019;22(1):96–100.
66. Mekhail NA, Argoff CE, Taylor RS, et al. High-frequency spinal cord stimulation at 10 kHz for the treatment of painful diabetic neuropathy: design of a multicenter, randomized controlled trial (SENZA-PDN). Trials. 2020;21(1):87.
67. Nevro Corp. CA2016-5 US SENZA-PDN-1 statistical analysis plan, version 3.0; 2019. Available from: https://clinicaltrials.gov/ProvidedDocs/20/NCT03228420/SAP_001.pdf.
68. Farrar JT, Young JP
69. Al-Kaisy A, Palmisani S, Smith TE, et al. Long-term improvements in chronic axial low back pain patients without previous spinal surgery: a cohort analysis of 10-kHz high-frequency spinal cord stimulation over 36 months. Pain Med. 2018;19(6):1219–1226.
70. Al-Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15(3):347–354.
71. Liem L, Russo M, Huygen FJ, et al. A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation. 2013;16(5):471–482; discussion 482.
72. Duarte RV, Nevitt S, Maden M, et al. Spinal cord stimulation for the management of painful diabetic neuropathy: a systematic review and meta-analysis of individual patient and aggregate data. Pain. 2021;162(11):2635–2643.
73. Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med. 2016;17(2):325–336.
74. Baron R, Maier C, Attal N, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158(2):261–272.
75. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
