Back to Journals » Psychology Research and Behavior Management » Volume 17
The Effectiveness of Cognitive Behavioral Therapy-Based Intervention on Improving Sleep-Related Outcomes for People with Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Authors Wang H, Li R, Ge L, Xu F, Wiley JA, Tang S, Sun M
Received 16 November 2023
Accepted for publication 14 February 2024
Published 9 March 2024 Volume 2024:17 Pages 957—972
DOI https://doi.org/10.2147/PRBM.S449577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gabriela Topa
Hongjuan Wang,1 Rongzhi Li,1 Lin Ge,2 Fan Xu,1 James Allen Wiley,3 Siyuan Tang,1 Mei Sun1,4
1Xiangya School of Nursing, Central South University, Changsha, Hunan Province, People’s Republic of China; 2Department of Alcohol Addiction and Internet Addiction, Brain Hospital of Hunan Province (The Second People’s Hospital of Hunan Province), Changsha, Hunan Province, People’s Republic of China; 3Department of Family and Community Medicine and Institute for Health Policy Research, University of California, San Francisco, CA, USA; 4School of Nursing, Changsha Medical University, Changsha, Hunan Province, People’s Republic of China
Correspondence: Siyuan Tang; Mei Sun, Xiangya School of Nursing, Central South University, Changsha, Hunan Province, 410013, People’s Republic of China, Email [email protected]; [email protected]
Background: Sleep-related outcomes in people with diabetes are poor, which is closely linked to reducing the development of diabetes. Cognitive behavioral therapy (CBT) based intervention presents innovative solutions that can help improve sleep-related outcomes.
Aim: This synthesis aims to assess the effectiveness of CBT-based intervention compared to controls in Randomized Controlled Trials (RCTs) for sleep-related outcomes among people with diabetes.
Methods: Eight electronic databases were systematically searched: PubMed, EMBASE, Cochrane library, Web of Science, PsycINFO, CINAHL, China National Knowledge Infrastructure (CNKI), and Wan Fang database. We examined CBT-based intervention’s effectiveness on sleep-related outcomes in people with diabetes in RCTs identified in these databases from their inception to 1st November 2023, and updated on 15 January 2024. The risk of bias was assessed using the Cochrane Risk of Bias tool by two reviewers. The meta-analysis of included studies was conducted by RevMan 5.3 software.
Results: Seven studies in total (n = 2633 participants) were included in this systematic review based on our inclusion criteria. The systematic review found CBT-based intervention significantly improved sleep quality (Pittsburgh Sleep Quality Index, PSQI scores) at immediate post-intervention [95% CI=(− 1.31 to -0.32), p = 0.001], six months [95% CI=(− 0.75 to − 0.22), p = 0.0003], and 12 months [95% CI=(− 0.72 to − 0.24), < 0.0001], compared to control groups. Furthermore, our findings demonstrated that six sessions [95% CI= (− 0.38 to − 0.13), p < 0.0001] or more than six sessions [95% CI=(− 1.76 to − 0.02), p = 0.05] of CBT-based intervention could improve sleep quality compared to controls (I2=0%). Interestingly, CBT-based intervention improves total sleep time at post-intervention in people with diabetes compared to the control group [95% CI= (− 0.57 to − 0.12), p = 0.003]. However, there was no significant that CBT-based intervention is beneficial to time to fall asleep [95% CI (− 1.89 to 0.43), p = 0.22] and sleep efficiency [95% CI (− 1.27 to 0.27), p = 0.20] after intervention, compared to control group.
Conclusion: CBT-based intervention appears to have a beneficial effect on improving sleep quality and total sleep time among people with diabetes. CBT-based intervention could be considered a strategy among healthcare providers to enhance sleep quality and total sleep time for people with diabetes. More RCTs with rigorous designs and long-term follow-up are warranted to provide conclusive evidence of the CBT-based intervention on sleep-related outcomes and to explore the mechanisms by which the CBT-based interventions improve sleep-related outcomes.
Keywords: diabetes, cognitive behavioral therapy, sleep, systematic review, meta-analysis
Introduction
World Health Organization reported that the number of people with diabetes is expected to increase to 642 million worldwide by 2030.1 According to the Centers for Disease Control and Prevention, an estimated 38.4 million people of all ages, which accounts for approximately 11.6% of the US population, were living with diabetes in 2021.2 The total estimated direct and indirect costs associated with diagnosed diabetes in the United States in 2022 amounted to a staggering $413 billion.2 An important but less-known risk factor for the development of diabetes is having a sleep disturbance.3,4
Sleep disturbance in people with diabetes is a complex symptom that refers to impaired sleep quality and/or abnormal sleep duration.5 Compared to other populations, people with Type 2 diabetes Mellitus (T2DM) might suffer from sleep disturbances due to diabetes symptoms such as frequency nocturnal urination, hyperglycemia, insulin resistance, which might influence the sleep variability.6 Evidence suggests that there is a bidirectional relationship between sleep disturbances and diabetes, implying sleep disturbances contribute to progression of diabetes.7,8 Several systematic reviews have highlighted the impact of sleep-related outcomes, eg, quantity and quality, on glycaemic control in people with diabetes.9,10 Optimal sleep outcomes among people with T2DM have been associated with a 3% reduction in deaths, a 2% reduction in myocardial infarction, and a 5% reduction in microvascular complications.9 Therefore, improving sleep-related outcomes might play an essential role in the prevention of diabetes progression and the improvement of health outcomes for people with diabetes.7
Few guidelines provided recommendations relating to assessment and management of sleep in people with diabetes.11 CBT-based interventions, including CBT and Cognitive Behavioral Therapy for Insomnia (CBT-I), aim to enhance sleep-related outcomes by targeting the underlying thoughts, behaviors, and emotions that contribute to sleep difficulties.12–15 Preliminary studies have demonstrated that CBT improves sleep-related outcomes in other chronic diseases, such as ovarian cancer and breast cancer,16–18 despite uncertainty about the pathways by which CBT addresses sleep disturbances. To date, there have been several RCTs investigating the impact of CBT-based intervention on sleep-related outcomes in individuals with diabetes. However, there is a lack of systematic reviews and meta-analyses that provide a comprehensive synthesis of the findings due to the limited number of participants and occasionally inconclusive results.
To address this gap, we performed a systematic review and meta-analysis of available evidence from RCTs to synthesize the effectiveness of CBT-based intervention compared to controls for sleep-related outcomes among people with diabetes. It is promising to aid in clinical decision-making and identify gaps where further research is needed.
Methods
Following the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions, we conducted this systematic review and meta-analysis.19 PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines were followed.20
Eligibility Criteria
Included original research met the following inclusion criteria: (1) RCT design (RCTs were included to reach a high-grade level of evidence); (2) Participants were over the age of 18 with diabetes; (3) A CBT-based intervention was performed; (4) Studies compared CBT-based intervention with usual care, waitlist control, health education; (5) Studies reported at least one outcome of sleep-related outcomes. Studies were excluded if (1) published articles are written not in English or Chinese; (2) CBT was not the primary intervention but only a component of a multimodal intervention; or were (3) conference papers, abstracts, book chapter reviews, letters and reviews.
Literature Search Strategy
Two reviewers searched six English databases (PubMed, EMBASE, Cochrane library, Web of Science, PsycINFO, CINAHL), and two Chinese databases (CNKI and WanFang) to identify relevant studies. We identified relevant RCTs from the databases from inceptions to 1st November 2023, and updated on 15 January 2024. Additionally, we conducted a thorough screening of the reference lists of included studies and reviewed the references from other relevant systematic reviews to identify potentially eligible RCTs. The search strategies employed a combination of Medical Subject Heading terms and keywords, with the following constructs: Diabetes, Cognitive Behavioral Therapy, Sleep, and Randomized Controlled Trials. The whole search strategy is provided in Appendix 1.
Study Selection
EndNote X9 Software was used to remove duplicate articles from search results. Two independent reviewers (H.J.W. and R.Z.L.) assessed all records for eligibility based on the titles and abstracts of studies during the first level screen. Disagreements about whether to include a paper were resolved through discussion. The second level screen was performed by two reviewers (H.J.W. and R.Z.L.) who independently assessed the full text of all relevant and potentially relevant articles to identify whether they met inclusion criteria. We removed articles for non-eligibility reasons, with detailed documentation. A third reviewer (S.Y.T.) was consulted to resolve disagreements between the two reviewers.
Data Collection Process and Data Extraction
The extraction of data was guided by an Excel template adapted from the Cochrane data extraction form.21 Two independent reviewers (H.J.W. and L.G.) extracted data independently, in order to minimize bias and prevent errors during data extraction.22 We extracted data from each included study, including author, publication year, study design, country, essential characteristics of participants (sample size, age, and distribution of groups), detail information about intervention (eg, type, duration, frequency, number of sessions, characteristics of interventionists, and setting), controls, outcomes (outcome indicators, measuring tools of the studies, follow-up time, attrition rate), adverse events and intention-to-treat analysis. When subjective and objective outcomes were reported in a study, we priority extracted objective outcomes for our review. For those reporting the same research in multiple articles, we will combine them into one research to summarize the intervention characteristics and data extraction. The discussion resolved the disagreement between the two reviewers regarding data extraction. Similarly, a third reviewer (M.S.) was consulted when no consensus was reached. The κ scores were calculated to estimate interrater reliability between reviewers, resulting in a good score of κ= 0.75.23 We contacted the original authors included in the study by email when the required data could not be extracted, or information was missing (up to three attempts).
Quality Assessment
Using the Cochrane Collaboration’s “risk of bias” tool for systematic reviews of interventions, two reviewers independently assessed the included studies (H.J.W. and R.Z.L.).21 There were seven items in this quality assessment tool to assess the potential for bias in trials: (1) Random sequence generation (how participants will be assigned to interventions is generated based on a process that includes an element of chance); (2) Allocation concealment (to prevent participants or trial personnel from knowing the forthcoming allocations until after recruitment has been confirmed); (3) Blinding of participants and personnel (to prevent participants or trial personnel from knowing the intervention contents); (4) Blinding of outcome assessment (to prevent the assessor from knowing the intervention details); (5) Incomplete outcome data (describe the integrity of outcome data for each primary outcome measure); (6) Selective reporting (the reported result is selected based on its direction, magnitude, or statistical significance); (7) Other bias (whether a pre-specified plan analyzed the trial). Each item was categorized as “low risk”, “unclear”, or “high risk” for bias. Each study was assessed for quality through a consensus between two reviewers or consultation with a third independent reviewer (M.S.).
Data Analysis
RevMan V.5.3 software was used to perform the meta-analysis. We performed a meta-analysis only when two or more intervention studies were available with similar participants and outcomes.24,25 When studies reported data at multiple follow-up timepoints, the post-intervention data was chosen for pooling to align with the other studies. All significance testing was 2-sided, and results were considered statistically significant if the P value was.05 or less. If heterogeneity is significant, random-effects models are used; otherwise, fixed-effects models are used.26 We quantified heterogeneity using I2 statistics. I2 < 25% indicates a low degree of heterogeneity, I2= 25–75% indicates moderate heterogeneity, and I2 >75% indicates a high degree of heterogeneity.27 A sensitivity analysis was conducted by removing one by one studies that had significantly contributed to the heterogeneity level when significant heterogeneity was detected. Additionally, a subgroup analysis was conducted to compare the effect of CBT-based interventions on sleep quality with different dosages and detect the source of heterogeneity. According to the recommendation in the Cochrane Handbook, Cohen’s criteria for effect size means that SMD = 0.20, 0.2 to 0.5, and more than 0.5 were considered to represent small, medium, and large effects, respectively.28 A narrative synthesis of the study findings was reported when included studies did not provide extractable outcome information (eg, means and SD) and studies for those only reported one outcome could not conduct meta-analysis.
Results
Search Process
Eight database searches yielded 6236 records: PubMed (n = 1170), Web of science (n = 2885), Cochrane Library (n = 305), PsycINFO (n = 42), EMBASE (n = 1627), CINAHL (n = 30), CNKI (n = 52) and Wan Fang (n = 125). Other sources of gray literature, and dissertations did not yield any additional eligible studies. A total of 3521 duplicates were removed from the database. Out of 2715 records remaining, 2615 were excluded after screening based on title and abstract screening. Two reviewers (H.J.W. and R.Z.L.) independently screened the full-text of the remaining 100 records retrieved. The following 93 studies were excluded from the review for the reasons listed in Figure 1. (see Appendix 2). Ultimately, seven studies were included in this review. Figure 1 illustrates the PRISMA Flowchart.
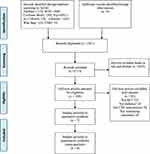 |
Figure 1 PRISMA flow diagram. |
Description of Included Studies
Three out of seven were multicenter RCT,29–31 the remaining four studies were single-center RCTs. Studies were conducted in China (n = 5, 71.4%),29–33 US (n =1, 14.3%)34 and Iran (n = 1, 14.3%). All studies were published between 2020 and 2023, three of the studies were published between 2022 and 2023, while four were published between 2020 and 2021.29–31,34 Five studies were published in English journals29,30,33–35 and two study was published in Chinese journal.31,32 Six studies did not report adverse events; only one reported that the adverse events 14.28% in the CBT group, while in the control group the rate of adverse event is 0.34 Two studies performed Intention-to-treat analysis,29,32 four studies were conducted under protocols guideline.29,30,34,35 Table 1 provides details of study characteristics.
 |
Table 1 Summary of Included Studies (n = 7) |
Seven studies29–35 reported the sleep quality in this systematic review. PSQI scale was used to evaluate sleep quality. The self-report questionnaire assessed patients’ sleep conditions over the past month, comprising 19 items and encompassing seven subscales related to subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. One study34 used the Actigraph to investigate the Epworth sleepiness, sleep self-efficacy, sleep latency, sleep efficiency, total sleep time, wake after sleep onset, and the number of awakenings. The Actigraph device, a validated tool for individuals with insomnia, is a compact and noninvasive device (specifically, a 3-axis accelerometer, Model wGT3X-BT, ActiGraph Corp, Pensacola, FL).36 One study used the subscales of the PSQI scale to evaluate subjective sleep quality, sleep latency, sleep duration, and sleep efficiency.33
Description of Participants
There were a total of 2633 participants across the studies, with individual sample sizes ranging from 2834 to 1142.29 A total of 1308 participants with diabetes in the intervention group, and 1325 in the control groups, were included. Among the participants in the study, there were a wide range of ages, from 18 to 90 years old. The mean age ranged from 44.45 ± 6.91, to 66.05 ± 13.08, respectively, in CBT-based intervention group and 43.03 ± 6.31 to 66.83 ± 13.78 years old in control group across all studies. Six studies consisted of T2DM populations accounting for 85.7% of the sample, and only one study reported people diagnosed with diabetes.32 A total of five studies reported poor sleep quality both among intervention and control participants at baseline.29–32,34
Description of Interventions
There were two types of CBT among the seven studies, including general CBT (n = 6)29–33,35 and CBT-I (n = 1)34 in our systematic review. Participants in CBT-I received several face-to-face interview techniques: sleep restriction therapy, stimulus control procedures, sleep hygiene, relaxation training and cognitive components. Three out of seven studies conducted six sessions of CBT in six weeks to three months,29,34,35 two studies conducted eight sessions in two months,30,31 and one study conducted 12 sessions in six months.33 Across all interventions, each session ranged from 20 min to 60 min, with the majority of sessions (n = 4) lasting 40–60 min. Two studies did not report the time per session.32,35 A 10–15 min discussion session was added in three studies29–31 after the end of the intervention as a means to boost CBT. CBT were delivered in a group format in two studies, 8–10 people per group,30,31 and three studies conducted CBT with an individual format. Two studies did not report the group size.29,34 The interventionists providing the CBT included general practitioners (n = 5),29–33 CBT-I instructors (n = 1),34 and research Investigators (n = 1).35 Six out of seven studies conducted CBT face-to-face, while one used a face-to-face and online intervention format.33 Three studies were conducted in community health service centers,29–31 one in the endocrine clinic and ward,33 one in the University Medical Centre and Hospital,34 and one CBT conducted at the diabetes ward.32 However, one study did not report the intervention setting.35 Six studies recruited participants with poor sleep quality at baseline, while one did not indicate.33 Because the cut-off score of five provides satisfactory diagnostic sensitivity (89.6%) and specificity (86.5%) in distinguishing good from poor sleep quality.37 However, no theoretical framework model was adopted by our included studies to support the intervention. Table 2 presents information about the characteristics of CBT-based intervention.
 |
Table 2 Characteristics of CBT-Based Intervention (n = 7) |
For the control group, usual care (n = 6, 85.7%),29–33,35 and health education (n = 1, 14.3%)34 were used in our included studies. Patients with T2DM usually receive advice on diet, medication, exercise, and blood glucose monitoring from their healthcare providers. The diabetes education was provided by research assistants covering foot care, sick-day management, taking insulin on vacation, and insurance coverage.
Risk of Bias
The risk of bias summary is provided in Figure 2. The random sequence generation process was described in the majority of studies (n = 5, 71.4%),29,31–34 and allocation concealment measures were described in three studies (42.9%).31,32,34 Due to the CBT-based intervention’s nature, blinding participants was difficult or impossible, resulting in an unclear or high-performance bias in most studies. In only one study, a masked assessor scored the sleep data without having any involvement with the interventions.34 A low risk of attrition bias and a low risk of selective reporting bias was observed in all seven studies. No other risk was observed in our included studies.
 |
Figure 2 The risk of bias summary. |
Effectiveness of CBT-Based Intervention on Outcomes
Effectiveness of CBT-Based Intervention on Sleep Quality at Different Time Points
Five studies evaluated sleep quality using the PSQI.29,30,32–34 Sleep quality was not statistically significantly different in the CBT group compared with the control group when immediate post-intervention effects of the intervention were measured (effect size=−0.81, 95% CI −1.31 to −0.32, Z = 3.23, p = 0.001, I2=93%). When we removed every study one by one, the heterogeneity level did not disappear. The CBT groups, however, showed a statistically significant improvement in sleep quality at 6-month follow-up with a moderate effect size compared with the control group (n = 4)29–31,33 (effect size=−0.48, 95% CI −0.75 to –0.22, Z = 3.60, p = 0.0003, I2=88%). When we deleted any of the four studies, significant heterogeneity remained. Similarly, sleep quality was also statistically significantly different between the two groups after 12 months (n = 2)29,31 (effect size=−0.48, 95% CI −0.72 to –0.24, Z = 3.90, p < 0.0001, I2=84%) (see Figure 3). Due to the limited studies (n = 2) at 12-month follow-up, we did not remove the study to examine the heterogeneity.
 |
Figure 3 Forest plot of CBT-based intervention on sleep quality at different time points. |
Effectiveness of CBT-Based Intervention on Sleep Quality with Different CBT Sessions
CBT significantly improved sleep quality with a moderate effect size compared to the control group when studies reported CBT-based intervention with six sessions (n = 2)29,34 (effect size=−0.25, 95% CI −0.38 to –0.13, Z = 4.13, p < 0.0001, I2=0%). Moreover, the CBT group showed statistically significant improvements in sleep quality with a large effect size compared with the control group when studies reported CBT-based intervention with more than six sessions (n = 2)30,33 (effect size=−0.89, 95% CI −1.76 to –0.02, Z = 2.00, p = 0.05, I2=95%) (see Figure 4).
 |
Figure 4 Forest plot of CBT-based intervention on sleep quality with different CBT sessions. |
Effectiveness of CBT-Based Intervention on Time to Fall Asleep
Two studies,33,34 including 307 participants, reported the time to fall asleep; there was no significant that CBT is beneficial to the time to fall asleep after the intervention, compared to the control group. (effect size=−0.73, 95% CI −1.89 to 0.43, Z = 1.23, p = 0.22, I2= 85%). (see Figure 5).
 |
Figure 5 Forest plot of CBT-based intervention on time to fall asleep. |
Effectiveness of CBT-Based Intervention on the Total Sleep Time
Two studies33,34 with 307 participants reported a statistically significant increase in sleep time in the CBT group with a moderate effect size compared with the control group. (effect size=−0.34, 95% CI −0.57 to −0.12, Z = 2.98, p = 0.003, I2= 0%). (see Figure 6).
 |
Figure 6 Forest plot of CBT-based intervention on total sleep time. |
Effectiveness of CBT-Based Intervention on the Sleep Efficiency
Two studies (n = 307)33,34 reported there was not a statistically significant increase in sleep efficiency in the CBT group with a large effect size compared with the control group (effect size=−0.50, 95% CI −1.27 to 0.27, Z = 1.28, p = 0.20, I2= 71%) (see Figure 7).
 |
Figure 7 Forest plot of CBT-based intervention on sleep efficiency. |
Narrative Summary of the Included Studies
One study reported the sleep disorder showed no statistically significant improvement in the CBT group compared with the control group (p = 0.679).33 Furthermore, participants in the CBT-I group showed higher improvements in insomnia symptoms (p < 0.001), Epworth sleepiness (p = 0.05), sleep latency (p = 0.01), wake after sleep onset (p < 0.001) and number of awakenings (p = 0.03), compared to the health education group.34
Publication Bias and Sensitivity Analyses
Figure 8 indicates that there was no potential publication bias from visual inspection of the funnel plot. We conducted a sensitivity analysis to investigate how the results changed with the removal studies and assessed the stability of the results. When we removed the study from the model and recalculated the combination estimate on the remaining studies, heterogeneity did not disappear or decrease, and the pooled results were consistent, indicating our results’ robustness.
 |
Figure 8 Funnel plot. |
Discussion
This systematic review is the first to comprehensively examine the effectiveness of CBT-based intervention on sleep-related outcomes in people with diabetes. Our study showed that the CBT-based intervention could improve sleep quality at immediate post-intervention effect, six months, and 12 months, compared to control groups. Meanwhile, CBT-based intervention has positive effects on total sleep time treatment response. In terms of the CBT sessions, six sessions or more than six sessions were beneficial to sleep quality among people with diabetes based on current data. No evidence was found to support that CBT-based intervention is beneficial for time to fall asleep and sleep efficiency after intervention. Collectively, a definitive conclusion cannot be drawn regarding the effectiveness of CBT-based intervention for sleep-related outcomes due to the small number of included studies and the heterogeneity among them. It is, therefore, important to interpret the results cautiously. Despite the promising effectiveness of CBT-based intervention on sleep quality and total sleep time, this meta-analysis emphasizes the need for further research, especially in the effectiveness of CBT on insomnia symptoms, sleep disorder, Epworth sleepiness, sleep self-efficacy, sleep latency, wake after sleep onset, and number of awakenings.
Methodological and clinical problems were reported in the studies, raising concerns about CBT-based interventions’ effectiveness in improving sleep-related outcomes. High heterogeneity has been detected from this systematic review, which may be due to the different intervention contents (CBT and CBT-I), settings (community health service centers, University Medical Centre, and endocrine clinic and ward), interventionists (general practitioner, research Investigators, and CBT-I instructor), and different numbers of CBT sessions (six sessions and more than six sessions). Meanwhile, detection bias was also a concern because many of the included studies predominantly failed to contain sufficient detail on using blinded assessors to collect self-report data or to score objective measures. This finding suggested that more research with higher quality is needed to improve sleep-related outcomes among people with diabetes. Consequently, future RCTs should strictly follow the research proposal and the Consolidated Standards of Reporting Trials (CONSORT) guidelines, improving literature quality. Moreover, all studies were conducted in China, the US, and the Iran. Comparatively, little attention has been paid to CBT-based intervention on sleep-related outcomes among people with diabetes in other countries. Yet sleep problems are a public health issue for people with diabetes around the world. Therefore, it is necessary to conduct more large-scale, high-quality, and rigorous randomized control trials worldwide on people with diabetes in order to generate scientific evidence.
In the meta-analysis, we firstly did find a significant effect of CBT-based intervention on sleep quality at immediate post-intervention effect in people with diabetes (n = 5 trails). However, due to the high heterogeneity in our meta-analysis, we removed each study one by one and recalculated the combination estimate on the remaining studies. It was found that heterogeneity did not disappear, and results were unchanged. One possible reason for improving the sleep quality after the intervention may be that the participants we included were diagnosed with poor sleep quality at baseline. Therefore, there was a big room for improvement after intervention. Similarly, CBT-based intervention improved sleep quality in people with diabetes at 6-month and 12-month follow-ups in our study, indicating that the benefits of CBT are generally maintained 6 to 12 months after completing treatment, consistent with previous findings for long-term in the general population.38 Meanwhile, our findings are line with a scoping review (n = 5 trails) of CBT improve sleep-related outcomes in individuals with traumatic brain injury.39 The possible reason for the maintenance of the effect is that the material for CBT training was retained, practiced, and consolidated for long enough for people with diabetes to have an impact on their sleep quality. Additionally, CBT-based interventions include sleep hygiene education, stimulus control, relaxation, and cognitive reconstruction, which need time to obtain and further relieve patients’ tension, help them develop sleep-wake biorhythms, and improve sleep quality.40 When we delete each study one by one from pooling effect in 6-month follow up, the heterogeneity did not disappear. Thus, we conducted the subgroup analysis to examine heterogeneity.
In the subgroup analyses, we found that six sessions of CBT (I2=0%) and more than six sessions of CBT had significantly improved sleep quality compared with the control group. Therefore, different CBT dosages may be considered a heterogeneous source of the effect of CBT on sleep quality. More importantly, these findings suggested that people with diabetes are favored to accept six to eight sessions of CBT-based intervention.
In our meta-analysis, we found that CBT-based intervention could improve total sleep time, with a moderate effect, which was line with a systematic review founding that participants who eventually increased sleep time until 3, 6, and up to 12 months after 6–8 sessions of CBT.41 It may be possible and appropriate to appreciably increase total sleep time through further intervention. Higher dose CBT (6–8 sessions) with a dedicated focus on the upward titration of sleep opportunity may be helpful to increase the magnitude of the sleep time gain and to extend the effect to a larger proportion of patients.41 However, our results did not echo a systematic review (37 studies) that show the efficacy of CBT-I for insomnia comorbid with psychiatric and/or medical conditions on total sleep time.42 This might be due to the limited included studies in our review, which caused the bias. Another possible reason is that sleep time was measured by subjective and objective methods in our included studies, which may confound our results.
Our meta-analysis revealed no significance in sleep efficiency after the intervention, which was not similar to a review among adults with insomnia, indicating that CBT-based intervention benefits sleep efficiency among older adults and adolescents.43 The possible reason is the different measurement tools for sleep efficiency and different populations. Actigraph and subscale of PSQI were used in our systematic review to evaluate sleep efficiency, and we included adults with diabetes. Our narrative summary demonstrated that only one study using objective measurement methods reported CBT-based intervention could improve insomnia symptoms, Epworth sleepiness, sleep self-efficacy, sleep latency, wake after sleep onset, and number of awakenings after the intervention. Thus, more large-scale studies to investigate the effect of CBT-based intervention on sleep in people with diabetes are needed to reach a conclusive result.
Implications
Implications for Practice
CBT has the potential to improve sleep quality and total sleep time in people with diabetes. Therefore, Information from the studies included in this review (eg, standard CBT contents, duration, and frequency) should be shared with CBT teachers who perform CBT-based intervention in people with diabetes who experience poor sleep quality and total sleep time. Although the studies we reviewed did not directly examine how many intervention sessions would be practical to improve sleep quality and total sleep time in people with diabetes, our findings showed that 6–8 sessions in 6–8 weeks were the most common, which can be easily translated to practice. CBT could be considered a strategy among healthcare providers to enhance sleep quality and total sleep time for people with diabetes.
Implications for Future Research
Our finding suggested that all studies included in our review were conducted between 2020 and 2023 and showed promising results, which offered new insight for future research in which we encouraged more researchers to investigate the effectiveness of CBT on sleep-related outcomes among people with diabetes. Most studies in this meta-analysis used general CBT, and only one used CBT-I. There is an urgent need to test the effects of CBT-I interventions with robust experimental designs under framework guidance on sleep-related outcomes in people with T1DM, especially in younger age groups. Other methods of CBT-based intervention, such as mHealth strategies, should be considered to provide cheaper and more accessible CBT-based interventions, eg, smartphone-based CBT or computer-based CBT or text messaging to supplement class content. To enhance data accuracy, incorporating objective measures such as actigraphy or polysomnography could be considered when assessing sleep-related outcomes during these interventions. Furthermore, psychiatrists and practitioners are scarce in developing countries, community-based health services, such as those in China, cannot provide sophisticated psychological services to individuals.44 Therefore, based on our findings from this systematic review, it may be possible to conduct nurse-led CBT training or implement task-sharing strategies to empower nurses to conduct CBT-based intervention at community-based health services to help people with diabetes manage sleep problems. CBT-based intervention methods must be standardized so that researchers can more easily integrate findings to draw definitive conclusions about the extent to which CBT-based interventions affect sleep-related outcomes and to increase the generalizability of results. Meanwhile, more studies need to be done under the protocol guide to assess fidelity. Comparing CBT’s effects on sleep-related outcomes between people with T1DM and T2DM is warranted for clinical recommendations. In future studies, researchers need to explore the underlying mechanisms (mediators and moderators) by which the CBT program influences sleep-related outcomes, eg, sleep quality.
Limitations
Some limitations should be noted. First, the lack of relevant literature prevents firm conclusions from being drawn about the effects of CBT-based interventions on sleep-related outcomes in people with diabetes. For example, some sleep-related outcomes were only reported in one study, which prevented conducting meta-analysis. Despite this lack of literature, these promising findings from this systematic review highlight the importance of further research. Second, the search strategy included only published literature; this may have resulted in publication bias and an overrepresentation of effective intervention. Third, since the language restrictions, the review was also limited to publications in English and Chinese, and research on CBT-based intervention’s effects on sleep-related outcomes may have been conducted in other languages. Fourth, the unclear risk for blinding outcome assessment was observed in several studies. Therefore, a detailed report on data collection procedures is necessary for future studies to assess bias and reliability accurately. Meanwhile, few studies reported fidelity assessments in our included studies. Thus, the quality of CBT-based intervention is an unpredictable factor. We should be cautious when concluding. Fifth, limited studies make it impossible to compare CBT-based intervention’s effects on sleep-related outcomes between people with T1DM and T2DM. Finally, there were insufficient studies reporting the effect of CBT-based intervention on the time to fall asleep, sleep time, and sleep efficiency at follow-up to examine the long-term effect.
Conclusion
The preliminary findings of the systematic review show that CBT-based intervention is a promising complementary therapy for improving sleep quality and total sleep time among people with diabetes despite the limited evidences. These findings provide empirical support for the recommendation of using CBT-based intervention as the treatment of choice for improving sleep-related outcomes. Healthcare professionals should regularly assess for sleep-related outcomes, and efforts should be directed at conducting CBT-based intervention as a model for implementing routine work for people with diabetes in future clinical practice. Studies with better designs, larger sample sizes, and long-term follow-up RCTs are warranted to draw conclusive evidence and explore how the program influences sleep-related outcomes.
Abbreviations
CBT, Cognitive-behavioral therapies; CBT-I, Cognitive Behavioral Therapy for Insomnia; CNKI, China National Knowledge Infrastructure; RCT, Randomized Controlled Trials; T1DM, Type 1 diabetes mellitus; T2DM, Type 2 diabetes mellitus; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis.
Funding
This research was supported by the science and technology innovation program of Hunan province (2022WZ1033). This funding source had no role in the design of the study and did not have any role in collection, analysis, and interpretation of data or in writing the manuscript.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. World Health Organization. Diabetes; 2017. Available from: http://www.who.int/news-room/fact-sheets/detail/diabetes.
2. Prevention CfDCa. National diabetes statistics report estimates of diabetes and its burden in the United States; 2021. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html#anchor_40084.
3. Holingue C, Wennberg A, Berger S, Polotsky VY, Spira AP. Disturbed sleep and diabetes: a potential nexus of dementia risk. Metabolism. 2018;84:85–93. doi:10.1016/j.metabol.2018.01.021
4. Zhu B, Hershberger PE, Kapella MC, Fritschi C. The relationship between sleep disturbance and glycaemic control in adults with type 2 diabetes: an integrative review. J Clin Nurs. 2017;26(23–24):4053–4064. doi:10.1111/jocn.13899
5. Zhu B, Vincent C, Kapella MC, et al. Sleep disturbance in people with diabetes: a concept analysis. J Clin Nurs. 2018;27(1–2):e50–e60. doi:10.1111/jocn.14010
6. Kluding P the effect of cognitive behavioral therapy for insomnia on type 2 diabetes health outcomes; 2019. Available from: https://clinicaltrials.gov/study/NCT03713996.
7. Schipper SBJ, Van Veen MM, Elders PJM, et al. Sleep disorders in people with type 2 diabetes and associated health outcomes: a review of the literature. Diabetologia. 2021;64(11):2367–2377. doi:10.1007/s00125-021-05541-0
8. Lamond N, Tiggemann M, Dawson D. Factors predicting sleep disruption in type II diabetes. Sleep. 2000;23(3):415–416. doi:10.1093/sleep/23.3.1i
9. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91–101. doi:10.1016/j.smrv.2016.02.001
10. Azharuddin M, Kapur P, Adil M, Ghosh P, Sharma M. The impact of sleep duration and sleep quality on glycaemic control in Asian population with type 2 diabetes mellitus: a systematic literature review and meta-analysis of observational studies. Clin Epidemiol Glob Health. 2020;8(3):967–975. doi:10.1016/j.cegh.2020.03.006
11. Smyth A, Jenkins M, Dunham M, Kutzer Y, Taheri S, Whitehead L. Systematic review of clinical practice guidelines to identify recommendations for sleep in type 2 diabetes mellitus management. Diabetes Res Clin Pract. 2020;170:108532. doi:10.1016/j.diabres.2020.108532
12. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204.
13. Silber MH. Clinical practice. Chronic insomnia. N Engl J Med. 2005;353(8):803–810. doi:10.1056/NEJMcp043762
14. Kalkman JS, Schillings ML, Zwarts MJ, van Engelen BG, Bleijenberg G. The development of a model of fatigue in neuromuscular disorders: a longitudinal study. J Psychosom Res. 2007;62(5):571–579. doi:10.1016/j.jpsychores.2006.11.014
15. Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American academy of sleep medicine report. Sleep. 2006;29(11):1415–1419. doi:10.1093/sleep/29.11.1415
16. Zachariae R, Amidi A, Damholdt MF, et al. Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: a randomized controlled trial. J Natl Cancer Inst. 2018;110(8):880–887. doi:10.1093/jnci/djx293
17. Chen HY, Chiang CK, Wang HH, et al. Cognitive-behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis: a pilot randomized controlled trial. Am J Kidney Dis. 2008;52(2):314–323. doi:10.1053/j.ajkd.2008.03.012
18. Terpstra JA, van der Vaart R, Ding HJ, Kloppenburg M, Evers AWM. Guided internet-based cognitive-behavioral therapy for patients with rheumatic conditions: a systematic review. Internet Interv. 2021;26:100444. doi:10.1016/j.invent.2021.100444
19. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.2. Cochrane; 2021. Available from: www.training.cochrane.org/handbook.
20. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
21. Higgins JP, Green S. Cochrane handbook for systematic review of interventions version 5.1. 0; 2011 [updated March, 2011]. Available from: https://www.cochrane-handbook.org.
22. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
23. Singla DR, Lawson A, Kohrt BA, et al. Implementation and effectiveness of nonspecialist-delivered interventions for perinatal mental health in high-income countries: a systematic review and meta-analysis. JAMA psychiatry. 2021;78(5):498–509. doi:10.1001/jamapsychiatry.2020.4556
24. Zhou T, Luo Y, Xiong W, Meng Z, Zhang H, Zhang J. Problem-solving skills training for parents of children with chronic health conditions: a systematic review and meta-analysis. JAMA pediatr. 2024. doi:10.1001/jamapediatrics.2023.5753
25. Joanne E, McKenzie SEB. Chapter 12: synthesizing and presenting findings using other methods. Available from: https://training.cochrane.org/handbook/archive/v6.1/chapter-12.
26. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. doi:10.1136/bmj.d5928
27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
28. Cohen J. Statistical Power for the Behavioural Sciences. Academic press 1988.
29. Zhang HZ, Zhang P, Chang GQ, et al. Effectiveness of cognitive behavior therapy for sleep disturbance and glycemic control in persons with type 2 diabetes mellitus: a community-based randomized controlled trial in China. World J Diabetes. 2021;12(3):292–305. doi:10.4239/wjd.v12.i3.292
30. Zuo X, Dong Z, Zhang P, et al. Effects of cognitive behavioral therapy on sleep disturbances and quality of life among adults with type 2 diabetes mellitus: a randomized controlled trial. Nutr Metab Cardiovasc Dis. 2020;30(11):1980–1988. doi:10.1016/j.numecd.2020.06.024
31. Zongmei D, Lou Peian, Zhang pan, et al. Effectiveness of cognitive-behavioral therapy on sleep quality and glycated hemoglobin A1c in patients with type 2 diabetes mellitus. J Chin J Diabetes Mellitus. 2020;12(7):525–529.
32. Dandan S, Sisi L, Yanyan Z, Yaqing Z. Effect of cognitive behavioral intervention based on accelerated rehabilitation concept on sleep quality and perceived burden in patients with ischemic stroke combined with insomnia and diabetes. Chin J Pract Nerv Dis. 2023;26(6):744–747.
33. Li Y, Buys N, Ferguson S, et al. The evaluation of cognitive-behavioral therapy-based intervention on type 2 diabetes patients with comorbid metabolic syndrome: a randomized controlled trial. Diabetol Metab Syndr. 2023;15(1):158. doi:10.1186/s13098-023-01100-2
34. Alshehri MM, Alenazi AM, Alothman SA, et al. Using cognitive behavioral therapy for insomnia in people with type 2 diabetes, pilot rct part I: sleep and concomitant symptom. Behav Sleep Med. 2021;19(5):652–671. doi:10.1080/15402002.2020.1831501
35. Irandoust K, Taheri M, Hamzehloo K, et al. The effects of cognitive behavioral therapy on selected physical, physiological parameters, exercise and nutritional behaviors in diabetic persons. Eur Rev Med Pharmacol Sci. 2022;26(18):6805–6812. doi:10.26355/eurrev_202209_29782
36. Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–239.
37. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
38. Koffel EA, Koffel JB, Gehrman PR. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6–16. doi:10.1016/j.smrv.2014.05.001
39. Ludwig R, Vaduvathiriyan P, Siengsukon C. Does cognitive-behavioural therapy improve sleep outcomes in individuals with traumatic brain injury: a scoping review. Brain Inj. 2020;34(12):1569–1578. doi:10.1080/02699052.2020.1831070
40. Newby JM, Mackenzie A, Williams AD, et al. Internet cognitive behavioural therapy for mixed anxiety and depression: a randomized controlled trial and evidence of effectiveness in primary care. Psychol Med. 2013;43(12):2635–2648. doi:10.1017/S0033291713000111
41. Scott H, Cheung JMY, Muench A, et al. Does total sleep time substantially increase after cognitive behavioral therapy for insomnia? J Clin Sleep Med. 2022;18(7):1823–1829. doi:10.5664/jcsm.10004
42. Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461–1472. doi:10.1001/jamainternmed.2015.3006
43. Haynes J, Talbert M, Fox S, Close E. Cognitive behavioral therapy in the treatment of insomnia. South Med J. 2018;111(2):75–80. doi:10.14423/SMJ.0000000000000769
44. Qingzhi Z, Yanling H, Zhengyu S, et al. A community-based controlled trial of a comprehensive psychological intervention for community residents with diabetes or hypertension. Shanghai Arch Psychiatry. 2016;28(2):72–85. doi:10.11919/j.issn.1002-0829.216016
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
