Back to Journals » Journal of Pain Research » Volume 17
A Systematic Guideline by the ASPN Workgroup on the Evidence, Education, and Treatment Algorithm for Painful Diabetic Neuropathy: SWEET
Authors Sayed D , Deer TR , Hagedorn JM , Sayed A, D'Souza RS , Lam CM , Khatri N , Hussaini Z , Pritzlaff SG , Abdullah NM, Tieppo Francio V , Falowski SM , Ibrahim YM, Malinowski MN , Budwany RR, Strand NH , Sochacki KM, Shah A , Dunn TM , Nasseri M, Lee DW, Kapural L , Bedder MD, Petersen EA , Amirdelfan K, Schatman ME , Grider JS
Received 21 November 2023
Accepted for publication 19 March 2024
Published 13 April 2024 Volume 2024:17 Pages 1461—1501
DOI https://doi.org/10.2147/JPR.S451006
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Dawood Sayed,1 Timothy Ray Deer,2 Jonathan M Hagedorn,3 Asim Sayed,4 Ryan S D’Souza,3 Christopher M Lam,1 Nasir Khatri,5 Zohra Hussaini,1 Scott G Pritzlaff,6 Newaj Mohammad Abdullah,7 Vinicius Tieppo Francio,1 Steven Michael Falowski,8 Yussr M Ibrahim,9 Mark N Malinowski,10 Ryan R Budwany,2 Natalie Holmes Strand,11 Kamil M Sochacki,12 Anuj Shah,13 Tyler M Dunn,11 Morad Nasseri,14 David W Lee,15 Leonardo Kapural,16 Marshall David Bedder,17,18 Erika A Petersen,19 Kasra Amirdelfan,20 Michael E Schatman,21,22 Jay Samuel Grider23
1Department of Anesthesiology and Pain Medicine, The University of Kansas Medical Center, Kansas City, KS, USA; 2Pain Services, Spine and Nerve Center of the Virginias, Charleston, WV, USA; 3Department of Anesthesiology and Perioperative Medicine, Mayo Clinic, Rochester, MN, USA; 4Podiatry/Surgery, Susan B. Allen Memorial Hospital, El Dorado, KS, USA; 5Interventional Pain Medicine, Novant Spine Specialists, Charlotte, NC, USA; 6Department of Anesthesiology and Pain Medicine, University of California, Davis, Sacramento, CA, USA; 7Department of Anesthesiology, University of Utah, Salt Lake City, UT, USA; 8Neurosurgery, Neurosurgical Associates of Lancaster, Lancaster, PA, USA; 9Pain Medicine, Northern Light Eastern Maine Medical Center, Bangor, ME, USA; 10OhioHealth Neurological Physicians, OhioHealth, Columbus, OH, USA; 11Anesthesiology and Pain Medicine, Mayo Clinic, Phoenix, AZ, USA; 12Department of Anesthesiology and Perioperative Medicine, Rutgers Robert Wood Johnson, New Brunswick, NJ, USA; 13Department of Physical Medicine and Rehabilitation, Detroit Medical Center, Detroit, MI, USA; 14Interventional Pain Medicine / Neurology, Boomerang Healthcare, Walnut Creek, CA, USA; 15Pain Management Specialist, Fullerton Orthopedic, Fullerton, CA, USA; 16Carolinas Pain Institute, Winston Salem, NC, USA; 17Chief of Pain Medicine Service, Augusta VAMC, Augusta, GA, USA; 18Associate Professor and Director, Addiction Medicine Fellowship Program, Department Psychiatry and Health Behavior, Medical College of Georgia at Augusta University, Augusta, GA, USA; 19Department of Neurosurgery, University of Arkansas for Medical Sciences, Little Rock, AR, USA; 20Director of Clinical Research, Boomerang Healthcare, Walnut Creek, CA, USA; 21Department of Anesthesiology, Perioperative Care & Pain Medicine, NYU Grossman School of Medicine, New York, NY, USA; 22Department of Population Health – Division of Medical Ethics, NYU Grossman School of Medicine, New York, NY, USA; 23Anesthesiology, Division of Pain Medicine, University of Kentucky College of Medicine, Lexington, KY, USA
Correspondence: Dawood Sayed, Anesthesiology and Pain Medicine, the University of Kansas Medical Center, Kansas City, KS, USA, Tel +1 785-550-5800, Email [email protected]
Introduction: Painful diabetic neuropathy (PDN) is a leading cause of pain and disability globally with a lack of consensus on the appropriate treatment of those suffering from this condition. Recent advancements in both pharmacotherapy and interventional approaches have broadened the treatment options for PDN. There exists a need for a comprehensive guideline for the safe and effective treatment of patients suffering from PDN.
Objective: The SWEET Guideline was developed to provide clinicians with the most comprehensive guideline for the safe and appropriate treatment of patients suffering from PDN.
Methods: The American Society of Pain and Neuroscience (ASPN) identified an educational need for a comprehensive clinical guideline to provide evidence-based recommendations for PDN. A multidisciplinary group of international experts developed the SWEET guideline. The world literature in English was searched using Medline, EMBASE, Cochrane CENTRAL, BioMed Central, Web of Science, Google Scholar, PubMed, Current Contents Connect, Meeting Abstracts, and Scopus to identify and compile the evidence for diabetic neuropathy pain treatments (per section as listed in the manuscript) for the treatment of pain. Manuscripts from 2000-present were included in the search process.
Results: After a comprehensive review and analysis of the available evidence, the ASPN SWEET guideline was able to rate the literature and provide therapy grades for most available treatments for PDN utilizing the United States Preventive Services Task Force criteria.
Conclusion: The ASPN SWEET Guideline represents the most comprehensive review of the available treatments for PDN and their appropriate and safe utilization.
Keywords: diabetes, painful diabetic neuropathy, neuropathy, spinal cord stimulation, chronic pain, diabetic neuropathy
Introduction and Methodology
Development Process
The American Society of Pain and Neuroscience (ASPN), through its mission to increase evidence-based access to treatment, has commissioned a systematic guideline process to outline the current state of the art in treatment of painful diabetic neuropathy (PDN) (SWEET guidelines). Members of the SWEET consensus group were selected from among the thought leaders across a broad spectrum of specialties interested in the treatment of diabetic neuropathy within both ASPN and other societies. A diverse authorship included experts from the specialties of Pain Medicine, Neurology, Podiatry, Primary Care, Neurosurgery, Physiatry, Psychology, and Anesthesiology. The current guideline will examine the evidence, education and current treatment options. The SWEET consensus work group was convened and at regular intervals, members have evaluated the level of current evidence in the peer-reviewed literature for topics that have been identified as critical for treatment.
Work groups were convened to conduct literature searches and examine the evidence for the topics developed by lead authors in outline form. After the literature search was completed, each author was asked to provide cited references, and evidence rank. The section leaders then formulated the recommendation grade, based on the evidence, which were reviewed by at least three different, nonconflicted SWEET working group members. If conflicts of interest were identified, recusal was required as outlined below. ASPN utilizes the United States Preventative Services Task Force (USPSTF) format with slight modification for interventional pain treatment. This process has been established in previous ASPN publications.1 Once literature was reviewed, consensus statements were created and graded based upon the ASPN-USPSTF criteria listed in Table 1. The process by which section leaders then created consensus points included in-person meetings, teleconference, or other electronic or audio-video communications to define the consensus; agreement by at least 80% of the contributing authors was considered a quorum. Consensus strength was defined, as described in previous ASPN guidelines.1 If a recommendation was proposed with <50% consensus, based on assigned evidence rank and recommendation grade, then no consensus was achieved.
This consensus guideline gives guidance to clinicians concerning painful PDN treatment and evidence-based practice and outcome optimization. However, these recommendations should not be construed as a standard of care, but instead represent best practices. This guidance is based on several factors and peer-reviewed evidence, and regardless of the strength of evidence, requires interpretation for clinical application.
Management of Conflict of Interest
All authors were required to disclose conflicts of interest prior to assignment of topics. The senior authors determined the extent of the conflict of interest ensuring balanced inquiry and evaluation for each manuscript section. One of the co-primary authors without conflict was identified for each section and is the adjudication determination official for any issues of potential conflict. All authors were asked to recuse themselves on any recommendation potentially affected by a disclosed conflict. Additionally, authors without conflict vetted all recommendations for bias.
Methodology: Literature Search, Evidence Ranking
The world literature in English was searched using Medline, EMBASE, Cochrane CENTRAL, BioMed Central, Web of Science, Google Scholar, PubMed, Current Contents Connect, Meeting Abstracts, and Scopus to identify and compile the evidence for diabetic neuropathy pain treatments (per section as listed in the manuscript) for the treatment of pain. Manuscripts from 2000-present were included in the search process. Search words were selected based upon the section represented. Identified peer-reviewed literature was critiqued using the USPSTF criteria for quality of evidence,2 with modifications for neuromodulation studies (Table 1). After USPSTF letter grading was assigned, the working subgroup then assigned the “level of certainty regarding benefit” as described in Table 2.
 |
Table 1 Quality of Evidence Ranking Using United States Preventative Services Task Force Criteria Modified for Therapy |
 |
Table 2 Levels of Certainty Regarding Net Benefit |
For each major section or topic, ASPN formulated consensus points. Consensus points should not be confused with recommendations based on consensus alone (Evidence Level II), which were rendered as clinical guidance in the situations where, due to the lack of evidence-based literature (such as randomized controlled trials [RCTs]), prospective observational studies, and retrospective cohort/case series), the best available guidance is expert opinion.
Painful Diabetic Neuropathy
Background
PDN is a common and distressing complication of diabetes mellitus (DM), characterized by chronic pain and sensory abnormalities in the extremities. PDN is reported in as many as 16%–26% of patients with diabetes, yet often not discussed with the patients and continues to remain untreated,3 negatively affecting the patient’s quality of life, both physically and psychologically.4
The pathophysiology of PDN is multifactorial, involving both metabolic and vascular mechanisms. Chronic hyperglycemia, oxidative stress, and neuroinflammation contribute to nerve damage in a length-dependent manner and subsequent development of pain, with variety of clinical manifestations, ranging from mild discomfort to severe debilitating pain, often accompanied by sleep disturbances, anxiety, and depression.
The diagnosis of PDN involves a comprehensive evaluation of a patient’s symptoms, medical history, physical examination, and additional diagnostic tests.
- Patient’s history: including detailed information about the symptoms, underlying medical conditions, and evaluation of potential causes for neuropathic pain.
- Physical examination: assessment of neurological function including sensory perception, reflexes, and motor strength.
- Diagnostic criteria, such as the Toronto Diabetic Neuropathy Expert Group criteria.5
- Nerve conduction studies: helps to assess the nerve damage and rule out other possible causes of neuropathy.
- Quantitative sensory testing: measuring the perception of various sensory stimuli to evaluate the function of small nerve fibers.
- Laboratory testing: to assess patient’s blood glucose control, kidney function, and rule out other potential causes of neuropathy such as vitamin deficiency and autoimmune disorders.
- Imaging studies: such as magnetic resonance imaging (MRI) or nerve ultrasound to assess for structural abnormalities or nerve compression.
The management of PDN requires a multimodal approach, that combines pharmacological, topical analgesics, interventional therapies, and non-pharmacological therapies. Pharmacological treatments include tricyclic antidepressants, anti-convulsants, serotonin-norepinephrine reuptake inhibitors, and opioids. Topical analgesics include 8% capsaicin, lidocaine and amitriptyline. Non-pharmacological interventions such as physical therapy, transcutaneous electrical nerve stimulation, and acupuncture can also provide some relief. Additionally, interventional procedures like nerve blocks and spinal cord stimulation have been on emerging rise, in treatment of refractory cases.
Despite a position statement from multiple national associations, including the Centers for Disease Control and Prevention (CDC) as well as American Academy of Neurology (AAN),6,7 opioids continue to remain the most commonly prescribed medication for PDN, followed by gabapentin, pregabalin, duloxetine, amitriptyline, and venlafaxine.8
PDN continues to remain a challenging condition to manage effectively. Tailoring treatment to individual patients, considering their comorbidities and preferences, is crucial for optimizing outcomes. Additionally, a multidisciplinary approach involving collaboration between primary care physicians, endocrinologists, interventional pain specialists, podiatrists, and other healthcare professionals is essential for comprehensive PDN management.
Physical Exam
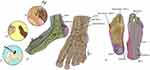
Early detection of PDN is challenging due to the insidious nature of PDN and nonspecific symptoms. Patients frequently experience symptoms such as pins and needles, shocks, numbness, a feeling of walking on sandpaper or extra socks, and burning sensations. These symptoms often worsen at night and follow a stocking-glove distribution, initially affecting the feet and toes before progressing proximally.9 Evaluating medical history, family history, alcohol use, and medications, along with thorough physical exam is vital for early stage PDN identification. The American Diabetes Association recommends sensory tests such as the 128 Hz tuning fork, monofilament testing, thermal testing, pinprick sensations, deep tendon reflexes, and proprioceptive testing; see Figure 1.10 Additionally, laboratory investigations, electrodiagnostic studies, and skin biopsies may aid diagnosis and assess disease progression.10
 |
Figure 2 Algorithmic approach to management of PDN. |
Diagnostic Approaches
Sensory Testing
Sensory testing plays a crucial role in evaluating peripheral nerve function in PDN. The 128 Hz tuning fork is utilized to assess vibratory sensation, reflecting the integrity of large sensory nerve fibers. The absence of perceived vibratory sensation indicates sensory impairment.11 The 10-g Semmes Weinstein monofilament test evaluates light touch perception and is used to assess large nerve fibers. The mono filament is designed to buckle under 10 g of pressure. Various testing sites, including the plantar aspect of the toes, metatarsal heads, dorsum of the hallux, midfoot, and plantar aspect of the heel, are recommended.12 Because the aforementioned exams test the large nerve fibers, positive tests are usually in the irreversible late stage of the disease progression.13
Thermal Testing
Thermal testing, which assesses temperature perception, has shown promise in early detection of PDN, as it tests the small nerve fibers that are affected early in the disease progression. Reduced temperature sensations have been associated with up to 93% of individuals with glucose intolerance or DM.13 However, standardization and subjectivity remain challenges in this diagnostic modality.
Pinprick Sensations
The evaluation of pinprick sensations provides insight into the functionality of small myelinated A-delta fibers. By applying pressure using a pin or small gauge needle, the ability to perceive sharp stimuli is assessed. Inability to perceive the sharp sensation indicates abnormal sensory function. Despite its subjective nature, the pinprick test demonstrates high positive predictive value in diagnosing PDN.14
Deep Tendon Reflexes
Deep tendon reflexes, particularly the Achilles and patellar reflexes, exhibit acceptable sensitivity, specificity, and predictive values associated with abnormal nerve conduction velocity (NCV). Impaired reflexes in these regions warrant further investigation, but evidence proposes deep tendon reflex testing as a valuable tool for early detection of PDN.15
Proprioceptive Testing
Assessment of joint position sense provides valuable information regarding the effect of PDN on proprioceptive accuracy. Studies have shown a correlation between proprioceptive inaccuracy and altered diabetic neuropathy scores, supporting its utility as a diagnostic tool.16
Laboratory Testing
Initial laboratory testing should include a complete blood count, comprehensive metabolic profile, fasting blood glucose, thyroid-stimulating hormone, and vitamin B12 levels.17 Serum protein electrophoresis with immunofixation is suggested by the AAN due to the association of monoclonal gammopathies with peripheral neuropathy. Laboratory testing should be performed on initial presentation/diagnosis. Although laboratory testing alone cannot diagnose PDN, it can be utilized as a screening test for etiologies attributed to peripheral neuropathy.17
Electrodiagnostic Studies
Nerve conduction velocities and electromyography can aid in treatment planning once a comprehensive history and physical examination have been conducted. However, the utility of electrodiagnostic studies in the absence of suspicion of demyelinating disorders, nerve entrapment, or nerve injury remains debatable.9
Skin Biopsies
Analysis of intraepidermal nerve fiber densities through small punch biopsy has emerged as a valuable and promising modality for the diagnosis and monitoring of early-stage PDN. This technique provides a distinct advantage over NCV studies, as it allows for the evaluation of small intraepidermal nerve fibers that are susceptible to early disease involvement.18,19 The biopsy procedure is easily performed, exhibits good reproducibility, and offers the capability to assess disease progression and potentially serve as an indicator of treatment response.18
Section 1. Pharmacotherapy
All patients with diabetes should be screened for PDN and, if present, should be appropriately treated. There are three classes of medications that are primarily used for the treatment of PDN. These classes include serotonin-norepinephrine reuptake inhibitors (SNRIs), gabapentinoids, and tricyclic antidepressants (TCAs).20 The individual medications belonging to each of these classes are presented later in this section. Of all these oral agents, duloxetine, pregabalin, and tapentadol are approved by the FDA for the treatment of painful diabetic neuropathy.21–24 Drugs belonging to the TCA class are often used and effective for various neuropathic pain conditions; however, these agents have not been studied in randomized control trials specifically for the treatment of PDN.20
Opioids, while effective in the short term, are discouraged for use in the treatment of chronic noncancer pain.6 In a systematic review by the CDC, there was weak to nonexistent evidence for the long-term efficacy of opioids for the treatment of chronic pain.6,7 Instead, long-term opioid use was found to be associated with adverse consequences including addiction and opioid-induced hyperalgesia. Interestingly, tapentadol which exerts its effect by both opioids and SNRI-based mechanisms was found to be effective in the treatment of painful diabetic neuropathy and has an FDA indication for this condition.25 However, due to the adverse consequences of long-term opioid use, this medication is discouraged by several societies.
At large, all the agents from the SNRI, gabapentinoids, and TCA classes have similar efficacy on neuropathic pain.20 However, there are agent-specific differences in side effects. Additionally, some of the agents have multiple indications, including depression and anxiety. Clinicians should be mindful of these differences in selecting appropriate medication. While many of these medications are effective for the treatment of PDN, complete elimination of pain is not realistic. Clinicians should have a discussion with patients in setting reasonable analgesic expectations. When initiating an agent, it is advisable to slowly titrate the agent to the effective dose to avoid intolerance and if therapeutic efficacy is not achieved after increasing the dose to the maximum allowable dose for the duration of 12 weeks, the medication can be considered inefficacious. Clinicians should not prematurely declare an agent ineffective when the dose has not been escalated to an appropriate level and the agent has not been tried for an appropriate duration.
Combination therapy with agents from different classes has not been studied extensively in well-designed trials. One study evaluated the efficacy of combined duloxetine (60 mg/day) and pregabalin (300 mg/day) to high-dose duloxetine (120 mg/day) and pregabalin (600 mg/day).26 The combination therapy was no more effective than individual agent therapy. Given the paucity of data on combination therapy, a clinician should carefully weigh the pros and cons before initiating such therapy.
Individual Pharmacotherapy Agents
Duloxetine
Duloxetine is a serotonin and norepinephrine reuptake inhibitor.21 It has a balanced activity when it comes to serotonin and norepinephrine reuptake inhibition. Serotonin and norepinephrine are important neurotransmitters and modulate descending inhibitory pain pathways in the brain and spinal cord, and this underlies the analgesic mechanism of duloxetine.27 Duloxetine is approved for use in PDN by the FDA.21 It is available in 20 mg, 30 mg, and 60 mg strength capsules. For the treatment of PDN, FDA recommends a target dose of 60 mg daily; however, in clinical practice, the dose varies between 20 mg and 120 mg daily. Numerous well-designed studies support the use of duloxetine for the treatment of PDN.28,29 In a parallel-group, double-blind, placebo-controlled study, various doses and regimens of duloxetine were compared to placebo in subjects with PDN. After 12 weeks of treatment, the 24-hour average pain score was significantly improved in subjects taking duloxetine 60 mg daily or 60 mg twice daily compared to those taking duloxetine 20 mg daily or placebo group. In another open-label, randomized, parallel study, subjects were randomized to receive either duloxetine 60 mg twice daily or 120 mg once daily.28 Subjects were followed for 28 weeks. Both regimens were equally effective as measured by the Brief Pain Inventory (BPI) scale. Additionally, both regimens were safe and well tolerated by patients. There is evidence that duloxetine may be a better option compared to other choices such as pregabalin.26 In a multicenter, double-blind, parallel-group study in PDN, subjects were randomized to duloxetine 60 mg daily or pregabalin 300 mg daily, and after 8 weeks those who received duloxetine performed significantly better than pregabalin on all domains of the BPI scales. In addition to PDN, duloxetine is approved for a few other indications including depression, anxiety, fibromyalgia, and generalized musculoskeletal pain. Therefore, duloxetine can be an ideal choice for individuals with PDN with concomitant depression, anxiety, fibromyalgia, or musculoskeletal pain.
Gabapentin
Gabapentin belongs to a class of medication called anticonvulsants. It exerts its analgesic effect by binding to and inhibiting α2δ-1 receptor which is a voltage-gated calcium channel.30,31 Gabapentin is approved for use in the treatment of seizures and post-herpetic neuralgia by the FDA. Gabapentin is often used for the treatment of PDN though it is not FDA-approved for PDN.30 Gabapentin is available in capsule form in 100 mg, 300 mg, and 400 mg strength, and in tablet form in 600 mg and 800 mg strength.30 Gabapentin also has a liquid formulation for those who cannot swallow pills and is available in 250 mg/5 mL strength.30 The efficacy of gabapentin for PDN has been studied in randomized control trials.32,33 In one trial, subjects with PDN were randomized to receive gabapentin or placebo.32 All patients were given 3600 mg of gabapentin each day divided into three doses. Subjects who could not tolerate the 3600 mg per day dose received a reduced dose (900 mg/day, 1200 mg/day, 1800 mg/day, and 2400 mg/day). After 8 weeks of treatment, gabapentin was found to be effective in reducing symptoms of PDN compared to placebo as measured on a VAS. In another trial, gabapentin was compared to amitriptyline and found to be superior in controlling pain and paresthesia on an NRS.33 Despite the analgesic advantages of gabapentin, amitriptyline should be considered in patients in patients with concomitant mood disorder because of its effectiveness in treating depression. Gabapentin dose and regimen vary from patient to patient when it comes to the treatment of PDN. In clinical practice, the dose and regimen range from 300 mg three times daily to 1200 mg three times daily. The maximum dose of gabapentin is 3600 mg/day.30 Gabapentin is primarily eliminated through the kidney; therefore, the dose needs to be adjusted in individuals with renal insufficiency.30 Gabapentin can lead to peripheral edema and should not be used in patients who have pre-existing peripheral edema.
Pregabalin
Pregabalin also belongs to the anticonvulsant categories of medication. Pregabalin acts similarly to gabapentin mechanistically.31 Pregabalin is FDA-approved for use in the treatment of neuropathic pain from PDN, post-herpetic neuralgia, and spinal cord injury.22 It is also used for fibromyalgia and partial onset seizure.2 Pregabalin is available in 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg, 225 mg, and 300 mg capsules as well as a liquid formulation in 20 mg/mL dosage.22 The FDA recommends a maximum of 300 mg per day divided into 3 doses for the treatment of PDN; however, in clinical practice, the regimen is more nuanced and varies with patients with a maximum of 300 mg per day. The effectiveness of pregabalin for the treatment of PDN is demonstrated in randomized clinical trials.34 In one trial, subjects with PDN were randomized to pregabalin 300 mg per day (100 mg three times daily) and a placebo group. After 8 weeks of treatment, the pregabalin group has significantly improved pain VAS scores compared to the placebo group.
Tricyclic Antidepressants
TCAs are a class of antidepressant medication that can be used to alleviate neuropathic pain. It is believed that the mechanism of action of TCAs in neuropathic pain involves multiple pharmacological effects. TCAs are believed to function primarily by inhibiting the reuptake of norepinephrine and serotonin, two neurotransmitters implicated in pain modulation. By increasing the concentrations of these neurotransmitters in the synaptic cleft, TCAs can enhance the inhibitory pathways that modulate pain transmission. This modulation of neurotransmitter activity may contribute to the analgesic properties of TCAs.35 Moreover, sodium channel-blocking properties have been discovered in TCAs. By inhibiting sodium channels, particularly voltage-gated sodium channels, TCAs can reduce the aberrant discharge of neurons in neuropathic pain states. By inhibiting the transmission of pain signals, this action functions to decrease pain perception.35
Amitriptyline was initially studied to evaluate the efficacy in individuals with PDN and either normal or depressed mood. A crossover study was done with 29 patients that were randomly assigned to receive amitriptyline or a placebo for six weeks. During the third to sixth week of treatment, amitriptyline was found to be more effective than the placebo at reducing discomfort. In addition, patients who could tolerate higher concentrations of amitriptyline experienced greater pain relief, with 150 mg per day identified as the optimal daily dose.36
In another study conducted by Max et al, there was a comparison of the efficacy of amitriptyline and desipramine in 38 PDN patients in a randomized, double-blind, crossover study. At 6 weeks 74% of patients treated with amitriptyline and 61% of patients treated with desipramine experienced moderate or greater pain relief. They concluded that both drugs were equally effective in treating PDN, with desipramine functioning as an alternative for patients who were unable to tolerate amitriptyline.37 Furthermore, a randomized double-blind study was conducted comparing amitriptyline and pregabalin for the pain relief of PDN. Fifty-one patients were administered either amitriptyline or pregabalin. Response rates of 73% for amitriptyline and 77% for pregabalin on the McGill and Likert pain scales revealed no statistically significant differences between the two treatments.38
Another study conducted, compared the effectiveness of amitriptyline and gabapentin in treating neuropathic pain in a prospective, randomized, double-blind study. Half of the 28 patients received amitriptyline (25 to 75 mg per day) for six weeks, while the other half received gabapentin (900 to 1800 mg per day). There was no significant difference in pain relief between the two regimens, according to the study.
As an alternative to other recommended treatments, TCAs such as amitriptyline may be considered. Despite the limited evidence, TCAs, particularly amitriptyline, may have a positive effect on neuropathic pain reduction. TCA therapy may be challenging for some patients, as one in five individuals cannot tolerate it. Clinicians need to be cautious and monitor for potential adverse effects, particularly in patients with specific conditions such as narrow-angle glaucoma, benign prostatic hypertrophy, orthostasis, urinary retention, impaired liver function, or thyroid disease.39 Patients with additional cardiovascular risk factors should have their QTc interval assessed to avoid the risk of torsades de pointes, and alternative treatments may be considered if prolongation is present.39
Tapentadol
Tapentadol is a centrally-acting analgesic agent with dual mechanisms of action. It is an m-opioid receptor agonist and norepinephrine reuptake inhibitor.25 Both mechanisms are well established for pain control.27 Tapentadol is approved for use in PDN by the FDA.25 There are two randomized control trials that evaluated the efficacy and tolerability of tapentadol in patients with PDN.25,40 In both trials, subjects with PDN were titrated to tapentadol during a three-week open-label period. Subjects with ≥1-point reduction in pain intensity on 11 NRS at the end of the titration period were randomized to tapentadol versus the placebo group. After 12 weeks of a double-blinded maintenance phase, both trials found tapentadol to be effective and safe for the management of moderate-to-severe PDN. Despite FDA indication, the American Association of Clinical Endocrinology clinical Practice Guideline recommends against the use of opioids (including tapentadol) for the treatment of PDN due to the risks of addiction with long-term use of opioids.41
Tables 3 and 4 summarize the literature and ASPN recommendations for this section.
 |
Table 3 Literature Summary for Pharmacotherapy in PDN |
 |
Table 4 ASPN Recommendations on Pharmacotherapy in PDN |
Section 2. Topical Treatments
While oral medications are commonly used to treat painful diabetic neuropathy, their use is typically limited by significant side effects, a high number-needed-to-treat (NNT), and a high non-responder rate. As such, the application of topical medications is gaining significant interest and has in many instances become a routine aspect of patient care in patients with PDN. While many medications are used in the topical treatment of PDN, this section focuses on the most commonly utilized medications including, lidocaine, capsaicin, and amitriptyline.
Topical Lidocaine
Though not specifically mentioned in the 2021 American Diabetes Association guidelines for standard of care management of PDN, topical lidocaine presents a low-risk option for patients refractory to FDA approved treatments.43 Lidocaine, an amide local anesthetic, mitigates neuropathic pain by reversibly blocking voltage-gated sodium channels on neuronal membranes.44 This action inhibits sodium influx during action potential generation, dampening nerve impulse transmission and inducing a numbing state. Lidocaine preferentially targets channels in hyperexcitable, pathologically signaling neurons, due to its higher affinity for open and inactivated states. This selective targeting lends to its effectiveness in managing chronic neuropathic pain.44,45
Topical lidocaine has been the subject of significant clinical research in the management of PDN for years. In 2004, Argoff et al performed an open-label, non-randomized prospective study with 41 subjects which demonstrated that a 5% lidocaine patch significantly improved pain scores as compared to baseline, with no systemic side effects or drug interactions over a 2-week observation period.44 Barbano et al observed a similar pain reduction (VAS 68.6 at baseline vs 42.4 at 3 weeks, p < 0.001) in a 3-week trial with 56 participants in an open-label, flexible-dosing prospective study.46 While effective as proof of concepts for the utilization of topical lidocaine in the management of PDN, both of these studies had significant limitations in that they were non-randomized, non-blinded, and did not have a control arm.
In 2009, Baronet al conducted the only study to-date evaluating the efficacy of topical lidocaine as a monotherapy for PDN. A randomized, open-label, multicenter, non-inferiority study was performed comparing 5% lidocaine plaster, which is commercially available in Europe, to pregabalin in patients with PDN.47–49 The results of this study were initially reported as an interim analysis, which found similar pain relief in the two groups but that the lidocaine plaster arm (n = 47) experienced fewer drug-related adverse events (DRAE, 3.9% in the lidocaine arm vs 39.2% in the pregabalin arm).47 There was also a substantially higher discontinuation rate due to DRAE in the pregabalin group (n = 44) as compared to the lidocaine group (20.3% vs 13.%).47 While the subsequently published full analysis substantiated the results found in the interim analysis, the authors also reported quality of life measures in the full analysis.48,49 Subjects in the lidocaine arm (n = 105) reported significantly greater improvements in quality of life (QOL) based on the EuroQOL 5-dimension questionnaire (EQ-5D) as compared to the pregabalin group (n = 105).48,49
Although significant limitations exist to the broad applicability of these results, there are sufficient data in the literature to support the use of topical lidocaine in an individualized treatment plan for patients with PDN. Given the low incidence of DRAE in studies evaluating the efficacy of topical lidocaine, it is a worthwhile consideration in patients who are either refractory to traditional FDA approved medications for PDN or in whom side effects limit their use (see Table 5).
 |
Table 5 Literature Summary of Topical Lidocaine in PDN |
Topical Capsaicin
Capsaicin, a naturally occurring compound of chili peppers, treats neuropathic pain by interacting with sensory neurons involved in pain transmission.50,51 It is a highly selective agonist for the transient receptor potential vanilloid 1 (TRPV1) receptor. Nociceptive (C and A-delta) fibers expressing TRPV1 are responsible for transmitting pain signals to the brain. Upon entering the epidermal layers, high concentrations of capsaicin (8% prescription dose) bind and activate the TRPV1 receptors.50
As a TRPV1 agonist, capsaicin initially causes depolarization and action potential propagation, which desensitizes the nociceptive fibers, triggering sensations of warmth, itching, or pain.50–52 This chemical cascade is associated with a marked increase of intracellular calcium, and sustained capsaicin exposure in high doses results in “defunctionalization”, or reversible ablation of the nociceptive fibers, characterized by loss of cell membrane potential, receptor desensitization, substance P depletion, and reduced nerve fiber density, thus decreasing pain signals sent to the brain.50 Additionally, capsaicin-activated TRPV1 in immune cells can mitigate inflammation, further contributing to its analgesic and potential anti-inflammatory effects.51
Topical capsaicin’s role in the management of PDN has been substantiated by many clinical trials. The Capsaicin Study Group and Tandan et al demonstrated that 0.075% capsaicin cream significantly improved pain compared to placebo in 8 and 12-week trials, respectively.53,54 Biesbroeck et al reported similar results in an 8-week trial.55 However, these studies noted high dropout rates due to side effects, such as skin irritation.51
While lower doses have been around for much longer, a critical advancement was reported by Mou et al in their comprehensive review.56 They presented evidence that a single application of a higher dose, 8% capsaicin patch, could provide pain relief for up to 12 weeks in patients with focal neuropathic pain.56 Subsequently, in a randomized controlled trial by Simpson et. al, in a study of 369 patients, the mean percentage change in “average daily pain” score from baseline to weeks 2–8 was significantly greater in the capsaicin group (−27.4%) than in the placebo group (−20.9%) (p<0.001).51 Moreover, the incidence of adverse events was similar in both groups, suggesting that this high-concentration capsaicin patch provided substantial pain relief without increasing side effects.51
Anand et al reported similar results in a randomized control trial with 50 participants in which patients were randomized to either 8% capsaicin patch along with standard of care (SOC) medication management or SOC alone. Patients in the capsaicin plus SOC group fared significantly better with a clinically and statistically meaningful improvement in NRS pain scores at 3-month follow-up, as compared to SOC alone.50
On the basis of this clinical evidence, 8% capsaicin patch is an FDA approved treatment for PDN and should be considered as a treatment option that can provide moderate relief, especially when initial oral agents are ineffective or provide only partial pain relief (see Table 6).
 |
Table 6 Literature Summary of Topical Capsaicin in PDN |
Topical Amitriptyline
Amitriptyline, a tricyclic antidepressant, is used topically for neuropathic pain, including diabetic neuropathy.57,58 Its analgesic action primarily stems from inhibiting norepinephrine and serotonin reuptake, enhancing their neurotransmission and reducing pain perception.57,58 It also antagonizes peripheral and central N-methyl-D-aspartate (NMDA) receptors, inhibiting excitatory neurotransmitter glutamate release. Furthermore, amitriptyline blocks voltage-gated sodium and calcium channels, reducing neuronal excitability, particularly relevant in hyperglycemia-induced neuropathic pain.57 The exact mechanism of the topical formulation remains unclear, but it is suggested that the drug penetrates the skin to exert local and potentially systemic effects.
The effectiveness of topical amitriptyline is not well delineated in the literature. In a double-blind, randomized, placebo-controlled crossover trial by Ho et al, 35 subjects with postsurgical neuropathic pain, postherpetic neuralgia, or PDN were randomized to receive either topical 5% amitriptyline or placebo.57 The study did not show any clinically meaningful difference in pain scores between the two groups. Certainly, these results are marred by a small sample size overall and an even smaller group of PDN patients within the overall cohort.57 However, a double-blind, randomized controlled trial by Kiani et al compared topical 2% amitriptyline to 0.75% capsaicin cream. The results showed that while both were effective in managing the painful symptoms of PDN, amitriptyline did so with fewer DRAE and improved patient compliance.58
Given there is conflicting evidence in the literature regarding the efficacy of topical amitriptyline, this medication should not be used as a first line treatment for PDN. However, its use may certainly be warranted in patients refractory to first- and second-line therapeutic agents, as the drug does have a favorable side effect profile (see Table 7). Overall, there is moderate evidence for topical medications in the treatment of PDN (see Table 8).
 |
Table 7 Literature Summary of Topical Amitriptyline in PDN |
 |
Table 8 ASPN Recommendations for Topical Medications in PDN |
Section 3. Neuromodulation
Spinal Cord Stimulation
Spinal cord stimulation (SCS) for the treatment of PDN was initially thoroughly described in three different studies, all published in 2014. De Vos and colleagues performed a multicenter randomized controlled trial investigating the effectiveness of traditional SCS in patients with PDN.59 Sixty patients with PDN in the lower extremities unresponsive to conventional medical therapy were enrolled and followed for six months. They were randomized 2:1 to conventional medical therapy with SCS (SCS group) or without SCS (control group) therapy. At each follow-up visit, the EQ-5D, the short form McGill Pain Questionnaire (SF-MPQ), and a 0–100 VAS to measure pain intensity were recorded. At baseline, the average VAS score for pain intensity was 73 in the SCS group and 67 in the control group. After six months of treatment, the average VAS score was reduced to 31 in the SCS group (P<0.001) and remained 67 in the control group (P = 0.97). The reported responder rate was 69% in the SCS group. Similarly, the EQ-5D and SF-MPQ questionnaires also showed that patients in the SCS group experienced reduced pain and improved health and quality of life after six months of treatment, while those in the control group did not.59
Slangen and colleagues published results from a two-center randomized controlled trial studying traditional SCS versus best conventional medical management for the treatment of PDN.60 Thirty-six PDN patients with severe lower limb pain not responding to conventional medical therapy were enrolled. Twenty-two patients were randomly assigned to conventional medical therapy with SCS (SCS group) and 14 to best medical therapy only (BMT group). The SCS system was implanted only if trial stimulation was successful. Treatment success was defined as ≥50% pain relief during daytime or nighttime or “(very) much improved” for pain and sleep on the patient global impression of change (PGIC) scale at six months. Trial stimulation was successful in 77% of the SCS patients. Treatment success was observed in 59% of the SCS and in 7% of the BMT patients (P < 0.01). Pain relief during daytime and during nighttime was reported by 41 and 36% in the SCS group and 0 and 7% in the BMT group, respectively (P < 0.05). Pain and sleep were “(very) much improved” in 55 and 36% in the SCS group, whereas no changes were seen in the BMT group, respectively (P < 0.001 and P < 0.05).60
Also in 2014, de Vos et al published results from a study in which patients with PDN or failed back surgery syndrome (FBSS) treated for six months with traditional SCS were transitioned to burst SCS for two weeks.61 A total of 48 patients were in the overall study, of which 12 patients were diagnosed with PDN. At baseline, these patients had a VAS of 70. With traditional SCS, they reported a VAS of 28 (P<0.001) and with burst SCS a VAS of 16 (P<0.001), and this difference was statistically significant (P<0.05). The effect of burst SCS was more evident in the PDN patients compared to the FBSS patients (77% vs 57%). Eight of the 12 patients had more pain reduction with burst stimulation as compared to traditional SCS.61 At this time, no other studies exploring burst SCS for use in PDN have been published.
In 2018, Van Beek et al reported the longest follow-up data for traditional SCS in treating PDN. Forty-eight patients with PDN were included in this prospective multicenter study.62 The Michigan Diabetic Neuropathy Score (MDNS) was used to assess the severity of neuropathy. During the five years of follow-up, the NRS score for pain, PGIC, and treatment success (50% reduction of NRS score or significant PGIC) were evaluated. Treatment success was observed in 55% of patients after five years. Of those patients that underwent a permanent implant, 80% were still using their SCS device after five years. Interestingly, higher MDNS was associated with treatment failure during the five-year follow-up time period (P = 0.014).62
Recently, Petersen et al explored the benefit of 10 kHz high frequency (HF)-SCS in treating patients with PDN in the SENZA-PDN study.63 In this multicenter, open-label, randomized controlled trial, a total of 216 patients were randomized 1:1 to either the HF-SCS with conventional medical management arm (treatment arm) or conventional medical management alone arm (control arm). Following randomization, 113 patients were included in the treatment arm and 103 in the control arm. They reported 78.9% (75/95) of the HF-SCS arm and 5.3% (5/94) of the conventional medical management (CMM) arm obtained >50% pain relief and had no neurologic deterioration at six months (P < 0.001). After allowing crossover from the control arm to the treatment arm at six months, the same researchers reported the 12-month results following implantation for all implanted patients.64 The average pain relief was 74.3%, and 85% of patients had >50% pain relief without further neurological decline. Additionally, health-related quality of life measures, including EQ-5D-5L (baseline overall health), Diabetes Quality of Life score, Pain and Sleep Questionnaire 3-Item Index (sleep quality), BPI (pain interference), and Global Assessment of Functioning (social, occupational, and psychological functioning) were all improved at six and 12 months. Recently, long-term efficacy of 10kHz was published in a 24-month publication on the previously described RCT. At 24 months, the investigators reported that 10kHz reduced pain by a mean of 79.9% compared to baseline, with 90.1% of participants experiencing greater than 50% pain relief. Participants also had improvements in quality of life, sleep, and neurological symptoms. Only 3.2% of implants were explanted due to infection, supporting the safety of implantable therapies in the diabetic population when appropriate measures are taken.65
The findings of these studies were substantiated by a recent systematic review assessing the use of SCS for treatment of pain in length-dependent peripheral neuropathy.66 Neuropathies included in this review extended beyond just PDN and included chemotherapy-induced neuropathy and other polyneuropathies. This systematic review comprising nineteen studies (376 participants who underwent SCS implantation) demonstrated that all studies reported significant improvement in pain intensity after 12 months of SCS therapy compared to baseline. However, the quality of evidence for this outcome per the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria was very low quality, indicating the need for future high-quality trials to strengthen the use of SCS for all neuropathies beyond just PDN.66
Overall, SCS is a well-studied and proven intervention for the treatment of PDN. Both traditional SCS and the more contemporary HF-SCS have been studied in randomized controlled trials and shown to be more efficacious than conventional medical management for the relief of pain related to PDN (see Tables 9 and 10).
 |
Table 9 Literature Summary for SCS in PDN |
 |
Table 10 ASPN Recommendations for SCS in PDN |
Peripheral Nerve Stimulation
Background
Peripheral nerve stimulation (PNS) has emerged as a potential treatment modality for PDN, offering an alternative to conventional pharmacological approaches. The utility of PNS in various pain conditions including neuropathic pain,67 complex regional pain syndrome (CRPS),68 phantom and residual limb pain,69 low back pain,70,71 and postoperative pain72 has been well described. The current state of the literature on PNS for PDN is limited; more study is needed to assess the utility of PNS in people with diabetes.
PNS may not be appropriate for managing symptoms in patients with diffuse PDN involving multiple nerve distributions with a sizeable dermatomal area. The limitation of PNS is that current systems can only accommodate 1 or 2 leads which may not be adequate to cover a patient’s pain. In some cases, larger mixed motor-sensory nerves can be targeted (ie, the sciatic nerve). However, it can be difficult to selectively target afferent Aβ fibers and avoid stimulating efferent Aα motor fibers.
Some of the benefits of PNS include use in patients who may not be candidates for SCS or dorsal root ganglion stimulation (DRG-S) because of complex spine anatomy, prior spinal surgery or fusion, or inability to discontinue anticoagulants. PNS is considered a low to intermediate risk depending on the location of lead placement and proximity to vasculature and other critical structures.73 In many cases, PNS can be done safely without discontinuing blood-thinning medications.74 Temporary and permanent options are commercially available, giving patients a variety of options for treatment.
Nerve Targets and Patient Considerations
PDN patients with relatively focal neuropathic pain may be good candidates for PNS. For example, if most of the pain is localized to the plantar surface of the foot, the tibial nerve could be targeted with PNS. The tibial nerve runs from the popliteal fossa through the deep posterior compartment of the leg before passing through the tarsal tunnel, dividing into the lateral plantar nerve (LPN), medial plantar nerve (MPN), and medial calcaneal nerve (MCN). Lead placement along the tibial nerve will generally occur proximal to the tarsal tunnel and posterior to the tibia.75 Other potential nerve targets for treating PDN in the lower extremity include the common peroneal, superficial peroneal, and sciatic nerves. It should be noted that other neuromodulation modalities (SCS and DRG-S) have focused mainly on PDN in the lower extremities.63,76 Another benefit to PNS is that targets in the upper extremity, notably the median and ulnar nerves, could also be considered.
Peripheral nerves in the lower extremity, including the tibial and common peroneal nerves, are prone to entrapment at the tarsal tunnel and fibular head, respectively. It has been proposed that diabetic patients are at increased risk of complications due to increased compression in these areas, leading to nerve damage and irreversible sensory changes. Decompression neurolysis has been performed in diabetics in the upper (median, ulnar) and lower extremities (tibial, common peroneal, deep peroneal, superficial peroneal nerves) with promising results.77,78 PNS can be an option for patients who have undergone decompression surgery and continue to have focal pain. When planning the location of PNS placement, it is advisable to consider placing leads proximally to the suspected lesion, entrapment, or injury.
Current Evidence for PNS in PDN
The evidence for using PNS in PDN is evolving, and several challenges still need to be addressed. One key challenge is the need for standardized protocols and parameters for PNS treatment. A recent review of neuromodulation in PDN79 found grade II-3 evidence across three retrospective and prospective cohort studies showing the benefit of using PNS to target the tibial nerve in the lower leg. In an older study examining percutaneous electrical nerve stimulation (PENS) of lower extremity nerves (tibial, deep peroneal) in PDN patients, positive improvements in pain were demonstrated.80 Larger-scale, well-designed clinical trials with extended follow-up periods are needed to determine the durability of pain relief and potential adverse effects associated with PNS.
Future Directions
In conclusion, the evidence for using PNS in PDN is evolving but shows promise as a potential therapeutic option for managing pain associated with PDN (see Table 11). Patients who may benefit the most are those with localized pain that can be attributed to a single nerve. However, further research is needed to establish standardized protocols, evaluate long-term efficacy and safety, and assess cost-effectiveness. With continued advancements in PNS technology and more robust clinical evidence, peripheral nerve stimulation has the potential to become an important tool in the multidisciplinary management of PDN. The SWEET Consensus Committee recommends consideration of PNS for patients with focal lower extremity PDN who have failed conservative therapies and are not candidates for SCS (see Table 12). (Grade C)
 |
Table 11 Literature Summary for PNS in PDN |
 |
Table 12 ASPN Recommendations for PNS in PDN |
DRG Stimulation
DRG-S is approved for CRPS and/or peripheral causalgia in the groin and lower limb. Application of DRG-S to other painful conditions of the lower extremity, such PDN, is actively developing. The ability to directly modulate the Aδ and C small fibers that innervate the skin and subcutaneous tissue, at level Aβ collaterals, and taking advantage of convergence in the dorsal horn, would appear to make DRG-S an optimal therapy for pain associated with peripheral neuropathic pain. Furthermore, DRG-S can normalize pathologic hypersensitivity of DRG neurons associated with neuropathic pain and suppress inflammatory responses, particularly those driven by glial cells. There are multiple experimental animal model studies testing stimulation paradigms at the DRG for PDN.83,84
Advantages of DRG-S relative to SCS are the ability to achieve focal coverage of concordant painful areas with minimal extraneous stimulation. This may be due to the direct recruitment of relevant primary sensory neurons that innervate the painful distal regions as opposed to more generalized stimulation of the dorsal column.
There is a small prospective study, three retrospective case series, and five case reports for the application of DRG-S for varying causes of peripheral neuropathy (PN).85–89 These studies included diagnoses of PDN, painful small-fiber PN, idiopathic PN, polysensory PN, hereditary sensory and autonomic PN, PN associated with Lyme’s disease, and chemotherapeutic agent-induced PN. There are two additional DRG-S case series that included patients with multiple concomitant pain etiologies which included peripheral neuropathy. There are two retrospective studies76,90 and one case report85 specifically for the indication of PDN.
Eldabe et al retrospectively reported on seven PDN patients having undergone device implantation.76 Of the seven subjects that had stimulators implanted, two had the devices explanted at or before the one-week follow-up for either poor painful area coverage or personal reasons. Of the seven patients who underwent full implantation, three achieved ≥50% pain reduction at the six-month follow-up, two of whom had a reduction of more than 80%. At 12-month follow-up, two patients achieved ≥50% pain reduction, with one patient lost to follow-up.
A retrospective analysis on PDN patients was completed by Falowski et al.90 Inclusion criteria included chronic intractable peripheral neuropathy of the legs and/or feet and responding successfully to a trial of DRG-S with leads at L4-S1. Eight consecutive patients across two study centers were included (7 males, 1 female; mean age: 64.8 ±10.2 years). Two of the eight patients had PDN. Data pertaining specifically to PDN patients was not included. Visual analog scale pain scores and pain medication usage were collected at the baseline visit and after six weeks of treatment. Two patients reported complete (100%) pain relief, two patients reported better than 80% pain relief, and another three patients reported better than 50% pain relief. A single patient reported better than 40% pain relief.
Chapman et al published a case report of a patient with both PDN and low back pain.85 He underwent a 7-day trial of unilateral DRG stimulation at T12 and S1, which allowed the untreated side to serve as the control. The trial resulted in significant pain relief in both feet and low back pain, with a VAS reduction from 9 to 0 for feet pain. Additional measures including the Oswestry Disability Index (ODI), EQ-5D and SF-36 all showed significant improvement. Implantation data was not made available.
Evidence for DRG-S for PDN is limited by retrospective nature of studies, lack of blinding, and limited number of patients. Despite these limitations, DRG-S is shown to be effective for not only pain but also function and quality of life measures (see Table 13). Further studies are needed to elucidate the efficacy of DRG-S applied to PDN. At this time, recommendation is for selective and judicious use of DRS-S to PDN patients based on professional judgment, along with previous treatments tried and failed (see Table 14).
 |
Table 13 Literature Summary for DRG-S in PDN |
 |
Table 14 ASPN Recommendations for DRG-S in PDN |
Intrathecal Drug Delivery
Ziconotide is a nonopioid analgesic medication that reversibly blocks pronociceptive neurotransmitter release from afferent nerves in the dorsal horn of the spinal cord via binding of N-type voltage-sensitive calcium channels.91 Specifically, glutamate, calcitonin gene-related peptide (CGRP), and substance P from primary nociceptive afferents terminating in the superficial layers of the spinal cord dorsal horn are blocked from being released.91 The only clinically available route of administration is via the intrathecal route. Ziconotide does not affect mu-opioid receptors, and typical reversal agents such as naloxone have no effect. There is no evidence of tolerance with long-term administration, and sudden cessation does not cause withdrawal syndrome.92
The medication’s on-label FDA approval is for administration via a microambulatory delivery device with a recommended starting dose of 0.5–1.2 mcg/day per the Polyanalgesic Consensus Conference (PACC) but can be as high as 2.4 mcg/day per the product labeling. Upward titration of 0.5–1.0 mcg/day every several days is recommended as ziconotide has a narrow therapeutic window and a max dose of 21.6 μg/day.93 Ziconotide carries a “black box warning” and is contraindicated in patients with a history of psychosis; thus, psychiatric evaluation should be completed before trialing medication. Side effects of ziconotide which are more common at higher doses include nausea, vomiting, confusion, postural hypotension, gait abnormality, urinary retention, nystagmus, drowsiness, dizziness, weakness, visual changes, and serum creatine kinase elevation.94
There are three randomized, double-blinded, placebo-controlled studies that support the use of intrathecal ziconotide for non-cancer-related pain,95–97 with approximately 75% of patients having a neuropathic pain condition. In a 2021 prospective study of 14 patients, it was shown that ziconotide improves pain and emotional components and function, specifically improving disability, emotional well-being, and catastrophizing.98 Based on the available evidence (see Table 15), the SWEET guidelines support careful selection of intrathecal therapy of diabetic neuropathic pain with ziconotide although there are no published studies that specifically investigate its effectiveness for PDN (see Table 16).
 |
Table 15 Literature Summary of Intrathecal Drug Delivery in PDN |
 |
Table 16 ASPN Recommendations for Intrathecal Drug Delivery in PDN |
Sub-Topic: Special Considerations for Implantable Therapies in the Diabetic Patient
DM is an increasingly prevalent chronic multisystemic condition that is associated with increased perioperative morbidity and mortality. During periods of heightened stress, such as surgery, significant changes may occur in glucose metabolism leading to acute hyperglycemia. This occurs in up to 40% of patients undergoing noncardiac surgery.99 Poorly controlled DM negatively impacts soft tissue and tendon healing, rendering diabetic patients at an increased risk of poor wound healing and surgical site infections. More specifically, chronic hyperglycemia is associated with impaired neutrophil phagocytic activity, increased inflammation and oxidative stress, and poor endothelial function.100 These variables contribute to a relative immunocompromised state. Decreased innate immunity has been found to be the key factor in diabetic patients that results in increased infection in the setting of implanted devices. Being aware of surgical technique is important not only to help mitigate postoperative infections but also seromas and wound dehiscence. Diabetic patients have been found to be at an increased risk of these postoperative complications.101
Utilizing the data gathered from orthopedic and cardiac surgery literature, we can deduce the implications of DM on surgical outcomes within pain medicine and neurosurgery. While the incidence of surgical site infections is variable amongst chronic pain implantable devices (1% to 17%), diabetic patients are at increased risk of severe infection.102 More recent studies have revealed that the rate of SCS infections can be as high as 3.11% within a 12-month period.103 Hoelzer et al reported an overall infection rate of 2.45% of SCS implants, which included diabetic patients, but did not further assess the role of uncontrolled versus controlled diabetes.104 The pathogenesis of an infection relates to the initial innate immune response to the formation of a biofilm around the implanted device.105 Multiple factors have been identified in impairing the innate immune response and include absence of vascularization at the interface with the device, disrupted blood flow in the vicinity of the device due to tissue damage during surgery, local hypoxia, dysregulation of phagocyte function on foreign materials, inadequate immune signaling between the inert biomaterial and host cells, and protection of contaminating microorganisms from phagocytosis due to attachment to the implant – all factors impacted by DM.105 This highlights the systemic micro- and macrovascular implications of DM.
Lab markers, primarily hemoglobin A1C or glycated hemoglobin, may be used to identify diabetic patients with poorly controlled DM. A1C is commonly used as a surrogate for glycemic control, but there remains no clear consensus on A1C cutoff values for elective surgery. This is partly due to an ongoing debate regarding whether long-standing hyperglycemia or acute perioperative hyperglycemia has more significant implications on surgical outcomes. A study by Underwood et al conveys that A1C >8% is related to poor surgical results.100 This cutoff was also found to be associated with an increased rate of postoperative surgical site infections by Gabriel et al.106 Nonetheless, while variable cutoff target values of A1C may exist, severe and prolonged perioperative hyperglycemia may result in greater operative complications than acute hyperglycemia alone.107 See Tables 17 and 18.
 |
Table 17 Literature Summary Special Considerations for Implantable Therapies in PDN |
 |
Table 18 ASPN Recommendations in Special Considerations for Implantable Therapies in PDN |
Non-Invasive Neuromodulation
The use of a time-varying magnetic field to induce a sufficiently strong current to stimulate living tissue was first reported by d’Arsonval in 1896.112 Magnetic stimulation of nerve tissue was demonstrated by Oberg in 1973.113 The first magnetic stimulation of peripheral nerves (mPNS) was reported by Polson in 1982.114 He established that mPNS, as compared to electrical peripheral nerve stimulation (ePNS), was pain free and could reach deep nerves. Another advantage over electrical stimulation is that higher current densities near the surface of the skin which can cause tissue damage are not seen in magnetic stimulation as there is no hydrolysis and pH changes. mPNS induces a larger E field due to its time-varying, 3D magnetic field, whereas ePNS generates a smaller more 2D electrical field due to being a line source electrode. Due to the differences in the resultant electrical fields, the stimulation threshold in mPNS recruits many more Aβ fibers without recruiting Aδ fibers as compared to ePNS.
The first pilot study using a new mPNS device involved 24 patients with neuropathic pain, including those with painful diabetic neuropathy.115 The active treatment wand was placed on or just over the patient’s skin. The treatment protocol consisted of three daily sessions in a row during the first week of therapy. This was followed by a weekly treatment for the remainder of the month totaling 6 treatments. There were treatments every second week in the second month. Monthly treatments were continued as needed for pain exacerbations. Two-thirds of patients were deemed responders, defined as 50% or more reduction in VAS pain scores, experiencing 87% reduction of pain scores. The average VAS pain reduction was 3.8 from baseline scores. Opioid reduction was achieved in 58.3% of responders.
A first randomized prospective study on mPNS, including patients with mono- and peripheral neuropathy, was recently conducted, and 90 days data are available in the form of an abstract to the 2023 ASPN meeting.116 There were 13 patients who had PDN in the active treatment arm (mPNS plus CMM), and 4 patients in the control arm (CMM only). Responders were defined as subjects who achieved >50% of pain relief at 3 months after the treatment initiation. There were 8 out of 8 responders in the treatment arm on a per-protocol basis, with reduction of their pain scores by 64.7%. In the control arm, 1 out of 4 were responders, with an average increase of the pain scores by 2.4%. mPNS appears to be a very promising noninvasive, painless initial neuromodulation therapy for PDN as well as other neuropathic conditions. A randomized sham-controlled prospective study on use of mPNS for the lower extremity peripheral neuropathy in diabetes is ongoing, and enrollment completion is expected by the end of the year. See Tables 19 and 20.
 |
Table 19 Literature Summary of Non-Invasive Neuromodulation in PDN |
 |
Table 20 ASPN Recommendations for Non-Invasive Neuromodulation in PDN |
Section 4. Alternative Approaches
Aside from implantable therapies for treatment of PDN, alternative treatment approaches have historically focused on non-implantable interventions, dietary supplementations, and lifestyle modifications. Such interventions trialed in the past include acupuncture, sympathetic nerve blocks, and botulinum toxin injections.
Moderate level evidence suggests favorable utilization of acupuncture as an optional treatment for PDN.117–120 Numerous prospective clinical studies and RCTs suggest a positive effect with relatively low adverse event rates.119,121–125 A single-blinded, placebo-controlled RCT compared acupuncture to sham acupuncture demonstrated low-moderate treatment effect without appreciable side effects, meanwhile another RCT found that pain was improved at week 12 of follow-up but efficacy waned by week 18 when acupuncture was compared to standard of care.123,124 Outside of traditional acupuncture, modifications of acupuncture have been proposed to be effective for treatment of PDN. Electroacupuncture whereby current applied to acupuncture needles was found to reduce neuropathic pain while improving sleep and overall quality of life. Laser acupuncture was found to improve nerve conduction velocities and patient reported outcomes compared to placebo for PDN.121,125 Other studies report improvement of PDN symptoms with injections of Saussureae Involucratae Herba (snow lotus; a herbal medicine suggested to accelerate blood circulation and have anti-oxidative properties) at acupuncture points and perineural platelet rich plasma, though further studies are needed to validate this approach.126,127 Meta-analyses support these findings, but suggestions have been made for further studies to assess the longitudinal efficacy of traditional acupuncture for treatment of PDN. If acupuncture is trialed, the semi-standardized acupuncture in diabetic peripheral neuropathy (ACUDPN) treatment protocol with bilateral acupuncture points is recommended.119
Few studies support the utilization of lumbar sympathetic ganglion block or neurolysis for PDN.128–131 One case report documented improvement in bilateral lower extremity PDN with a series of nine continuous lumbar sympathetic blocks over a 26-month period.129 Another RCT suggested combining treatment with continuous lumbar sympathetic block followed by alcohol neurolysis provided more benefit in pain scores versus sympathetic alcohol neurolysis alone, with benefits sustained at 6 months post treatment.130 Similarly, another RCT compared alcohol neurolysis with radiofrequency thermocoagulation or both for treatment of PDN. Postoperative pain scores were significantly decreased from baseline with a 66.7%, 73.3% and 93.3% complete remission rate, respectively, without severe complications. However, pain returned at 3 months after alcohol neurolysis, 6 months after radiofrequency thermocoagulation and 1 year after combined treatment. The authors concluded that lumbar sympathetic ganglion block combined with radiofrequency was safe and effective in managing PDN.131 These interventions may be a viable option for selected patients with refractory PDN prior to other more invasive interventions.128
Similarly, few studies have demonstrated optimistic results utilizing botulinum toxin injection for PDN without major complications. Botulinum toxin type A inhibits the release of acetylcholine at the neuromuscular junctions and may also have a modulatory effect on afferent sensory fiber firing by inhibiting glutamate release, decreasing calcitonin gene related peptide and substance P release.132–135 A double-blind RCT demonstrated that intradermal botulinum toxin type A injection into the foot reduced neuropathic pain and improved quality of life and sleep in people with PDN compared to control.132 Similarly, other studies have found that botulinum toxin type A is well tolerated and significantly reduced PDN, compared to normal saline placebo injections.133,134 One meta-analysis concluded that there are level I studies to support a correlation between botulinum toxin A injection and a small improvement in pain in diabetic neuropathy, particularly in the dorsum of the feet. However, given the small effect size, botulinum toxin A should only be considered as an adjunctive treatment to first-line modalities and further studies are needed.135 Of note, botulinum toxin injection for PDN is not an FDA-approved indication.
The majority of supplements utilized to address PDN fall into the category of antioxidants and neuroprotective cofactors.136 Alpha-lipoic acid, an antioxidant and chemoprotective compound, has been investigated in treatment of PDN. Studies have shown improvement of PDN symptoms with long-term use, however efficacy of this has yet to be compared to typical pharmacologic agents used for treatment of PDN.137–139 Acetyl-L-carnitine, an acetylated amino acid, has also been implicated in the treatment of PDN. It has been used for treatment of various forms neuropathic pain including PDN with success.140–142 However, high-grade evidence is not available to evaluate its efficacy compared to other pharmacologic agents. Some studies have evaluated the use of the vitamin B complex (B1, B6, B12) in treatment of PDN due to its antioxidant effects as well as its function in neural metabolism and neural protection. However, no recommended dosing is available for safe treatment as increased levels can cause neuropathy.143 Benfotiamine, a lipid formulation of vitamin B1 has also been evaluated for treatment of PDN. High-level studies to validate its use are also lacking.136
Lifestyle modifications remain one of the oldest and most traditional means of management for PDN, focusing on improved glycemic control, particularly in patients with type 1 DM (T1DM).144 Though intensive glycemic control can help delay the development and progression of PDN in patients with T1DM, it has been found to have little effect on PDN in patients with type 2 DM (T2DM).145–148 The Diabetes Control and Complications Trial (DCCT) emphasized enhanced glycemic control in patients with T1DM with a −1.84 annualized risk difference with tight glycemic control, whereas the ACCORD and VADT studies reported an annualized risk difference of −0.058 for T2DM which was not statistically significant.144,145,149,150 Moreover, tight glycemic control of hemoglobin A1C less than 6.0 has been associated with increased mortality. As such, improved glycemic control with hemoglobin A1C between 7.0 and 8.0 has been recommended instead.146 Weight loss as well as exercise has been shown to increase intraepidermal nerve fiber density which is normally reduced during PDN.151,152 In combination, an improved diet with active lifestyle has been advocated to help reduce the severity of PDN symptoms.
A summary of the literature cited in this section appears in Table 21 and ASPN recommendations are in Table 22.
 |
Table 21 Literature Summary for Alternative Approaches in PDN |
 |
Table 22 ASPN Recommendations for Alternative Approaches in PDN |
Psychological/Behavioral
PDN is a complex condition with physical, social and psychological consequences.153 Given its complexity, multidisciplinary treatment approaches are likely to be more effective than any monotherapy.
For many years, PDN’s comorbidities with depression,154,155 anxiety,156,157 catastrophization,158,159 and impairment of patients’ QOL160,161 have been identified in the literature, with these psychological factors thought to play a potential mediating role in patient’s pain experiences. As a result, logic would suggest that these psychological sequelae of PDN should be treated in order to optimize patients’ emotional comfort levels, at the least. For this review, however, the focus will be on the literature addressing psychological treatments aimed at reducing patients’ pain severity, pain experience and levels of pain interference.
Psychological approaches commonly studied in the treatment of PDN include cognitive behavior therapy (CBT), mindfulness therapy (MT) (either mindfulness meditation (MM) or mindfulness-based stress reduction (MBSR)) and, most recently, Acceptance and Commitment Therapy (ACT).
RCTs
The initial RCT on MM in PDN was a pilot published by Teixeira in 2010.162 In a small study, the author determined that subjects experienced significant improvements in QOL compared to controls, although relative changes in pain severity and pain unpleasantness were insignificant. The study’s methodology was weak, with the author noting numerous limitations.
The initial RCT on CBT for PDN was published in 2013 by Otis and colleagues.163 RCT patients reported significantly reduced pain severity and pain interference from pretreatment to 4-month follow-up, unlike treatment-as-usual controls. Interestingly, however, neither group reported a significant change in depressive symptoms. This finding is surprising, as CBT has its roots in the treatment of depression. Any minimal and insignificant decreases in pain severity and pain interference cannot be considered particularly strong evidence, as this was a pilot involving only 12 subjects in the treatment group and 8 control group subjects.
Of note regarding ACT for chronic pain is a 2020 Iranian RCT in which pain perception and pain acceptance were significantly better both immediately following a course of ACT and at 3-month follow-up when compared to control patients.164 The authors, however, noted a small sample size due to a high dropout rate based on their inclusion-exclusion criteria in discussing the study’s limitations, which may have resulted in sampling bias. This is the only RCT in the extant literature that has considered ACT’s impact on pain perception.
All published RCTs of psychological treatments for reduction of pain severity, pain experience and levels of pain interference are summarized in Table 23, and group recommendations are given in Table 24.
 |
Table 23 Literature Summary for Psychological Treatment of PDN |
 |
Table 24 ASPN Recommendations for Psychological Interventions in PDN |
Systematic Reviews
Because of the paucity of meaningful RCTs on psychological treatments that alter PDN pain experience, the limited systematic reviews that have been attempted tell us little of value. For example, a 2015 Cochrane review on psychological treatments for chronic neuropathic pain written by esteemed authors169 chose to include only 2 studies, neither of which was PDN. A 2016 systematic review from an Italian consensus conference on pain in neurorehabilitation170 considered only a single study,163 and concluded, “For the treatment of diabetic neuropathy and neuropathic pain associated with cancer or HIV [human immunodeficiency virus], CBT may be used”. The authors graded their evidence as a D [evidence level 3 or 4 or extrapolated evidence from studies rated as 2+]. (GPP [recommended best practice based on the clinical experience of the guideline development group]). The most recent systematic review and meta-analysis of CBT and MT in the treatment of PDN171 determined that at the conclusion of treatment, experimental groups reported significantly less pain than control group patients, although at follow-up at ≥2 weeks, no significant differences between groups were evident. Further, the authors determined that although there were no immediate post-treatment differences between groups in terms of pain interference, the CBT/MT groups experienced significantly less pain interference than did control group patients at 24-week follow-up. Unfortunately, this systematic review and meta-analysis was seriously flawed methodologically in a number of ways, with selection of articles for inclusion in their analysis questionable.
Section 5. Algorithmic Approach
We describe an algorithm (see Figure 2) that can be utilized to treat PDN, although treatment options should be specific and individualized to the patient, accounting for comorbidities, type and severity of diabetes, and other patient-related variables.
The initial tier of treatment modalities centers on preventive strategies and conservative treatment. The natural history of PDN in patients with diabetes is variable,172 yet studies suggest that persistent hyperglycemia may be a primary cause of nerve dysfunction, resulting in hyperexcitable painful pathways in the peripheral and central nervous system. Therefore, a reasonable first step in the treatment of PDN is to treat the major underlying cause of neuropathy – hyperglycemia. Glycemic control may not only prevent PDN, but in those who already have established PDN symptoms, glycemic control may partially reverse or modulate these painful symptoms.172 This is particularly notable in type 1 diabetes with high-quality evidence supporting that intensive glycemic control is associated with lower odds of distal symmetric polyneuropathy compared to conventional insulin therapy.24,173 However, intensive glycemic control in type 2 diabetes has not consistently shown benefit in preventing or treating PDN symptoms, and may increase the risk for hypoglycemia. In terms of additional conservative treatment modalities, physical therapy including long-term aerobic exercise, weight-bearing exercise, and massage therapy should be offered early in the course of PDN as these modalities may improve motor and sensory neuropathy, balance, and quality of life.174–176
Oral analgesics are commonly trialed thereafter, or concomitantly with, conservative therapy. First-line oral analgesics include gabapentinoids (pregabalin and gabapentin) and serotonin-norepinephrine reuptake inhibitors (duloxetine).24 Careful titration and monitoring for side effects (eg, dizziness, sedation) are warranted. If the patient does not obtain meaningful pain relief or experiences intolerable side effects, other second-line and third-line oral analgesics may be trialed. For instance, tapentadol is approved by the FDA for treatment of PDN, although it is an opioid analgesic with risk for opioid-related side effects. Tricyclic anti-depressants such as amitriptyline and nortriptyline may also provide analgesia, although they commonly manifest with neurologic and cardiac side effects. Opioids are generally not recommended177 due to modest short-term analgesia and an unfavorable safety profile consisting of respiratory depression, sedation, opioid use disorder, and constipation.24,178 When conservative treatment options and oral analgesics fail, clinicians may consider offering topical therapy. Eight percent capsaicin patch is approved by the FDA for treatment of PDN of the feet.51 The risk of application site reactions is substantial and may impact over 30% of patients, although strategies exist to improve patient tolerability. Given its non-invasiveness and safety profile, 8% topical capsaicin is a very reasonable first-line option after the failure of at least two proven oral pharmacological agents.
If the patient continues to experience refractory pain that is unresponsive to conservative modalities and pharmacologic agents (eg, trial of at least 1–2 analgesics) and topicals, consideration of interventional options is warranted.79
Neuromodulation interventions may alleviate symptoms of PDN by intervening within the ascending pathways that lead to perception, cognition, and awareness of the symptoms. Therapeutic disruption of this pathway may be in the periphery, the dorsal root ganglion, and in the dorsal columns of the spinal cord. These interventions can be categorized into traditional spinal cord stimulation (t-SCS), 10-kHz spinal cord stimulation (10-kHz SCS) and target-based neuromodulation (brain, dorsal root ganglion, and peripheral).
t-SCS, synonymous with tonic spinal cord stimulation, intrinsically refers to stimulation cycle frequency that is considered “low” and above the threshold of perception. The data to support t-SCS for PDN suggests modest to moderate efficacy.59,60 Subthreshold spinal cord stimulation signifies stimulation which is not cognitively perceived, and these include 10-kHz SCS and burst spinal cord stimulation (burst-SCS), among others. Recent level 1 evidence highlights significant efficacy of 10-kHz SCS in the treatment of patients with PDN when compared to CMM alone.63 When compared to CMM, test subjects demonstrated substantial improvement in secondary outcomes as well as an overall health-related quality of life to 12 months.64
Burst-SCS (with passive recharge) is known in the literature as proprietary high-frequency spike trains cycled at a specific frequency, and this pattern is designed to replicate Theta burst patterning in the thalamus. In a crossover comparative study, De Vos demonstrated that burst-SCS is more effective that t-SCS in a subset of test subjects who received burst stimulation after at least six months of continuous treatment with t-SCS,61 although this has not been investigated extensively in patients with PDN.
Similarly, limited evidence exists for dorsal root ganglion stimulation (DRG-S). Eldabe and colleagues reported a series of 10 patients who received DRG-S for PDN. Overall pain reduction was followed at one month and six months, each showing a decline of 48 and 49 on the VAS scale, respectively. This study was subject to significant attrition among the test subjects.76
Noninvasive stimulation (NIS) or mPNS has been examined to improve symptoms and function related to PDN. However, to date, there is still only limited data to support the use of exogenous stimulation or magnetism for the treatment of this syndrome.179 Current and ongoing studies on mPNS may lead to this therapy option becoming a more frontline treatment option given its favorable safety profile and noninvasive nature.
Intrathecal drug delivery (IDD) is a well-established treatment for chronic intractable pain. Ziconotide and morphine are currently the only FDA-approved agents for the treatment of chronic intractable pain. There is no existing evidence supporting the use of IDD for the treatment of PDN, however there are well-supported guidelines for the care of patients experiencing chronic intractable pain of the axial spine and legs.93
In summary, spinal cord stimulation is superior to conventional medical management alone for the treatment of PDN. Studies have demonstrated modest to substantial efficacy of 10-kHz SCS and t-SCS, and this therapy may be considered when conventional medical management has failed to achieve benefit. The ability to subject patients to a temporary trial of approximately 7 days prior to surgical implantation of the permanent implant to gauge efficacy, is another considerable advantage of SCS as a treatment option for PDN.
Conclusion
The ASPN SWEET Guideline provides the first comprehensive clinical tool encompassing both pharmacological, interventional, and alternative approaches to PDN. As PDN continues to be a difficult and under-treated condition, the SWEET Guideline is intended to improve appropriate and safe treatment of patients suffering from PDN. Many recent interventional and pharmacological agents have significantly improved the ability to improve pain and suffering in PDN. Further research continues to be developed, which should only help in further identifying the optimal treatment of patients with PDN. In addition to global open access to this guideline for all clinicians involved in the care of those suffering from PDN, ASPN aims to disseminate the awareness of these important guidelines via social media, webinars, annual conferences, and other media forms. The ASPN SWEET guideline is intended to be a living document and will be updated at appropriate intervals as the research and science around PDN continues to evolve. The ASPN guidelines will be shared on the society’s website at aspnpain.com. The guidelines will be updated at a minimum of every 12 months and as impactful evidence is published relevant to the content of the SWEET guideline.
Abbreviation
A1c/ HbA1c, Glycated hemoglobin; AAN, American Academy of Neurology; ACCORD, Action to Control Cardiovascular Disease in Diabetes (study); ACT, Acceptance and Commitment Therapy; ACUDPN, Acupuncture in diabetic peripheral neuropathy; ASPN, American Society of Pain and Neuroscience; BDI, Beck Depression Inventory; BMT, Best medical therapy; BPI, Brief Pain Inventory; CBT, Cognitive behavior therapy; CDC, Centers for Disease Control and Prevention; CGRP, Calcitonin gene-related peptide; CI, Confidence interval; CMM, Conventional medical management; CPAQ, Chronic Pain Acceptance Questionnaire; CRPS, Complex regional pain syndrome; DCCT, Diabetes Control and Complications Trial (study); DM, Diabetes mellitus; DRAE, Drug-related adverse event; DRG-S, Dorsal root ganglion stimulation; ePNS, Electrical peripheral nerve stimulation; EQ-5D, EuroQOL 5-dimension questionnaire; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue Scale; FBSS, Failed back surgery syndrome; FDA, Food and Drug Administration (United States); HF, High frequency; HIV, Human immunodeficiency virus; IDD, IDT, Intrathecal drug delivery, intrathecal drug therapy; IENFD, Intraepidermal nerve fiber density; IT, Intrathecal; LOS, Length of stay; LPN, Lateral plantar nerve; MBSR, Mindfulness-based stress reduction; MCN, Medial calcaneal nerve; MDNS, Michigan Diabetic Neuropathy Score; MM, Mindfulness meditation; MPN, Medial plantar nerve; mPNS, Magnetic peripheral nerve stimulation; MRI, Magnetic resonance imaging; MT, Mindfulness therapy; NCV, Nerve conduction velocity; NePIQOL, Neuropathic Pain Impact on Quality of Life Questionnaire; NeuroQoL, Neuropathy-Specific Quality of Life Questionnaire; NIS, Noninvasive stimulation; NMDA, N-methyl-D-aspartate (glutamate receptor); NNT, Number-needed-to-treat; NPS, Neuropathic Pain Scale; NRS, Numeric rating scale; NPRS, Numeric pain rating scale; ODI, Oswestry Disability Index; PACC, Polyanalgesic Consensus Conference; PCS, Pain Catastrophizing Scale; PDN, Painful diabetic neuropathy; PENS, Percutaneous electrical nerve stimulation; PGIC, Patient Global Impression of Change; PHQ9, Patient Health Questionnaire-9; PJI, Periprosthetic joint infection; PN, Peripheral neuropathy; PNS, Peripheral nerve stimulation; POMS, Profile of Mood States; PROM, Patient-reported outcome measure; PRP, Platelet rich plasma; PSQI, Pittsburgh Sleep Quality Index; PSS, Perceived Stress Scale; QOL, Quality of life; QST, Quantitative sensory test; RCT, Randomized controlled trial; SCS, Spinal cord stimulation; SD, Standard deviation; SF-12/ SF-36, Short Form questionnaire; health-related quality of life (12-/ 36-item); SF-MPQ, Short form McGill Pain Questionnaire; SNAP, Sensory nerve action potential; SNRI, Serotonin-norepinephrine reuptake inhibitor; SOC, Standard of care; SSI, Surgical site infection; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; SWEET, Systematic guideline process to outline the current state of the art in treatment of painful diabetic neuropathy; T1DM, Type 1 diabetes mellitus; T2DM, Type 2 diabetes mellitus; TAU, Treatment as usual; TCA, Tricyclic antidepressant; TRPV1, Transient receptor potential vanilloid 1; USPSTF, United States Preventative Services Task Force; VADT, Veterans Affairs Diabetes Trial (study); VAS, Visual analog scale; VASPI, Visual analog scale of pain intensity; WHYMPI, West Haven Yale Multidimensional Pain Inventory.
Acknowledgments
Editing was provided by Allison Foster, PhD, of Foster Medical Communications.
Funding
The SWEET guideline was supported by unrestricted educational grants from Abbott, Averitas, and Nevro.
Disclosure
DS is a consultant to Abbott, Painteq, Saluda, Mainstay, Surgentec, Nevro, and holds stock options with Painteq, Neuralace, Mainstay, Vertos, and SPR. TRD is a consultant for Abbott, Vertos, SpineThera, Saluda Medical, Cornerloc, SPR Therapeutics, Boston Scientific, PainTeq, Spinal Simplicity, and Biotronik; an advisory board member for Abbott, Vertos, SPR Therapeutics, and Biotronik; has a pending patent with Abbott; and has received research funding from Abbott, Vertos, Saluda, Mainstay, SPR Therapeutic, Boston Scientific, and PainTeq. RSD receives investigator-initiated research grant funding from Nevro Corp and Saol Therapeutics that is paid to his institution. NK is a consultant to Saluda Medical and serves on an advisory board for Vertos Inc. ZH has agreements with Averitas, PainTEQ, SPR, Vertos, and Nevro. SGP is consultant to Bioness, SPR Therapeutics, Nalu Medical, and EBT Medical; receives royalties from Oxford University Press and Wolters Kluwer, and receives research grants from Biotronik, Medtronic, Nevro Corp, and Abbott. VTF receives research funding from Nevro Corporation part of an investigator-initiated study grant that is not related to this manuscript. SMF is a consultant to Abbott, Medtronic, Saluda, Vertos, CornerLoc, and Mainstay; has equity in SurgenTec, SynerFuse, Aurora Spine, Thermaquil, SPR Therapeutics, Saluda, CornerLoc, PainTeq, SpineThera, and Celeri; and has research agreements with Aurora, Mainstay, Medtronic, Abbott, Vertiflex, Saluda, CornerLoc, and Biotronik. MNM has consulting agreements with Abbott, Nalu Medical, BiotronikNEURO, and SI Bone Inc.; and serves on the scientific advisory board of BiotronikNEURO. MN is a consultant for Nevro. DWL is a consultant for Abbott, Medtronic, Boston Scientific, Biotronik, Mainstay Medical, and Petal Surgical. LK serves on advisory boards for Avanos, Biotronik, Medtronic, Gimer, Neuralace, Neuros, Nevro, PainTEQ, and Presidio; and has research agreements with Avanos, Neuros, Nevro, Fus Mobile, Saluda, and Nalu. MDB is a consultant for Neuralace Medical and Boston Scientific. EAP has received research support from Mainstay, Medtronic, Nalu, Neuros Medical, Nevro Corp, ReNeuron, SPR, and Saluda, as well as personal fees from Abbott Neuromodulation, Biotronik, Medtronic Neuromodulation, Nalu, Neuros Medical, Nevro, Presidio Medical, Saluda, and Vertos; and holds stock options from SynerFuse and neuro42. KA is a consultant for Nevro, Saluda, Biotronik, Boston Scientific, and Presidio. MES is a consultant with Modoscript, Collegium, and Syneos Health (all outside of the scope of this work). The authors report no other conflicts of interest in this work.
References
1. Sayed D, Grider J, Strand N, et al. The American Society of Pain and Neuroscience (ASPN) Evidence-Based Clinical Guideline of Interventional Treatments for Low Back Pain. J Pain Res. 2022;15:3729–3832. doi:10.2147/JPR.S386879
2. Harris RP, Helfand M, Woolf SH, et al. Current methods of the U.S. preventive services task force: a review of the process. Am J Preventive Med. 2001;20(3 Suppl):21–35. doi:10.1016/S0749-3797(01)00261-6
3. Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004;21(9):976–982. doi:10.1111/j.1464-5491.2004.01271.x
4. Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35(3):206–213. doi:10.1016/j.diabet.2008.11.004
5. Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi:10.2337/dc10-1303
6. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016;315(15):1624–1645. doi:10.1001/jama.2016.1464
7. Franklin GM; American Academy of Neurology. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology. 2014;83(14):1277–1284. doi:10.1212/WNL.0000000000000839
8. Callaghan BC, Reynolds E, Banerjee M, Kerber KA, Skolarus LE, Burke JF. Longitudinal pattern of pain medication utilization in peripheral neuropathy patients. Pain. 2019;160(3):592–599. doi:10.1097/j.pain.0000000000001439
9. Castelli G, Desai KM, Cantone RE. Peripheral Neuropathy: evaluation and Differential Diagnosis. Am Fam Physician. 2020;102(12):732–739.
10. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic Neuropathy: a Position Statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi:10.2337/dc16-2042
11. Meijer JWG, Smit AJ, Lefrandt JD, van der Hoeven JH, Hoogenberg K, Links TP. Back to basics in diagnosing diabetic polyneuropathy with the tuning fork! Diabetes Care. 2005;28(9):2201–2205. doi:10.2337/diacare.28.9.2201
12. Feng Y, Schlösser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vascular Surg. 2009;50(3):675–682.e1. doi:10.1016/j.jvs.2009.05.017
13. Vlckova-Moravcova E, Bednarik J, Belobradkova J, Sommer C. Small-fibre involvement in diabetic patients with neuropathic foot pain. Diabet Med. 2008;25(6):692–699. doi:10.1111/j.1464-5491.2008.02446.x
14. Chan AW, MacFarlane IA, Bowsher D, Campbell JA. Weighted needle pinprick sensory thresholds: a simple test of sensory function in diabetic peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1992;55(1):56–59. doi:10.1136/jnnp.55.1.56
15. Li ZF, Niu XL, Nie LL, Chen LP, Cao CF, Guo L. Diagnostic value of clinical deep tendon reflexes in diabetic peripheral neuropathy. Arch Med Sci. 2023;19(5):656. doi:10.5114/aoms.2020.100656
16. Maras O, Dulgeroglu D, Cakci A. Ankle Proprioception in Patients with Type 2 Diabetes Mellitus. J Am Podiatr Med Assoc. 2021;111(4):8. doi:10.7547/18-178
17. England JD, Gronseth GS, Franklin G, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72(2):185–192. doi:10.1212/01.wnl.0000336370.51010.a1
18. Timar B, Popescu S, Timar R, et al. The usefulness of quantifying intraepidermal nerve fibers density in the diagnostic of diabetic peripheral neuropathy: a cross-sectional study. Diabetol Metab Syndr. 2016;8:31. doi:10.1186/s13098-016-0146-4
19. Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem. 2008;110(5):351–362. doi:10.1016/j.acthis.2007.12.004
20. Price R, Smith D, Franklin G, et al. Oral and Topical Treatment of Painful Diabetic Polyneuropathy: practice Guideline Update Summary: report of the AAN Guideline Subcommittee. Neurology. 2022;98(1):31–43. doi:10.1212/WNL.0000000000013038
21. Cymbalta Package Insert; 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021427s049lbl.pdf.
22. Lyrica Package Insert. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021446s035,022488s013lbl.pdf.
23. Nucynta Package Insert; 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/200533s014lbl.pdf.
24. D’Souza RS, Barman R, Joseph A, Abd-Elsayed A. Evidence-Based Treatment of Painful Diabetic Neuropathy: a Systematic Review. Curr Pain Headache Rep. 2022;26(8):583–594. doi:10.1007/s11916-022-01061-7
25. Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin. 2011;27(1):151–162. doi:10.1185/03007995.2010.537589
26. Tesfaye S, Wilhelm S, Lledo A, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study”--a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154(12):2616–2625. doi:10.1016/j.pain.2013.05.043
27. Marks DM, Shah MJ, Patkar AA, Masand PS, Park GY, Pae CU. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7(4):331–336. doi:10.2174/157015909790031201
28. Raskin J, Wang F, Pritchett YL, Goldstein DJ. Duloxetine for patients with diabetic peripheral neuropathic pain: a 6-month open-label safety study. Pain Med. 2006;7(5):373–385. doi:10.1111/j.1526-4637.2006.00207.x
29. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1–2):109–118. doi:10.1016/j.pain.2005.03.029
30. Neurontin Package Insert; 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020235s041,020882s028,021129s027lbl.pdf.
31. Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010;49(10):661–669. doi:10.2165/11536200-000000000-00000
32. Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280(21):1831–1836. doi:10.1001/jama.280.21.1831
33. Dallocchio C, Buffa C, Mazzarello P, Chiroli S. Gabapentin vs. amitriptyline in painful diabetic neuropathy: an open-label pilot study. J Pain Symptom Manage. 2000;20(4):280–285. doi:10.1016/s0885-3924(00)00181-0
34. Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110(3):628–638. doi:10.1016/j.pain.2004.05.001
35. Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399–409. doi:10.1111/j.1742-7843.2005.pto_96696601.x
36. Max MB. Endogenous monoamine analgesic systems: amitriptyline in painful diabetic neuropathy. Anesth Prog. 1987;34(4):123–127.
37. Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250–1256. doi:10.1056/NEJM199205073261904
38. Bansal D, Bhansali A, Hota D, Chakrabarti A, Dutta P. Amitriptyline vs. pregabalin in painful diabetic neuropathy: a randomized double blind clinical trial. Diabet Med. 2009;26(10):1019–1026. doi:10.1111/j.1464-5491.2009.02806.x
39. Cohen K, Shinkazh N, Frank J, Israel I, Fellner C. Pharmacological treatment of diabetic peripheral neuropathy. P T. 2015;40(6):372–388.
40. Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014;37(8):2302–2309. doi:10.2337/dc13-2291
41. Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update. Endocr Pract. 2022;28(10):923–1049. doi:10.1016/j.eprac.2022.08.002
42. Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med. 1999;159(16):1931–1937. doi:10.1001/archinte.159.16.1931
43. American Diabetes Association. Chapter 11. Microvascular Complications and Foot Care: standards of Medical Care in Diabetes—2021. Diabetes Care. 2020;44(Supplement_1):S151–S167. doi:10.2337/dc21-S011
44. Argoff CE, Galer BS, Jensen MP, Oleka N, Gammaitoni AR. Effectiveness of the lidocaine patch 5% on pain qualities in three chronic pain states: assessment with the Neuropathic Pain Scale. Curr Med Res Opin. 2004;20(Suppl 2):S21–S28. doi:10.1185/030079904X12960
45. Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003;106(1–2):151–158. doi:10.1016/s0304-3959(03)00317-8
46. Barbano RL, Herrmann DN, Hart-Gouleau S, Pennella-Vaughan J, Lodewick PA, Dworkin RH. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol. 2004;61(6):914–918. doi:10.1001/archneur.61.6.914
47. Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of 5% lidocaine (lignocaine) medicated plaster in comparison with pregabalin in patients with postherpetic neuralgia and diabetic polyneuropathy: interim analysis from an open-label, two-stage adaptive, randomized, controlled trial. Clin Drug Investig. 2009;29(4):231–241. doi:10.2165/00044011-200929040-00002
48. Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. 5% lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: an open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25(7):1663–1676. doi:10.1185/03007990903047880
49. Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of combination therapy with 5% lidocaine medicated plaster and pregabalin in post-herpetic neuralgia and diabetic polyneuropathy. Curr Med Res Opin. 2009;25(7):1677–1687. doi:10.1185/03007990903048078
50. Anand P, Privitera R, Donatien P, et al. Reversing painful and non-painful diabetic neuropathy with the capsaicin 8% patch: clinical evidence for pain relief and restoration of function via nerve fiber regeneration. Front Neurol. 2022;13:998904. doi:10.3389/fneur.2022.998904
51. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% Patch in Painful Diabetic Peripheral Neuropathy: a Randomized, Double-Blind, Placebo-Controlled Study. J Pain. 2017;18(1):42–53. doi:10.1016/j.jpain.2016.09.008
52. Capsaicin Study Group. Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. Diabetes Care. 1992;15(2):159–165. doi:10.2337/diacare.15.2.159
53. The Capsaicin Study Group. Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, double-blind, vehicle-controlled study. Arch Intern Med. 1991;151(11):2225–2229. doi:10.1001/archinte.151.11.2225
54. Tandan R, Lewis GA, Krusinski PB, Badger GB, Fries TJ. Topical capsaicin in painful diabetic neuropathy. Controlled study with long-term follow-up. Diabetes Care. 1992;15(1):8–14. doi:10.2337/diacare.15.1.8
55. Biesbroeck R, Bril V, Hollander P, et al. A double-blind comparison of topical capsaicin and oral amitriptyline in painful diabetic neuropathy. Adv Ther. 1995;12(2):111–120.
56. Mou J, Paillard F, Turnbull B, Trudeau J, Stoker M, Katz NP. Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154(9):1632–1639. doi:10.1016/j.pain.2013.04.044
57. Ho KY, Huh BK, White WD, Yeh CC, Miller EJ. Topical amitriptyline versus lidocaine in the treatment of neuropathic pain. Clin J Pain. 2008;24(1):51–55. doi:10.1097/AJP.0b013e318156db26
58. Kiani J, Ahmad Nasrollahi S, Esna-Ashari F, Fallah P, Sajedi F. Amitriptyline 2% cream vs. capsaicin 0.75% cream in the treatment of painful diabetic neuropathy (Double blind, randomized clinical trial of efficacy and safety). Iran J Pharm Res. 2015;14(4):1263–1268.
59. de Vos CC, Meier K, Zaalberg PB, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain®. 2014;155(11):2426–2431. doi:10.1016/j.pain.2014.08.031
60. Slangen R, Schaper NC, Faber CG, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Dia Care. 2014;37(11):3016–3024. doi:10.2337/dc14-0684
61. de Vos CC, Bom MJ, Vanneste S, Lenders MWPM, de Ridder D. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation. 2014;17(2):152–159. doi:10.1111/ner.12116
62. van Beek M, Geurts JW, Slangen R, et al. Severity of Neuropathy Is Associated With Long-term Spinal Cord Stimulation Outcome in Painful Diabetic Peripheral Neuropathy: five-Year Follow-up of a Prospective Two-Center Clinical Trial. Diabetes Care. 2018;41(1):32–38. doi:10.2337/dc17-0983
63. Petersen EA, Stauss TG, Scowcroft JA, et al. Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy: a Randomized Clinical Trial. JAMA Neurol. 2021;78(6):687–698. doi:10.1001/jamaneurol.2021.0538
64. Petersen EA, Stauss TG, Scowcroft JA, et al. High-Frequency 10-kHz Spinal Cord Stimulation Improves Health-Related Quality of Life in Patients With Refractory Painful Diabetic Neuropathy: 12-Month Results From a Randomized Controlled Trial. Mayo Clin Proc Innov Qual Outcomes. 2022;6(4):347–360. doi:10.1016/j.mayocpiqo.2022.05.003
65. Petersen EA, Stauss TG, Scowcroft JA, et al. Long-term efficacy of high-frequency (10 kHz) spinal cord stimulation for the treatment of painful diabetic neuropathy: 24-Month results of a randomized controlled trial. Diabet Res Clin Pract. 2023;203:110865. doi:10.1016/j.diabres.2023.110865
66. D’Souza RS, ElSaban M, Martinez Alvarez GA, Jin MY, Kubrova E, Hassett LC. Treatment of pain in length-dependent peripheral neuropathy with the use of spinal cord stimulation: a systematic review. Pain Med. 2023;24(Supplement_2):S24–S32. doi:10.1093/pm/pnad091
67. Deer T, Pope J, Benyamin R, et al. Prospective, Multicenter, Randomized, Double-Blinded, Partial Crossover Study to Assess the Safety and Efficacy of the Novel Neuromodulation System in the Treatment of Patients With Chronic Pain of Peripheral Nerve Origin. Neuromodulation. 2016;19(1):91–100. doi:10.1111/ner.12381
68. Chmiela MA, Hendrickson M, Hale J, et al. Direct Peripheral Nerve Stimulation for the Treatment of Complex Regional Pain Syndrome: a 30-Year Review. Neuromodulation. 2021;24(6):971–982. doi:10.1111/ner.13295
69. Gilmore C, Ilfeld B, Rosenow J, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: a multicenter, randomized, placebo-controlled trial. Reg Anesth Pain Med. 2019;44(6):637–645. doi:10.1136/rapm-2018-100109
70. Gilligan C, Volschenk W, Russo M, et al. Three-Year Durability of Restorative Neurostimulation Effectiveness in Patients With Chronic Low Back Pain and Multifidus Muscle Dysfunction. Neuromodulation. 2023;26(1):98–108. doi:10.1016/j.neurom.2022.08.457
71. Gilmore CA, Kapural L, McGee MJ, Boggs JW. Percutaneous Peripheral Nerve Stimulation (PNS) for the Treatment of Chronic Low Back Pain Provides Sustained Relief. Neuromodulation. 2019;22(5):615–620. doi:10.1111/ner.12854
72. Ilfeld BM, Plunkett A, Vijjeswarapu AM, et al. Percutaneous Peripheral Nerve Stimulation (Neuromodulation) for Postoperative Pain: a Randomized, Sham-controlled Pilot Study. Anesthesiology. 2021;135(1):95–110. doi:10.1097/ALN.0000000000003776
73. Narouze S, Benzon HT, Provenzano D, et al. Interventional Spine and Pain Procedures in Patients on Antiplatelet and Anticoagulant Medications (Second Edition): guidelines From the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43(3):225–262. doi:10.1097/AAP.0000000000000700
74. Strand N, D’Souza RS, Hagedorn JM, et al. Evidence-Based Clinical Guidelines from the American Society of Pain and Neuroscience for the Use of Implantable Peripheral Nerve Stimulation in the Treatment of Chronic Pain. J Pain Res. 2022;15:2483–2504. doi:10.2147/JPR.S362204
75. Hanyu-Deutmeyer A, Pritzlaff SG. Peripheral Nerve Stimulation for the 21st Century: sural, Superficial Peroneal, and Tibial Nerves. Pain Med. 2020;21(Suppl 1):S64–S67. doi:10.1093/pm/pnaa202
76. Eldabe S, Espinet A, Wahlstedt A, et al. Retrospective Case Series on the Treatment of Painful Diabetic Peripheral Neuropathy With Dorsal Root Ganglion Stimulation. Neuromodulation. 2018;21(8):787–792. doi:10.1111/ner.12767
77. Dellon AL. Treatment of symptomatic diabetic neuropathy by surgical decompression of multiple peripheral nerves. Plast Reconstr Surg. 1992;89(4):689–697.
78. Aszmann OC, Kress KM, Dellon AL. Results of decompression of peripheral nerves in diabetics: a prospective, blinded study. Plast Reconstr Surg. 2000;106(4):816–822. doi:10.1097/00006534-200009040-00010
79. D’Souza RS, Langford B, Dombovy-Johnson M, Abd-Elsayed A. Neuromodulation Interventions for the Treatment of Painful Diabetic Neuropathy: a Systematic Review. Curr Pain Headache Rep. 2022;26(5):365–377. doi:10.1007/s11916-022-01035-9
80. Hamza MA, White PF, Craig WF, et al. Percutaneous electrical nerve stimulation: a novel analgesic therapy for diabetic neuropathic pain. Diabetes Care. 2000;23(3):365–370. doi:10.2337/diacare.23.3.365
81. Dabby R, Sadeh M, Goldberg I, Finkelshtein V. Electrical stimulation of the posterior tibial nerve reduces neuropathic pain in patients with polyneuropathy. JPR. 2017;10:2717–2723. doi:10.2147/JPR.S137420
82. Sokal P, Harat M, Zieliński P, Kieronska S. Tibial nerve stimulation with a miniature, wireless stimulator in chronic peripheral neuropathic pain. JPR. 2017;10:613–619. doi:10.2147/JPR.S128861
83. Franken G, Debets J, Joosten EAJ. Nonlinear Relation Between Burst Dorsal Root Ganglion Stimulation Amplitude and Behavioral Outcome in an Experimental Model of Painful Diabetic Peripheral Neuropathy. Neuromodulation: journal of the International Neuromodulation. Society. 2020;23(2):158–166. doi:10.1111/ner.13070
84. Franken G, Douven P, Debets J, Joosten EAJ. Conventional Dorsal Root Ganglion Stimulation in an Experimental Model of Painful Diabetic Peripheral Neuropathy: a Quantitative Immunocytochemical Analysis of Intracellular γ-Aminobutyric Acid in Dorsal Root Ganglion Neurons. Neuromodulation. 2021;24(4):639–645. doi:10.1111/ner.13398
85. Chapman KB, Van Roosendaal BKW, Van Helmond N, Yousef TA. Unilateral Dorsal Root Ganglion Stimulation Lead Placement With Resolution of Bilateral Lower Extremity Symptoms in Diabetic Peripheral Neuropathy. Cureus. 2020;12(9):e10735. doi:10.7759/cureus.10735
86. Groenen PS, Van Helmond N, Chapman KB, et al. Chemotherapy-Induced Peripheral Neuropathy Treated with Dorsal Root Ganglion Stimulation. Pain Med. 2019;20(4):857–859. doi:10.1093/pm/pny209
87. Maino P, Koetsier E, Kaelin-Lang A, Gobbi C, Perez R. Efficacious Dorsal Root Ganglion Stimulation for Painful Small Fiber Neuropathy: a Case Report. Pain Physician. 2017;20(3):E459–E463.
88. Grabnar M, Kim C. Dorsal Root Ganglion Stimulation for Treatment of Chemotherapy-Induced Neuropathy. Am J Phys Med Rehabilitation. 2020;1(4):2020–2022. doi:10.1097/phm.0000000000001542
89. Karri J, Bruel B. Dorsal Root Ganglion Stimulation for Post-Lyme Disease Chronic Peripheral Neuropathic Pain. Neuromodulation. 2020. doi:10.1111/ner.13136
90. Falowski S, Pope JE, Raza A. Early US Experience With Stimulation of the Dorsal Root Ganglia for the Treatment of Peripheral Neuropathy in the Lower Extremities: a Multicenter Retrospective Case Series. Neuromodulation. 2019;22(1):96–100. doi:10.1111/ner.12860
91. Pope JE, Deer TR, Amirdelfan K, McRoberts WP, Azeem N. The Pharmacology of Spinal Opioids and Ziconotide for the Treatment of Non-Cancer Pain. Curr Neuropharmacol. 2017;15(2):206–216. doi:10.2174/1570159x14666160210142339
92. Pope JE, Deer TR. Ziconotide: a clinical update and pharmacologic review. Expert Opin Pharmacother. 2013;14(7):957–966. doi:10.1517/14656566.2013.784269
93. Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): recommendations on Intrathecal Drug Infusion Systems Best Practices and Guidelines. Neuromodulation. 2017;20(2):96–132. doi:10.1111/ner.12538
94. Smith HS, Deer TR. Safety and efficacy of intrathecal ziconotide in the management of severe chronic pain. Ther Clin Risk Manag. 2009;5(3):521–534. doi:10.2147/tcrm.s4438
95. Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291(1):63–70. doi:10.1001/jama.291.1.63
96. Wallace MS. Ziconotide: a new nonopioid intrathecal analgesic for the treatment of chronic pain. Expert Rev Neurother. 2006;6(10):1423–1428. doi:10.1586/14737175.6.10.1423
97. Rauck RL, Wallace MS, Leong MS, et al. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31(5):393–406. doi:10.1016/j.jpainsymman.2005.10.003
98. Shao MM, Khazen O, Hellman A, et al. Effect of First-Line Ziconotide Intrathecal Drug Therapy for Neuropathic Pain on Disability, Emotional Well-Being, and Pain Catastrophizing. World Neurosurg. 2021;145:e340–e347. doi:10.1016/j.wneu.2020.10.079
99. Mendez CE, Wainaina N, Walker RJ, et al. Preoperative Diabetes Optimization Program. Clin Diabetes. 2018;36(1):68–71. doi:10.2337/cd17-0088
100. Underwood P, Askari R, Hurwitz S, Chamarthi B, Garg R. Preoperative A1C and clinical outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care. 2014;37(3):611–616. doi:10.2337/dc13-1929
101. Deer TR, Russo MA, Grider JS, et al. The Neurostimulation Appropriateness Consensus Committee (NACC): recommendations for Surgical Technique for Spinal Cord Stimulation. Neuromodulation. 2022;25(1):1–34. doi:10.1016/j.neurom.2021.10.015
102. Goel V, Kumar V, Agrawal SN, et al. Outcomes Associated With Infection of Chronic Pain Spinal Implantable Electronic Devices: insights From a Nationwide Inpatient Sample Study. Neuromodulation. 2021;24(1):126–134. doi:10.1111/ner.13263
103. Falowski SM, Provenzano DA, Xia Y, Doth AH. Spinal Cord Stimulation Infection Rate and Risk Factors: results From a United States Payer Database. Neuromodulation. 2019;22(2):179–189. doi:10.1111/ner.12843
104. Hoelzer BC, Bendel MA, Deer TR, et al. Spinal Cord Stimulator Implant Infection Rates and Risk Factors: a Multicenter Retrospective Study. Neuromodulation. 2017;20(6):558–562. doi:10.1111/ner.12609
105. Stewart PS, Bjarnsholt T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin Microbiol Infect. 2020;26(8):1034–1038. doi:10.1016/j.cmi.2020.02.027
106. Gabriel RA, Hylton DJ, Burton BN, Schmidt H, Waterman RS. The association of preoperative haemoglobin A1c with 30-day postoperative surgical site infection following non-cardiac surgery. J Perioper Pract. 2019;30(10):320–325. doi:10.1177/1750458919886183
107. Sethuraman RM, Parida S, Sethuramachandran A, Selvam P. A1C as a Prognosticator of Perioperative Complications of Diabetes: a Narrative Review. Turk J Anaesthesiol Reanim. 2022;50(2):79–85. doi:10.5152/TJAR.2021.854
108. Walid MS, Newman BF, Yelverton JC, Nutter JP, Ajjan M, Robinson JSJ. Prevalence of previously unknown elevation of glycosylated hemoglobin in spine surgery patients and impact on length of stay and total cost. J Hosp Med. 2010;5(1):E10–14. doi:10.1002/jhm.541
109. Adams AL, Paxton EW, Wang JQ, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001 to 2009. J Bone Joint Surg Am. 2013;95(6):481–487. doi:10.2106/JBJS.L.00109
110. Bock M, Johansson T, Fritsch G, et al. The impact of preoperative testing for blood glucose concentration and haemoglobin A1c on mortality, changes in management and complications in noncardiac elective surgery: a systematic review. Eur J Anaesthesiol. 2015;32(3):152–159. doi:10.1097/EJA.0000000000000117
111. Maradit Kremers H, Lewallen LW, Mabry TM, Berry DJ, Berbari EF, Osmon DR. Diabetes mellitus, hyperglycemia, hemoglobin A1C and the risk of prosthetic joint infections in total Hip and knee arthroplasty. J Arthroplasty. 2015;30(3):439–443. doi:10.1016/j.arth.2014.10.009
112. Geddes LA. History of magnetic stimulation of the nervous system. J Clin Neurophysiol. 1991;8(1):3–9. doi:10.1097/00004691-199101000-00003
113. Malmivuo J. Magnetic Stimulation of Neural Tissue. In: Malmivuo J, Plonsey R editors. Bioelectromagnetism: Principles and Applications of Bioelectric and Biomagnetic Fields. Oxford University Press; 1995:45. doi:10.1093/acprof:oso/9780195058239.003.0022
114. Polson MJ, Barker AT, Freeston IL. Stimulation of nerve trunks with time-varying magnetic fields. Med Biol Eng Comput. 1982;20(2):243–244. doi:10.1007/BF02441362
115. Bedder M, Parker L Magnetic Peripheral Nerve Stimulation (mPNS) for Chronic Pain.
116. Kapural L, Rosenberg J, Li S Safety and Efficacy of Axon Therapy (SEAT Study).
117. Zhou M, Zhang Q, Huo M, et al. The mechanistic basis for the effects of electroacupuncture on neuropathic pain within the central nervous system. Biomed. Pharmacother. 2023;161:114516. doi:10.1016/j.biopha.2023.114516
118. Dimitrova A, Murchison C, Oken B. Acupuncture for the Treatment of Peripheral Neuropathy: a Systematic Review and Meta-Analysis. J Altern Complement Med. 2017;23(3):164–179. doi:10.1089/acm.2016.0155
119. Dietzel J, Habermann IV, Horder S, et al. Acupuncture in Patients with Diabetic Peripheral Neuropathy-Related Complaints: a Randomized Controlled Clinical Trial. J Clin Med. 2023;12(6):2103. doi:10.3390/jcm12062103
120. Feng Z, Cui S, Yang H, et al. Acupuncture for neuropathic pain: a meta-analysis of randomized control trials. Front Neurol. 2023:13. doi:10.3389/fneur.2022.1076993
121. Shin KM, Lee S, Lee EY, et al. Electroacupuncture for Painful Diabetic Peripheral Neuropathy: a Multicenter, Randomized, Assessor-Blinded, Controlled Trial. Diabetes Care. 2018;41(10):e141–e142. doi:10.2337/dc18-1254
122. Abuaisha BB, Costanzi JB, Boulton AJ. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: a long-term study. Diabet Res Clin Pract. 1998;39(2):115–121. doi:10.1016/s0168-8227(97)00123-x
123. Garrow AP, Xing M, Vere J, Verrall B, Wang L, Jude EB. Role of acupuncture in the management of diabetic painful neuropathy (DPN): a pilot RCT. Acupunct Med. 2014;32(3):242–249. doi:10.1136/acupmed-2013-010495
124. Chao MT, Schillinger D, Nguyen U, et al. A Randomized Clinical Trial of Group Acupuncture for Painful Diabetic Neuropathy Among Diverse Safety Net Patients. Pain Med. 2019;20(11):2292–2302. doi:10.1093/pm/pnz117
125. Meyer-Hamme G, Friedemann T, Greten J, Gerloff C, Schroeder S. Electrophysiologically verified effects of acupuncture on diabetic peripheral neuropathy in type 2 diabetes: the randomized, partially double-blinded, controlled ACUDIN trial. J Diabetes. 2021;13(6):469–481. doi:10.1111/1753-0407.13130
126. Zheng H-T, Li Y-F, Yuan S-X, Zhang C-G, Chen G-M, Zhang L-F. Observations on 52 patients with diabetic peripheral neuropathy treated by needling combined with drug. J Acupuncture Tuina Sci. 2004;2(6):24–26. doi:10.1007/BF02848393
127. Hassanien M, Elawamy A, Kamel EZ, et al. Perineural Platelet-Rich Plasma for Diabetic Neuropathic Pain, Could It Make a Difference? Pain Med. 2020;21(4):757–765. doi:10.1093/pm/pnz140
128. Xu L, Sun Z, Casserly E, Nasr C, Cheng J, Xu J. Advances in Interventional Therapies for Painful Diabetic Neuropathy: a Systematic Review. Anesth Analg. 2022;134(6):1215–1228. doi:10.1213/ANE.0000000000005860
129. Cheng J, Daftari A, Zhou L. Sympathetic blocks provided sustained pain relief in a patient with refractory painful diabetic neuropathy. Case Rep Anesthesiol. 2012;2012:285328. doi:10.1155/2012/285328
130. Sun H, He M, Pang J, Guo X, Huo Y, Ma J. Continuous Lumbar Sympathetic Blockade Enhances the Effect of Lumbar Sympatholysis on Refractory Diabetic Neuropathy: a Randomized Controlled Trial. Diabetes Ther. 2020;11(11):2647–2655. doi:10.1007/s13300-020-00918-7
131. Ding Y, Yao P, Li H, Zhao R, Zhao G. Evaluation of combined radiofrequency and chemical blockade of multi-segmental lumbar sympathetic ganglia in painful diabetic peripheral neuropathy. J Pain Res. 2018;11:1375–1382. doi:10.2147/JPR.S175514
132. Salehi H, Moussaei M, Kamiab Z, Vakilian A. The effects of botulinum toxin type A injection on pain symptoms, quality of life, and sleep quality of patients with diabetic neuropathy: a randomized double-blind clinical trial. Iran J Neurol. 2019;18(3):99–107.
133. Yuan RY, Sheu JJ, Yu JM, et al. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009;72(17):1473–1478. doi:10.1212/01.wnl.0000345968.05959.cf
134. Ghasemi M, Ansari M, Basiri K, Shaigannejad V. The effects of intradermal botulinum toxin type a injections on pain symptoms of patients with diabetic neuropathy. J Res Med Sci. 2014;19(2):106–111.
135. Lakhan SE, Velasco DN, Tepper D. Botulinum Toxin-A for Painful Diabetic Neuropathy: a Meta-Analysis. Pain Med. 2015;16(9):1773–1780. doi:10.1111/pme.12728
136. Zaheer A, Zaheer F, Saeed HY, Tahir Z, Tahir MW. A Review of Alternative Treatment Options in Diabetic Polyneuropathy. Cureus. 2021;13(4). doi:10.7759/cureus.14600
137. Ziegler D, Ametov A, Barinov A, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29(11):2365–2370. doi:10.2337/dc06-1216
138. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34(9):2054–2060. doi:10.2337/dc11-0503
139. Garcia-Alcala H, Santos Vichido CI, Islas Macedo S, et al. Treatment with α-Lipoic Acid over 16 Weeks in Type 2 Diabetic Patients with Symptomatic Polyneuropathy Who Responded to Initial 4-Week High-Dose Loading. J Diabetes Res. 2015;2015:189857. doi:10.1155/2015/189857
140. Chiechio S, Copani A, Gereau RW 4th, Nicoletti F. Acetyl-L-carnitine in neuropathic pain: experimental data. CNS Drugs. 2007;21 Suppl 1:31–38. doi:10.2165/00023210-200721001-00005
141. De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3(4):223–231. doi:10.2165/00126839-200203040-00001
142. Sima AAF, Calvani M, Mehra M, Amato A. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28(1):89–94. doi:10.2337/diacare.28.1.89
143. Baltrusch S. The Role of Neurotropic B Vitamins in Nerve Regeneration. Biomed Res Int. 2021;2021:9968228. doi:10.1155/2021/9968228
144. Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. doi:10.1038/s41572-019-0092-1
145. Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. doi:10.1016/S0140-6736(10)60576-4
146. Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99(12A):21i–33i. doi:10.1016/j.amjcard.2007.03.003
147. Qaseem A. Hemoglobin A1c Targets for Glycemic Control With Pharmacologic Therapy for Nonpregnant Adults With Type 2 Diabetes Mellitus: a Guidance Statement Update From the American College of Physicians. Int J Med. 2018:168:58. doi:10.7326/M17-0939
148. Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care. 2011;34(10):2244–2249. doi:10.2337/dc11-0903
149. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi:10.1056/NEJM199309303291401
150. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi:10.1056/NEJMoa0808431
151. Singleton JR, Marcus RL, Jackson JE, Lessard K, Graham T, Smith TE. Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann Clin Transl Neurol. 2014;1(10):844–849. doi:10.1002/acn3.125
152. Singleton JR, Marcus RL, Lessard MK, Jackson JE, Smith AG. Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann Neurol. 2015;77(1):146–153. doi:10.1002/ana.24310
153. van Laake-Geelen CCM, Smeets RJEM, Quadflieg SPAB, Kleijnen J, Verbunt JA. The effect of exercise therapy combined with psychological therapy on physical activity and quality of life in patients with painful diabetic neuropathy: a systematic review. Scand J Pain. 2019;19(3):433–439. doi:10.1515/sjpain-2019-0001
154. Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28(10):2378–2383. doi:10.2337/diacare.28.10.2378
155. Yoshida S, Hirai M, Suzuki S, Awata S, Oka Y. Neuropathy is associated with depression independently of health-related quality of life in Japanese patients with diabetes. Psychiatry Clin Neurosci. 2009;63(1):65–72. doi:10.1111/j.1440-1819.2008.01889.x
156. Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005;30(4):374–385. doi:10.1016/j.jpainsymman.2005.04.009
157. Jain R, Jain S, Raison CL, Maletic V. Painful diabetic neuropathy is more than pain alone: examining the role of anxiety and depression as mediators and complicators. Curr Diab Rep. 2011;11(4):275–284. doi:10.1007/s11892-011-0202-2
158. Sullivan MJL, Lynch ME, Clark AJ. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain. 2005;113(3):310–315. doi:10.1016/j.pain.2004.11.003
159. Geelen CC, Kindermans HP, van den Bergh JP, Verbunt JA. Perceived Physical Activity Decline as a Mediator in the Relationship Between Pain Catastrophizing, Disability, and Quality of Life in Patients with Painful Diabetic Neuropathy. Pain Pract. 2017;17(3):320–328. doi:10.1111/papr.12449
160. Benbow SJ, Wallymahmed ME, MacFarlane IA. Diabetic peripheral neuropathy and quality of life. QJM. 1998;91(11):733–737. doi:10.1093/qjmed/91.11.733
161. Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabet Res Clin Pract. 2000;47(2):123–128. doi:10.1016/s0168-8227(99)00112-6
162. Teixeira E. The effect of mindfulness meditation on painful diabetic peripheral neuropathy in adults older than 50 years. Holist Nurs Pract. 2010;24(5):277–283. doi:10.1097/HNP.0b013e3181f1add2
163. Otis JD, Sanderson K, Hardway C, Pincus M, Tun C, Soumekh S. A randomized controlled pilot study of a cognitive-behavioral therapy approach for painful diabetic peripheral neuropathy. J Pain. 2013;14(5):475–482. doi:10.1016/j.jpain.2012.12.013
164. Taheri AA, Foroughi AA, Mohammadian Y, et al. The Effectiveness of Acceptance and Commitment Therapy on Pain Acceptance and Pain Perception in Patients with Painful Diabetic Neuropathy: a Randomized Controlled Trial. Diabetes Ther. 2020;11(8):1695–1708. doi:10.1007/s13300-020-00851-9
165. Higgins DM, Heapy AA, Buta E, et al. A randomized controlled trial of cognitive behavioral therapy compared with diabetes education for diabetic peripheral neuropathic pain. J Health Psychol. 2022;27(3):649–662. doi:10.1177/1359105320962262
166. Izgu N, Gok Metin Z, Karadas C, Ozdemir L, Metinarikan N, Corapcıoglu D. Progressive Muscle Relaxation and Mindfulness Meditation on Neuropathic Pain, Fatigue, and Quality of Life in Patients With Type 2 Diabetes: a Randomized Clinical Trial. J Nurs Scholarsh. 2020;52(5):476–487. doi:10.1111/jnu.12580
167. Nathan HJ, Poulin P, Wozny D, et al. Randomized Trial of the Effect of Mindfulness-Based Stress Reduction on Pain-Related Disability, Pain Intensity, Health-Related Quality of Life, and A1C in Patients With Painful Diabetic Peripheral Neuropathy. Clin Diabetes. 2017;35(5):294–304. doi:10.2337/cd17-0077
168. Hussain N, Said ASA. Mindfulness-Based Meditation Versus Progressive Relaxation Meditation: impact on Chronic Pain in Older Female Patients With Diabetic Neuropathy. J Evid Based Integr Med. 2019;24:2515690X19876599. doi:10.1177/2515690X19876599
169. Eccleston C, Hearn L, Williams AC. Psychological therapies for the management of chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2015;2015(10):CD011259. doi:10.1002/14651858.CD011259.pub2
170. Castelnuovo G, Giusti EM, Manzoni GM, et al. Psychological Treatments and Psychotherapies in the Neurorehabilitation of Pain: evidences and Recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Front Psychol. 2016;7:115. doi:10.3389/fpsyg.2016.00115
171. Bai Y, Ma JH, Yu Y, Wang ZW. Effect of Cognitive-Behavioral Therapy or Mindfulness Therapy on Pain and Quality of Life in Patients with Diabetic Neuropathy: a Systematic Review and Meta-Analysis. Pain Manag Nurs. 2022;23(6):861–870. doi:10.1016/j.pmn.2022.05.005
172. Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9(6):660–674. doi:10.1111/j.1526-4637.2007.00347.x
173. Callaghan BC, Little AA, Feldman EL, Hughes RAC. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6(6):CD007543. doi:10.1002/14651858.CD007543.pub2
174. Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20(4):216–223. doi:10.1016/j.jdiacomp.2005.07.005
175. Holmes CJ, Hastings MK. The Application of Exercise Training for Diabetic Peripheral Neuropathy. J Clin Med. 2021;10(21). doi:10.3390/jcm10215042
176. Chatchawan U, Eungpinichpong W, Plandee P, Yamauchi J. Effects of Thai foot massage on balance performance in diabetic patients with peripheral neuropathy: a randomized parallel-controlled trial. Med Sci Monit Basic Res. 2015;21:68–75. doi:10.12659/MSMBR.894163
177. Busse JW, Wang L, Kamaleldin M, et al. Opioids for Chronic Noncancer Pain: a Systematic Review and Meta-analysis. JAMA. 2018;320(23):2448–2460. doi:10.1001/jama.2018.18472
178. Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758–1765. doi:10.1212/WNL.0b013e3182166ebe
179. Liampas A, Rekatsina M, Vadalouca A, Paladini A, Varrassi G, Zis P. Non-Pharmacological Management of Painful Peripheral Neuropathies: a Systematic Review. Adv Ther. 2020;37(10):4096–4106. doi:10.1007/s12325-020-01462-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.