Back to Journals » International Journal of Women's Health » Volume 14
Leiomyoma with Bizarre Nuclei: A Current Update
Authors Guo E , Li C , Hu Y, Zhao K, Zheng Q, Wang L
Received 4 September 2022
Accepted for publication 17 November 2022
Published 25 November 2022 Volume 2022:14 Pages 1641—1656
DOI https://doi.org/10.2147/IJWH.S388278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Elie Al-Chaer
Enhui Guo,1,2,* Chengqian Li,2,3,* Yanjiao Hu,4 Kongyuan Zhao,1,2 Qingmei Zheng,1,* Liming Wang1,*
1Department of Gynecology, The Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 2Qingdao Medical College, Qingdao University, Qingdao, People’s Republic of China; 3Department of Neurology, The Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 4Department of Pathology, The Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qingmei Zheng; Liming Wang, Department of Gynecology, The Affiliated Hospital of Qingdao University, No. 1677, Wutaishan Road, Huangdao District, Qingdao, Shandong, 266000, People’s Republic of China, Tel +86-18661806725 ; +86-18661807912, Email [email protected]; [email protected]
Abstract: Leiomyoma with bizarre nuclei (LBN), also known as symplastic leiomyoma, is a histological subtype of benign leiomyoma with bizarre cells and nuclear atypia. Differentiating LBN from other benign leiomyoma subtypes, uterine smooth muscle tumors of uncertain malignant potential (STUMP), or leiomyosarcoma (LMS) can be diagnostically challenging owing to overlapping features in clinical presentation and pathologic morphological analysis. The difficulty of distinguishing LBN from other lesions, especially from LMS, and the potential of LBN for subsequent malignant transformation make LBN an important topic of research. Herein, we review the definition, diagnosis, treatment, and prognosis of LBN. Histopathological examination is essential for distinguishing LBN from other diseases. Pathology sampling and morphological examination remain the key to diagnosis. The newly established ancillary immunohistochemical (IHC) and molecular genetic analysis can be useful tools for differential diagnosis. Furthermore, serum biomarkers and imaging examination may also be useful diagnostic tools. Attention should be paid to the differentiation between LBN and LMS because morphological diagnosis may still be challenging in some cases. Some IHC markers of LBN have been identified, which may be helpful for differential diagnosis. Furthermore, the use of IHC panels as diagnostic markers may be advocated. Molecular genetic studies suggest that some genes can aid with the differential diagnosis between LBN and LMS. However, increasing evidence support the idea that LBN and LMS are molecularly related, indicating that LBN may represent a potentially malignant stage of precancerous progression. At present, conservative treatment is recommended for primary LBN, especially for patients desiring to retain fertility, but close follow-up with imaging examinations is required.
Keywords: diagnosis, histopathology, molecular genetics, imaging examination, treatment, prognosis
Introduction
Uterine smooth muscle tumors (USMTs) are mesenchymal tumors that develop from smooth muscle cells. The precise pathogenesis that led to the growth of USMTs is not totally understood. Previous studies have demonstrated that USMTs growth was depended on the estrogen and progesterone.1 Recently, Ura et al suggested that phosphorylation of protein was involved in leiomyoma growth.2 USMTs have tissue heterogeneity and can present as various histological subtypes. Based on morphological criteria, USMTs are divided into 5 groups in the 2020 World Health Organization (WHO) tumor classification system (Table 1).3 Uterine leiomyomas, including its subtypes, are currently the most common uterine tumors, usually occurring in patients between 30 and 50 years of age.4 Leiomyosarcoma (LMS) is the most common uterine sarcoma (accounting for approximately 40–50% of uterine sarcomas) but only makes up 2–5% of all uterine malignancies,5 with patients typically younger than 50 years in age. LMS can present in 1 of 200–800 hysterectomies for USMT and is clinically aggressive with early metastasis, high recurrence rate, and poor overall prognosis.6 Therefore, correct diagnosis of LMS is critical for patient care.
 |
Table 1 Classification of Uterine Smooth Muscle Tumors |
Leiomyoma with bizarre nuclei (LBN) is a histological subtype of leiomyoma with atypical histological characteristics. LBN was originally described by Kelly and Cullen in 1909 as a benign uterine leiomyoma with “sarcomatous degeneration”.7 The term “bizarre leiomyoma” was first used in 1972 by Christopherson et al,8 and in 1994, these tumors were defined as “atypical leiomyoma” by Bell et al.9 Atypical leiomyoma has been recognized and defined as a USMT with moderate-to-severe and pleomorphic nuclear atypia, no more than 9 mitoses/10 high power fields (HPF), and no tumor necrosis.9–11 Until 2014, the WHO adopted the terminology “leiomyoma with bizarre nuclei” and classified it as a leiomyoma variant; other names were no longer recommended.12 LBN can show focal or diffuse nuclear atypia, with or without increased mitosis (an average of 1–2 mitoses/10 HPF, although it may be up to 7–8 mitoses/10 HPFs focally, but not more than 10 mitoses/HPF). LBN presents with occasional recurrence and rare reports of malignant transformation.13,14 However, studies on selected genes or biomarkers indicate that LBN shares more molecular characteristics with LMS than with leiomyoma, leading to the hypothesis that LBN has the potential for subsequent malignant transformation.15–17 The 2020 WHO classification defined LBN as a leiomyoma subtype with bizarre cells arranged in a multifocal to diffuse distribution in a background of typical leiomyoma.3 The mitotic count is <5 mitoses/10 HPF of 0.55-mm diameter and 0.24-mm2 area. LBN with 5–9 mitoses/10 HPF may be considered smooth muscle tumors of uncertain malignant potential (STUMP). Uterine smooth muscle tumors can show two types of atypia: LBN, characterized by remarkable bizarre cells (striking pleomorphism, multinucleation, eosinophilic or globular cytoplasm, smudged chromatin, and nuclear pseudoinclusions) but devoid of malignant potential, and STUMP, which may be less remarkable but reflects a more aggressive behavior. According to the diagnostic criteria of the 2020 WHO guidelines, Travaglino et al conducted a mate-analysis on the recurrence rate of LBN. They reported that the recurrence rate of LBN was 1.9% after excluding STUMP.18 Their results indicate that the biological behavior of LBN is consistent with that of a benign lesion.
LBN is a diagnostically challenging tumor and is usually presumed to be a usual-type leiomyoma (ULM) preoperatively and suspected to be LMS in postoperative diagnosis.19,20 Accurate diagnosis is mainly based on clinical information, gross appearance, and strict utilization of morphologic criteria, including cytologic atypia, mitotic activity, and tumor cell necrosis.16,19 However, the overlapping clinical symptoms and histopathologic features between LBN and Fumarate hydratase-deficient leiomyoma (FH-LM), STUMP, and LMS in particular, often cause diagnostic problems.21,22 This may lead to pathological diagnostic problems and cause serious clinical consequences, especially when LBN is misdiagnosed as LMS. Therefore, improvement in diagnostic accuracy is required for better prognosis and treatment. Recent progress has been made on diagnostic accuracy based on the specific histology and molecular alterations of LBN. In addition, an exploration of preoperative diagnosis is necessary because some occult LMS patients who wish to preserve fertility may delay surgery or choose myomectomy, owing to the absence of clinical and radiological signs that are different from those of benign myoma. Therefore, it is necessary to explore the preoperative diagnosis including clinical, serological, and imaging features.
In this review, we present the histopathological, molecular, clinical and imaging features of LBN. We focus on the methods and new advances in the differential diagnosis of LBN. In particular, we highlight the current progress made in histology and molecular genetics that has improved LBN diagnosis. Finally, we summarize the clinical prognosis of LBN and provide suggestions for patient management in clinical practice.
Different Diagnostic Tools for LBN
Histopathological Examination
Histologic Evaluation
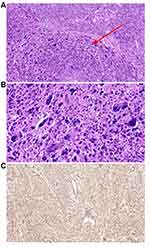
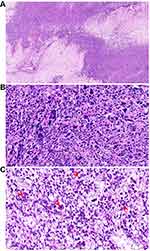
Histologic evaluation remains the key method in LBN diagnosis. LBN is defined by focal, multifocal, or diffused bizarre cells on a background of typical leiomyoma cells and is characterized by moderate-to-severe nuclear atypia, low mitotic count (≤5 mitoses/10 HPFs) but with karyorrhectic nuclei, and no tumor cell necrosis.3 The bizarre cells may be mono- or multinucleated and may have eosinophilic or globular cytoplasm, smudged chromatin, and nuclear pseudoinclusions. These tumors have well-circumscribed borders and occasionally have areas of infarct-type (hyaline) necrosis. Macroscopically, LBN may appear in various colors and consistencies (slightly soft and less bulging) with a mean diameter of 7 cm. Helpful features to establish LBN diagnosis include a patchy distribution of the bizarre cells, eosinophilic or globular cytoplasm, a large nuclear size (10–100 µm), spindled or elongated nuclei, irregular nuclear membrane, dark and smudgy chromatin, nuclear pseudoinclusions, and low mitotic count (≤5 mitoses/10 HPFs) (Figure 1).19 Nucleoli are usually small or absent, but eosinophilic giant nucleoli are occasionally seen. The latter should be carefully evaluated to exclude FH-LM. The characteristics of FH-LM are as follows: staghorn vessels, alveolar-type edema, scattered bizarre nuclei, eosinophilic cytoplasmic (rhabdoid) inclusions, eosinophilic giant nucleoli with a perinucleolar halo, ovoid nuclei sometimes arranged in chains, and immunohistochemical (IHC) staining showing negative fumarate hydratase (FH) expression in tumor cells (Figure 2). By carefully evaluating nuclear features, most LBN can be distinguished from FH-LM. Furthermore, FH deficiency may be helpful for differential diagnosis.23
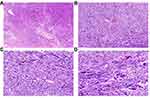
The mitotic index of LBN was previously defined as <10 mitoses/10 HPF.9,14 Stanford investigators and others found that LBN with 6–9 mitoses/10 HPF reflects a more aggressive behavior and often reclassified it as an intermediate grade USMT.24,25 The 2020 WHO classification established an objective criterion, and indicated a mitotic index of 0–4/10 HPF for LBN and 5–9/10 HPF for STUMP.3 Therefore, previously classified LBN groups with 5–9 mitoses/10 HPF is currently diagnosed as STUMP. However, the difference between LBN and STUMP may not be obvious. Other features such as atypical mitoses, infiltrative margins, and vascular intrusion may justify a STUMP diagnosis (Figure 3).26 Karyorrhectic nuclei with coarse and smudgy chromatin, which may mimic atypical mitoses, should be noted in LBN and may complicate the differential diagnosis between LBN and STUMP.11,19 In uncertain cases, mitotic counts in more sections and immunostaining for Ki-67 or phosphorylated histone H3 (PHH3) may be helpful.27,28 Furthermore, it is worth noting that infarct-type (hyaline) necrosis can occasionally be observed in LBN.
Most LMS are single tumors found in the uterine wall, with an average tumor size of 10 cm.29 They are grayish yellow or red, have a soft or fleshy consistency, and macroscopic hemorrhage and necrosis are often observed on the tumor surface. LMS tumors are usually histologically heterogeneous, consisting of leiomyoma-like, bizarre leiomyoma-like, and malignant regions.30 The wide range of histologic features often lead to a significant diagnostic challenge. Typical LMS is characterized by hypercellular spindle cells, diffuse moderate-to-severe cell atypia, a high mitotic index, atypical mitosis, and tumor cell necrosis (Figure 4).9 To establish a diagnosis of LMS requires the presence of two of the following features: moderate-to-severe cytologic atypia, high active mitosis (≥10 mitoses/10 HPFs), and tumor cell necrosis.31 However, similar atypical cells can be found in LBN, increasing the difficulty in distinguishing between LBN and LMS. In addition, karyorrhectic nuclei with coarse chromatin, which simulates atypical mitosis, and focal increased mitotic activity may also be noted in LBN.11,19 The determination of necrosis type is of great significance. Infarct-type (hyaline) necrosis, which occasionally occurs in LBN, should be distinguished from coagulative tumor cell necrosis; however, this can be difficult, especially at an early stage of LMS. In addition, determining the type of necrosis can be very challenging in patients receiving various treatment modalities for symptomatic fibroids before myomectomy. These tumors are often associated with vascular changes, including fibrinoid necrosis of the vessel wall, vessel lumen obliteration, and perivascular inflammatory infiltrates.11 Moreover, it is important to be aware of the factors influencing histological changes in leiomyomas.4 Medical treatments, including hormonal and non-hormonal drugs and uterine artery embolization, can alter the histological appearance of tumors by inducing infarct-type necrosis, mitosis, cellular atypia, and marked acute inflammation, further complicating the accurate diagnosis of USMTs. For example, gonadotropin-releasing hormone agonist (GnRH-a) may cause an irregular border, increased cell proliferation, focal infarction, hyaline degeneration, substantial lymphoid infiltration, and vascular changes. High progesterone levels may affect mitotic activity. Another factor that changes the appearance of leiomyomas is pregnancy, during which entities can often enlarge and may exhibit edema, bizarre cytologic atypia, infarction, hemorrhage, and hyalinization. Thus, extensive sampling may be necessary in these tumors to exclude malignancy or other tumors.
LBN pose diagnostic challenges owing to overlapping features with FH-LM, STUMP, and LMS in pathologic morphological analysis. In particular, the differential diagnosis between LBN and LMS is crucial. Despite careful evaluation of the aforementioned histologic features, many LBN cases can be misclassified as LMS.11 For example, Toledo et al reported that as many as 17% of LBN cases (10/59) were originally misclassified as LMS.30 In 2020, the WHO tumor classification system established the basic diagnostic criteria rendered by a global expert panel of pathologists (Table 2). Most smooth muscle tumors can be differentiated and classified in strict accordance with the diagnostic criteria, but there are still some cases presenting with morphological diagnostic challenges. Therefore, ancillary histopathological examinations, including IHC and genetic examinations, are necessary for LBN diagnosis.
 |
Table 2 Morphological Diagnostic Criteria for LBN and Other Uterine Smooth Muscle Tumors That Need to Be Differentiated from LBN |
Available IHC Markers
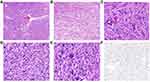
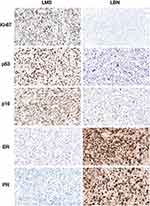
IHC markers may be useful to overcome the diagnostic challenges for LBN. FH and S‐(2‐succino)‐cysteine (2SC) have been shown to be sensitive and specific IHC markers to detect FH alterations.32,33 IHC examination of 2SC or FH can be used as a reliable auxiliary diagnostic method, aiding in the differential diagnosis between FH-LM and LBN. FH expression is completely absent and 2SC is diffusely positive in FH-LM. Other IHC markers, such as estrogen receptor (ER), progesterone receptor (PR), Ki-67, p53, and p16 have been studied as potential diagnostic and prognostic markers for LMS, which may help to differentiate it from LBN (Figure 5). Lusby et al have reported that IHC panels with the loss of ER and PR expression, overexpression of Ki-67, and altered p53, retinoblastoma protein (RB), and p16 expression may contribute to the accurate diagnosis of questionable cases.34 It is noteworthy that positive p16 and p53 expression is common in LBN and shows significant overlap with that in LMS.14,35 Recently, Cao et al demonstrated that the digital quantification of Ki-67 could assist in LMS diagnosis and higher Ki-67 values may indicate lower progression-free survival.27
Moreover, several studies have reported that the expression of PHH3, cyclin-dependent kinase inhibitor 1A (p21), RB, proliferating cell nuclear antigen (PCNA), epidermal growth factor receptor (EGFR), major vault protein (MVP), catechol-O-methyltransferase (COMT), and alpha-thalassemia/intellectual disability syndrome X-linked (ATRX) is significantly different between LBN and LMS. Furthermore, the distinction between LBN and LMS largely depends on mitotic count.36 PHH3, a mitosis-specific IHC marker, may be a more helpful marker than Ki-67 when it is difficult to determine mitotic rate based on routine examination.37 Liang et al suggested that PHH3 expression is significantly higher in LMS than in LBN,38 which is helpful for the differential diagnosis of the two lesions. Moreover, Chow et al reported that PHH3 is useful in differentiating LMS from LBN and serves as a prognostic indicator.39 PHH3 positive cells ≥7/10 HPFs are highly associated with LMS, whereas counts ≥29/10 HPFs are independently associated with worse clinical outcomes. Moreover, p21 showed diffused immunoreactivity in more than 40% of LBN cases and was positive in 14% of LMS cases. The increased expression of p21 in LBN may indicate a protective effect by preventing invasive tumor growth.40 RB showed a strong and diffused immune response in 13% of LMS cases but was rarely found in LBN (4%).40 Soltan et al found that EGFR was significantly overexpressed in LMS compared to that in LBN.41 Zhu et al reported that PCNA expression is higher in LMS than in LBN.42 Elevated PCNA levels together with reduced ER and PR levels can help differentiate between LBN and LMS. Nicholas et al found that the expression of MVP and COMT in LMS was 3.05 and 13.94 times higher than that in leiomyoma, respectively.22 Ahvenainen et al investigated ATRX expression in USMTs, and a loss of ATRX was detected in 16% of LBN cases.21 Recently, Gao et al reported that the combination of 6 IHC markers, including anterior gradient-2 (AGR2), ATPase family AAA domain-containing protein 3 (ATAD3), dual specificity phosphatase 2 (DUSP2), nuclear receptor subfamily 4, group A (NR4A2), p16, and polypyrimidine tract-binding protein 1 (PTBP1), was the optimal diagnostic model to discriminate between LBN and LMS.6 AGR2, DUSP2, NR4A2, and PTBP1 levels were significantly lower in LMS than in LBN. Conversely, ATAD3 and p16 levels were significantly higher in LMS than in LBN. Keyhanian et al demonstrated that the combination of diffuse strong minichromosome maintenance complex component 2 (MCM2) and p16 reactivity with an increased Ki-67 index was reliable to discriminate between LBN and LMS.43 Furthermore, MCM2 staining was diffusely and strongly positive in LMS.
Genetic Examination
Uterine leiomyoma has complex genetic alterations, with at least four distinct molecular subclasses, including mediator complex subunit 12 (MED12) mutations, high-mobility group AT-hook 2 (HMGA2) rearrangements, COL4A5/COL4A6 deletions, and FH mutations. Interestingly, these genetic alterations have been reported to be mutually exclusive in uterine leiomyomas.44,45 FH-LM is caused by biallelic inactivation of FH and induced by either germline or somatic FH alterations.32 More recently, the detection of biallelic alterations in FH made it possible to distinguish FH-LM from LBN and LMS. MED12 mutations were the most common alterations in usual-type leiomyomas, mitotically active leiomyomas, and LMS. Mäkinen et al reported that MED12 mutations were found in 56.9% of usual-type leiomyoma (37/65), 16.7% of LBN (3/18), 16% of cellular leiomyoma (4/25), and 21.6% of LMS (11/51) cases.15 The frequencies in LBN as well as LMS are significantly lower than those in usual-type leiomyoma.46–48 HMGA2 overexpression is the most common alteration in cellular leiomyomas.15,44 Mäkinen et al reported that cellular leiomyoma exhibited the highest HMGA2 mutation frequency of 32% (8/25), followed by usual-type leiomyomas (24.6%, 16/65), LBN (0%, 0/18), and LMS (5.9%, 3/51).15 These studies indicate that LMS and LBN are genetically more closely related than they are to other leiomyoma subtypes.21,47,48
LMS harbors complex numerical and structural chromosomal aberrations. Some genes, including TP53, RB1, ATRX, PTEN, CDKN2A, and KIT, have been reported to be associated with LMS.49,50 Recently, Gao et al reported five genes that showed differential copy number alterations between LBN and LMS,6 including ATAD3, PTBP1, RB1, p16, and progesterone receptor gene (PGR). The frequency of copy number loss in PTBP1, RB1, and PGR was higher in LMS than in LBN. In contrast, the frequency of copy number loss in ATAD and p16 was lower in LMS than in LBN. These findings may aid with the differential diagnosis between LBN and LMS. However, increasing evidence support the idea that LBN and LMS are molecularly related. Liegl-Atzwanger et al compared LBN and LMS using array comparative genomic hybridization (array-CGH).16 They found that all LBN and LMS cases showed a large number of copy number variations (CNV) involving almost all chromosomes, whereas usual-type leiomyoma had a lower frequency of CNV with a few cases (20%) lacking any gains or losses. Moreover, they reported that it was impossible to differentiate between LBN and LMS by only evaluating CNV. When comparing CNV patterns among LBN and LMS,6 37 common CNV peaks, including 29 deletions and 8 gains, were shared and principal component analysis (PCA) showed that they had overlapping features.
Increasing evidence has shown that LBN are molecularly related to LMS; distinguishing it from other subtypes. Typically, LMS is considered to develop as a de novo tumor. However, microscopically visible colocalization and shared molecular changes with LBN suggest that LBN may have some potential for malignant transformation.15,16,21 Recently, Fischer et al reported two cases of LBN that progressed to LMS.17 Furthermore, molecular genetic studies have suggested that the morphological changes in LBN most likely reflect severe genomic abnormalities instead of simple degenerative alterations, which may represent a potential malignant stage of precancerous progression.16,48,51 However, large-scale clinical trials are needed for further verification.
Clinical Features and Serum Biomarkers
LBN is a rare type of leiomyoma occurring between 43 and 50 years of age. On average, the onset of LBN is about 15 years earlier than that of LMS. The clinical features of LBN are similar to that of LMS and other leiomyoma subtypes. Most cases of LBN are asymptomatic; about one-third of patients may present pelvic pain, abnormal uterine bleeding (menorrhagia), and abdominal bloating. Tumor necrosis is associated with intravascular thrombosis and local strong inflammation. Therefore, related markers such as D-dimer, lactate dehydrogenase (LDH), and C-reactive protein (CRP) might be useful for differentiating LMS from LBN.52 Nishigaya et al assessed the diagnostic value of a combination serum assay of LDH, D-dimer, and CRP for USMTs.53 They reported that the positive rates of all three markers were high in the order of LMS, LBN, and usual-type leiomyoma. When all parameters were positive, the specificity and positive predictive value in differentiating LMS from LBN were 100%. Goto et al reported that the combination of serum LDH isoenzymes and dynamic magnetic resonance imaging (MRI) was helpful to distinguish LMS from degenerating leiomyoma.54 Di Cello et al first proposed the Uterine mass Magna Graecia (UMG) risk index, LDH3 + (24/LDH1) to predict LMS.55 When the index was >29, the sensitivity and specificity for LMS were 100% and 99.6%, respectively. Spivack et al determined the UMG index cutoff of 29 had a high specificity of 91.1% and could be a cost-effective tool for preoperative screening for LMS.56 However, it should be noted that higher BMI may be related to the increase of UMG index. It is worth noting that liquid biopsies can be very helpful in early diagnosis and differential diagnosis owing to the aggressive nature of LMS. The following indicators can be analyzed through liquid biopsies: circulating tumor DNA, circulating tumor cells, exosomes, circulating RNAs (including mRNA, microRNA, and long non-coding RNA), proteins, peptides, and metabolites.57
Imaging Examination
Ultrasound is the most common imaging examination for leiomyoma, but ultrasonic evaluation is subjective and has low accuracy. Ludovisi et al have reported that, on ultrasound, sarcomas usually present as solitary large solid masses with uneven echoes in solid tissues;58 they sometimes include cystic (often irregular) areas and usually do not show shadows or calcifications. They also reported that the original sonographers classified 20% of sarcomas as benign. The misclassification of LMS as benign myomas may lead to delayed treatment or improper surgical treatment, which is highly likely to result in a poor prognosis.
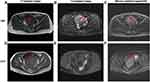
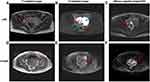
Recently, more researchers have demonstrated the use of MRI in the differential diagnosis between benign uterine leiomyomas, LBN, and LMS. In MRI, LBN and benign uterine leiomyomas usually present as well-defined large uterine masses, while LMS usually presents as large uterine masses with ill-defined, irregular margins.59 The MRI characteristics of different types of USMTs are shown in Figures 6 and 7. In T1-weighted images, LBN is typically isointense to hypointense to muscle, while benign uterine leiomyomas are isointense to muscle.60 STUMP may exhibit interspersed hyperintensities due to hemorrhage, whereas LMS is typically hyperintense to muscle due to hemorrhage and necrosis. In T2-weighted images, LBN may exhibit lace-like heterogeneous hyperintensity, whereas benign uterine leiomyomas are homogeneously hypointense to muscle and a T2-hyperintense rim may be observed due to edema.61 STUMP exhibits hyperintensity on T2-weighted images, and LMS is hyperintense to muscle owing to hemorrhage and necrosis.62 In diffusion weighted images (DWI), both LBN and benign uterine leiomyomas are hypointense,63 while LMS and STUMP exhibit hyperintensities in the solid components.64 Bi et al concluded that abnormal vaginal bleeding (3 points), tumors mainly located in the uterine cavity (3 points), ill-defined tumor margins (4 points), and mean apparent diffusion coefficient (ADCmean) values <1.272 × 10–3 mm2/s (5 points) are sensitive indicators for preoperative prediction of LMS.65 When the total score of the four predictive factors was ≥7, the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value for distinguishing between LMS and LBN were 88.9%, 99.9%, 95.7%, 97.0%, and 95.1%, respectively.
On enhanced MRI, LBN could exhibit inhomogeneous enhancement, while benign uterine leiomyomas show homogeneous enhancement in most cases and STUMP could exhibit heterogeneous enhancement due to necrosis. Rapid early enhancement followed by rapid washout is commonly seen in LMS on dynamic enhanced MRI.66 Both LMS and STUMP could exhibit central non-enhancement at the equilibria phase, which is useful to distinguish LBN from LMS and STUMP (Figure 8).67,68 Lakhman et al demonstrated that there are four qualitative MRI features most closely related to LMS: nodular bounders, hemorrhage, “T2 dark” areas, and central unenhanced areas.68 When a lesion exhibits at least three of the four features, the sensitivity and specificity of distinguishing LMS from LBN can reach 95%. Furthermore, Malek et al reported that comparing the myometrium contrast ratio, which is calculated using enhanced MRI images and T2-weighted images, could distinguish LBN from LMS with a sensitivity of 100% and a specificity of 89%.69
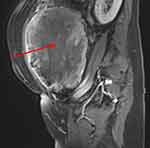 |
Figure 8 Sagittal contrast-enhanced T1-weighted fat saturated image shows the presence of central non-enhancement (red arrow). |
Treatment and Prognosis
Downes et al conducted a study with the longest follow-up time in published literature, where the average follow-up period for 24 LBN patients was 11.2 years with no recurrence or metastasis.70 Similar results have been found in other large case series, where the recurrence rate of LBN after treatment (myomectomy or hysterectomy) ranged from 0% to 8%, with no metastasis or death attributable to it.9–11,13,14 Travaglino et al analyzed all studies on LBN from 1994 to April 2020 and conducted a meta-analysis to define the clinical outcomes of LBN.18 They found that the recurrence risk of LBN was 1.9%; there was no concern for distant spread, and the overall risk of local recurrence was very low. However, with the expansion of molecular research, several studies have shown that LBN and LMS may have some shared molecular changes.15,16,21 These findings suggest that these two tumor types share a common pathogenesis and that LBN may be a potential malignant progression of LMS in some cases. At present, the potential association between LBN and LMS or the risk of LBN transforming into LMS is largely unknown. Several studies have reported cases with typical LBN histology and molecular evidence that are associated with malignant counterparts, which progress to LMS. Bennett et al reported a case of LMS within LBN lesions.71 Mittal et al found that copy number alteration (CNA) levels were different in fully malignant and benign appearing regions, and high CNAs were enriched in the former; suggesting potential tumor progression.72 Fischer et al reported two cases of LBN associated with LMS.17 The first case described a gross and histologically clear transition between LBN and LMS components. Subsequent molecular analysis found that there were common CNAs and loss of heterozygosity (LOH) regions in LMS and LBN, and additional CNAs were observed in LMS. In the second case, multiple leiomyomas, including an LBN lesion, were removed and metastatic LMS was found in the patient’s lungs four years later. Molecular analysis showed that LMS had common chromosomal losses and LOH areas with the original LBN, in addition to other chromosomal aberrations. These findings support that there is a common origin and connection between LBN and LMS development from a histologic and genomic perspective.
Although the histologic and genomic relationship between LBN and LMS is occasionally reported, it suggests the possibility for LMS progression from existing LBN, even if the process is still unclear. In addition, the risk of LBN recurrence should not be ignored. Therefore, clinical management of patients with LBN is still controversial, especially for those who want to retain fertility. As the average age of onset of LBN is 42.5–49.8 years and most patients are of reproductive age, conservative treatment is recommended.7 Previously, LBN was treated with surgery alone, without additional postoperative monitoring or routine imaging in clinical practice.73,74 The current recommendation is that appropriate follow-up is required, especially for patients with a large tumor size, high density and diffuse nuclear atypia, infiltrating borders, atypical mitoses, a high Ki-67 index (>30%), and P53 mutations.7,40 Imaging examinations should be performed at least once a year. Pelvic ultrasound, computed tomography (CT), or MRI can be used to detect new lesions. For recurrent LBN, hysterectomy is the preferred treatment for patients without fertility requirements and myomectomy for those who want to retain fertility. Uncontained power morcellation should be avoided as it may lead to the spread of benign conditions or malignant occult cancer cells, leading to a worse prognosis.75 Furthermore, patients with fertility requirements should be informed about the possibility of recurrence and should be closely followed up with imaging examinations.
Discussion
LBN are rarely encountered, but commonly present diagnostic challenges when distinguishing between FH-LM, STUMP, and LMS owing to overlapping features in clinical presentation and pathologic morphological analysis. At present, pathology sampling and morphological examination are still the key to accurate diagnosis. However, histologic evaluation alone may not be sufficient for definitive diagnosis. Newly established ancillary IHC and molecular tests can be valuable tools to facilitate differential diagnosis. We highlighted many recent advances made in histology and molecular genetics which have improved LBN diagnosis. To help reduce the incidence of occult sarcoma and improve patient prognosis, we also discuss the practicability of preoperative diagnosis including clinical, serological, and imaging features to distinguish LBN and LMS from other benign myomas.
Currently, LBN identification mainly depends on exclusion diagnosis. According to its typical histological characteristics and the lack of FH expression, FH-LM can easily be distinguished from LBN. The differentiation between STUMP and LBN mainly depends on mitotic count; however, this is challenging as the karyorrhectic nuclei can easily be confused with atypical mitoses. Nonetheless, there are a few useful IHC and molecular markers to distinguish between STUMP and LBN, such as IHC staining of Ki-67 and PHH3.27,28 The differentiation between LBN and LMS is the focus of this review because LBN is often misdiagnosed as LMS,30,60 leading to serious clinical consequences, such as overtreatment. Furthermore, some LMS patients may be misdiagnosed because of the lack of histologic evaluation. Although the median age for USMT is 40 years, the average childbearing age is increasing and the preservation of fertility should be considered when evaluating treatment options. For patients with symptomatic USMTs, there are various treatment options to preserve fertility, including myomectomy, gonadotropin-releasing hormone analog treatment, uterine artery embolization, and focused ultrasound surgery. However, excluding myomectomy, these treatments prevent further histopathological examination, which delays the optimal time for treatment, leading to a poor prognosis. Additionally, the use of laparoscopic power morcellation during myomectomy might increase the probability of dissemination of unexpected LMS.60 Therefore, it is necessary to improve the differential diagnosis between LBN and LMS from both histopathological and preoperative aspects.
There are similarities in clinical features between LBN, LMS, and other benign tumors. The main manifestations are pelvic pain, abnormal uterine bleeding, or abdominal bloating. The average age at LMS diagnosis is 10–15 years older than that for LBN.7 As ultrasonographic manifestations of LMS closely resemble those of leiomyoma, it is difficult to make a differential diagnosis.76 Recently, an increasing number of researchers have switched to the use of MRI in the differential diagnosis of USMTs.60 Additionally, inflammatory markers or liquid biopsy may contribute to preoperative diagnosis.52,57 When the combination of clinical feature evaluation, imaging examination, and serum biomarker detection shows atypical findings, biopsy, such as endometrial biopsy, should be performed. Intraoperative frozen sections offer another opportunity to identify underlying malignancy.76 However, because of sampling limitations, the accuracy often needs to be improved. Correct diagnosis often requires more extensive postoperative pathological sampling, as well as the pathological and IHC evaluation of more sections. For postoperative pathological evaluation, morphological diagnosis remains the gold standard. Immune and molecular indicators are auxiliary diagnostic measures that have been considerably developed in recent years. However, to date, none of these markers can individually clearly differentiate between LMS and LBN. Increasing studies have advocated the use of IHC panels as diagnostic markers.6,34,43 If LMS is diagnosed, surgery aiming at complete resection seems to be the best strategy.29 In addition, chemotherapy, endocrine therapy, or radiotherapy is essential. If STUMP is diagnosed, the uterus should be removed completely.29 If the patient insists on retaining the uterus because of fertility requirements, they should be informed of the risk of malignant tumor progression and close follow-up is required. For diagnosed LBN, patients with fertility requirements can opt for myomectomy, but power morcellation without protective measures is not recommended. First, power morcellation may lead to the spread of occult sarcoma, which seriously affects the prognosis. Second, some studies have shown that LBN has the possibility of recurrence.10,48 Increasing evidence shows that LBN may represent a potential stage of malignant tumor progression.17,72 Therefore, it is necessary to prevent the spread of tumor cells. For recurrent LBN, hysterectomy is the treatment of choice for patients that have completed their families and follow-up imaging should be carried out at least once a year.
Although various recent studies have described the genomic landscape of USMT, the histogenesis and molecular characteristics of LBN and LMS remain largely unknown. Cytogenetic studies have reported that both LBN and LMS showed genomic instability, and there was significant overlap in CNAs and LOH regions.60 Furthermore, LBN may share some common molecular and biomarker alterations with LMS,16,40,50 raising the question of whether LBN has a common pathogenesis with LMS or represents a potential stage of malignant tumor progression in certain cases. While LBN shares some genetic and histological characteristics with LMS, there is still no direct evidence of tumor progression. Future research should focus on the mechanisms behind the genomic instability observed in LBN and LMS and reveal the causes of genomic instability or related histological and molecular changes. High-resolution mapping for gene mutations, gene expression, gene methylation, and genomic DNA copy number changes could be a future research direction. In addition, it is of great significance to further improve the auxiliary diagnosis method of LBN. Finally, the treatment and prognosis of LBN still needs to be further evaluated by large-scale clinical trials.
Abbreviations
LBN, leiomyoma with bizarre nuclei; FH-LM, fumarate hydratase-deficient leiomyoma; STUMP, smooth muscle tumor of uncertain malignant potential; LMS, leiomyosarcoma; HPF, high power field.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This research was funded by the Special Project of Benefiting the People with Science and Technology in Qingdao West Coast New Area, grant number 2019-54 and 2019-55.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344–1355.
2. Ura B, Monasta L, Arrigoni G, et al. Phosphoproteins involved in the inhibition of apoptosis and in cell survival in the leiomyoma. J Clin Med. 2019;8:5. doi:10.3390/jcm8050691
3. Board WCoTE. Female Genital Tumours. International Agency for Research on Cancer, World Health Organization; 2020.
4. Hanley KZ, Birdsong GG, Mosunjac MB. Recent developments in surgical pathology of the uterine corpus. Arch Pathol Lab Med. 2017;141(4):528–541. doi:10.5858/arpa.2016-0284-SA
5. Byar KL, Fredericks T. Uterine leiomyosarcoma. J Adv Pract Oncol. 2022;13(1):70–76. doi:10.6004/jadpro.2022.13.1.6
6. Gao T, Finkelman BS, Ban Y, et al. Integrated histologic and molecular analysis of uterine leiomyosarcoma and 2 benign variants with nuclear atypia. Cancer Sci. 2021;112(5):2046–2059. doi:10.1111/cas.14775
7. Wei JJ. Leiomyoma with nuclear atypia: rare diseases that present a common diagnostic problem. Semin Diagn Pathol. 2022;39(3):187–200.
8. Christopherson WM, Williamson EO, Gray LA. Leiomyosarcoma of the uterus. Cancer. 1972;29(6):1512–1517. doi:10.1002/1097-0142(197206)29:6<1512::AID-CNCR2820290615>3.0.CO;2-K
9. Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18(6):535–558. doi:10.1097/00000478-199406000-00001
10. Ly A, Mills AM, McKenney JK, et al. Atypical leiomyomas of the uterus: a clinicopathologic study of 51 cases. Am J Surg Pathol. 2013;37(5):643–649. doi:10.1097/PAS.0b013e3182893f36
11. Croce S, Young RH, Oliva E. Uterine leiomyomas with bizarre nuclei: a clinicopathologic study of 59 cases. Am J Surg Pathol. 2014;38(10):1330–1339. doi:10.1097/PAS.0000000000000249
12. Kurman R, Carcangiu M, Herrington C, Young R. WHO Classification of Tumours of Female Reproductive Organs. Lyon: IARC Publications; 2014.
13. Kefeli M, Caliskan S, Kurtoglu E, Yildiz L, Kokcu A. Leiomyoma with bizarre nuclei: clinical and pathologic features of 30 patients. Int J Gynecol Pathol. 2018;37(4):379–387. doi:10.1097/PGP.0000000000000425
14. Ubago JM, Zhang Q, Kim JJ, Kong B, Wei JJ. Two subtypes of atypical leiomyoma: clinical, histologic, and molecular analysis. Am J Surg Pathol. 2016;40(7):923–933. doi:10.1097/PAS.0000000000000646
15. Mäkinen N, Kämpjärvi K, Frizzell N, Bützow R, Vahteristo P. Characterization of MED12, HMGA2, and FH alterations reveals molecular variability in uterine smooth muscle tumors. Mol Cancer. 2017;16(1):101. doi:10.1186/s12943-017-0672-1
16. Liegl-Atzwanger B, Heitzer E, Flicker K, et al. Exploring chromosomal abnormalities and genetic changes in uterine smooth muscle tumors. Mod Pathol. 2016;29(10):1262–1277. doi:10.1038/modpathol.2016.107
17. Fischer JV, Mejia-Bautista M, Vadasz B, et al. Uterine leiomyosarcoma associated with leiomyoma with bizarre nuclei: histology and genomic analysis of 2 cases. Int J Gynecol Pathol. 2022;41(6):552–565. doi:10.1097/PGP.0000000000000837
18. Travaglino A, Raffone A, Santoro A, et al. Risk of recurrence in uterine leiomyoma with bizarre nuclei: a systematic review and meta-analysis. Geburtshilfe Frauenheilkd. 2021;81(11):1217–1223. doi:10.1055/a-1533-1651
19. Oliva E. Practical issues in uterine pathology from banal to bewildering: the remarkable spectrum of smooth muscle neoplasia. Mod Pathol. 2016;29(Suppl 1):S104–S120. doi:10.1038/modpathol.2015.139
20. Porter AE, Kho KA, Gwin K. Mass lesions of the myometrium: interpretation and management of unexpected pathology. Curr Opin Obstet Gynecol. 2019;31(5):349–355. doi:10.1097/GCO.0000000000000569
21. Ahvenainen TV, Makinen NM, von Nandelstadh P, et al. Loss of ATRX/DAXX expression and alternative lengthening of telomeres in uterine leiomyomas. Cancer. 2018;124(24):4650–4656. doi:10.1002/cncr.31754
22. Lintel NJ, Luebker SA, Lele SM, Koepsell SA. MVP immunohistochemistry is a useful adjunct in distinguishing leiomyosarcoma from leiomyoma and leiomyoma with bizarre nuclei. Hum Pathol. 2018;73:122–127. doi:10.1016/j.humpath.2017.12.020
23. Siegler L, Erber R, Burghaus S, et al. Fumarate hydratase (FH) deficiency in uterine leiomyomas: recognition by histological features versus blind immunoscreening. Virchows Arch. 2018;472(5):789–796. doi:10.1007/s00428-018-2292-6
24. Berretta R, Rolla M, Merisio C, Giordano G, Nardelli GB. Uterine smooth muscle tumor of uncertain malignant potential: a three-case report. Int J Gynecol Cancer. 2008;18(5):1121–1126. doi:10.1111/j.1525-1438.2007.01125.x
25. Sung CO, Ahn G, Song SY, Choi YL, Bae DS. Atypical leiomyomas of the uterus with long-term follow-up after myomectomy with immunohistochemical analysis for p16INK4A, p53, Ki-67, estrogen receptors, and progesterone receptors. Int J Gynecol Pathol. 2009;28(6):529–534. doi:10.1097/PGP.0b013e3181a2b8d3
26. Gupta M, Laury AL, Nucci MR, Quade BJ. Predictors of adverse outcome in uterine smooth muscle tumours of uncertain malignant potential (STUMP): a clinicopathological analysis of 22 cases with a proposal for the inclusion of additional histological parameters. Histopathology. 2018;73(2):284–298. doi:10.1111/his.13515
27. Cao CD, Rico-Castillo J, De Cotiis D, Richard SD, Rosenblum NG, Chan JSY. Digital quantification of Ki-67 and PHH3 in the classification of uterine smooth muscle tumors. Int J Gynecol Pathol. 2021;40(6):549–555. doi:10.1097/PGP.0000000000000739
28. Rubisz P, Ciebiera M, Hirnle L, et al. The usefulness of immunohistochemistry in the differential diagnosis of lesions originating from the myometrium. Int J Mol Sci. 2019;20:5. doi:10.3390/ijms20051136
29. Juhasz-Böss I, Gabriel L, Bohle RM, Horn LC, Solomayer EF, Breitbach GP. Uterine leiomyosarcoma. Oncol Res Treat. 2018;41(11):680–686. doi:10.1159/000494299
30. Toledo G, Oliva E. Smooth muscle tumors of the uterus: a practical approach. Arch Pathol Lab Med. 2008;132(4):595–605. doi:10.5858/2008-132-595-SMTOTU
31. George S, Serrano C, Hensley ML, Ray-Coquard I. Soft tissue and uterine leiomyosarcoma. J Clin Oncol. 2018;36(2):144–150. doi:10.1200/JCO.2017.75.9845
32. Zhang Q, Poropatich K, Ubago J, et al. Fumarate hydratase mutations and alterations in leiomyoma with bizarre nuclei. Int J Gynecol Pathol. 2018;37(5):421–430. doi:10.1097/PGP.0000000000000447
33. Joseph NM, Solomon DA, Frizzell N, Rabban JT, Zaloudek C, Garg K. Morphology and Immunohistochemistry for 2SC and FH aid in detection of fumarate hydratase gene aberrations in uterine leiomyomas from young patients. Am J Surg Pathol. 2015;39(11):1529–1539. doi:10.1097/PAS.0000000000000520
34. Lusby K, Savannah KB, Demicco EG, et al. Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: a single institution’s experience. Ann Surg Oncol. 2013;20(7):2364–2372. doi:10.1245/s10434-012-2834-0
35. Chen L, Yang B. Immunohistochemical analysis of p16, p53, and Ki-67 expression in uterine smooth muscle tumors. Int J Gynecol Pathol. 2008;27(3):326–332. doi:10.1097/PGP.0b013e31815ea7f5
36. Idriss MH, Kazlouskaya V, Malhotra S, Andres C, Elston DM. Phosphohistone-H3 and Ki-67 immunostaining in cutaneous pilar leiomyoma and leiomyosarcoma (atypical intradermal smooth muscle neoplasm). J Cutan Pathol. 2013;40(6):557–563. doi:10.1111/cup.12127
37. Veras E, Malpica A, Deavers MT, Silva EG. Mitosis-specific marker phospho-histone H3 in the assessment of mitotic index in uterine smooth muscle tumors: a pilot study. Int J Gynecol Pathol. 2009;28(4):316–321. doi:10.1097/PGP.0b013e318193df97
38. Liang Y, Zhang X, Chen X, Lu W. Diagnostic value of progesterone receptor, p16, p53 and pHH3 expression in uterine atypical leiomyoma. Int J Clin Exp Pathol. 2015;8(6):7196–7202.
39. Chow KL, Tse KY, Cheung CL, et al. The mitosis-specific marker phosphohistone-H3 (PHH3) is an independent prognosticator in uterine smooth muscle tumours: an outcome-based study. Histopathology. 2017;70(5):746–755. doi:10.1111/his.13124
40. Zhang Q, Kanis MJ, Ubago J, et al. The selected biomarker analysis in 5 types of uterine smooth muscle tumors. Hum Pathol. 2018;76:17–27. doi:10.1016/j.humpath.2017.12.005
41. Soltan MM, Albasry AM, Eldosouky MK, Abdelhamid HS. Immunoexpression of progesterone receptor, epithelial growth factor receptor and galectin-3 in uterine smooth muscle tumors. Cell Mol Biol. 2018;64(5):7–12. doi:10.14715/cmb/2018.64.5.2
42. Zhu XQ, Shi YF, Cheng XD, Wu YZ. 子宫平滑肌肉瘤与特殊类型良性平滑肌瘤的鉴别 [The differential diagnosis between uterine leiomyosarcoma and the special subtypes of leiomyoma]. Zhonghua Yi Xue Za Zhi. 2003;83(16):1419–1421. Chinese.
43. Keyhanian K, Lage JM, Chernetsova E, Sekhon H, Eslami Z, Islam S. Combination of MCM2 with Ki67 and p16 immunohistochemistry can distinguish uterine leiomyosarcomas. Int J Gynecol Pathol. 2020;39(4):354–361. doi:10.1097/PGP.0000000000000616
44. Mehine M, Kaasinen E, Heinonen HR, et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc Natl Acad Sci U.S.A. 2022;113(5):1315–1320.
45. Kämpjärvi K, Mäkinen N, Mehine M, et al. MED12 mutations and FH inactivation are mutually exclusive in uterine leiomyomas. Br J Cancer. 2016;114(12):1405–1411. doi:10.1038/bjc.2016.130
46. Makinen N, Vahteristo P, Kampjarvi K, Arola J, Butzow R, Aaltonen LA. MED12 exon 2 mutations in histopathological uterine leiomyoma variants. Eur J Hum Genet. 2013;21(11):1300–1303. doi:10.1038/ejhg.2013.33
47. Mäkinen N, Aavikko M, Heikkinen T, et al. Exome sequencing of uterine leiomyosarcomas identifies frequent mutations in TP53, ATRX, and MED12. PLoS Genet. 2016;12(2):e1005850. doi:10.1371/journal.pgen.1005850
48. Zhang Q, Ubago J, Li L, et al. Molecular analyses of 6 different types of uterine smooth muscle tumors: emphasis in atypical leiomyoma. Cancer. 2014;120(20):3165–3177. doi:10.1002/cncr.28900
49. Tsuyoshi H, Yoshida Y. Molecular biomarkers for uterine leiomyosarcoma and endometrial stromal sarcoma. Cancer Sci. 2018;109(6):1743–1752. doi:10.1111/cas.13613
50. Chudasama P, Mughal SS, Sanders MA, et al. Integrative genomic and transcriptomic analysis of leiomyosarcoma. Nat Commun. 2018;9(1):144. doi:10.1038/s41467-017-02602-0
51. Holzmann C, Markowski DN, Koczan D, Küpker W, Helmke BM, Bullerdiek J. Cytogenetically normal uterine leiomyomas without MED12-mutations - a source to identify unknown mechanisms of the development of uterine smooth muscle tumors. Mol Cytogenet. 2014;7(1):88. doi:10.1186/s13039-014-0088-1
52. Suh DS, Song YJ, Roh HJ, et al. Preoperative blood inflammatory markers for the differentiation of uterine leiomyosarcoma from leiomyoma. Cancer Manag Res. 2021;13:5001–5011. doi:10.2147/CMAR.S314219
53. Nishigaya Y, Kobayashi Y, Matsuzawa Y, et al. Diagnostic value of combination serum assay of lactate dehydrogenase, D-dimer, and C-reactive protein for uterine leiomyosarcoma. J Obstet Gynaecol Res. 2019;45(1):189–194. doi:10.1111/jog.13792
54. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002;12(4):354–361. doi:10.1046/j.1525-1438.2002.01086.x
55. Di Cello A, Borelli M, Marra ML, et al. A more accurate method to interpret lactate dehydrogenase (LDH) isoenzymes’ results in patients with uterine masses. Eur J Obstet Gynecol Reprod Biol. 2019;236:143–147. doi:10.1016/j.ejogrb.2019.03.017
56. Spivack LE, Glantz JC, Lennon C, Bhagavath B. Specificity of the lactate dehydrogenase isoenzyme index as a preoperative screen for uterine sarcoma before myomectomy. Fertil Steril. 2021;115(1):174–179. doi:10.1016/j.fertnstert.2020.07.020
57. Dvorská D, Škovierová H, Braný D, Halašová E, Liquid DZ. Biopsy as a tool for differentiation of leiomyomas and sarcomas of corpus uteri. Int J Mol Sci. 2019;20:15. doi:10.3390/ijms20153825
58. Ludovisi M, Moro F, Pasciuto T, et al. Imaging in gynecological disease (15): clinical and ultrasound characteristics of uterine sarcoma. Ultrasound Obstet Gynecol. 2019;54(5):676–687. doi:10.1002/uog.20270
59. Aminzadeh P, Alibrahim E, Dobrotwir A, Paul E, Multiparametric GS. MR evaluation of uterine leiomyosarcoma and STUMP versus leiomyoma in symptomatic women planned for high frequency focussed ultrasound: accuracy of imaging parameters and interobserver agreement for identification of malignancy. Br J Radiol. 2021;94(1119):20200483. doi:10.1259/bjr.20200483
60. Lin Y, Wu RC, Huang YL, et al. Uterine fibroid-like tumors: spectrum of MR imaging findings and their differential diagnosis. Abdominal Radiol. 2022;26:1–2.
61. Ando T, Kato H, Furui T, Morishige KI, Goshima S, Matsuo M. Uterine smooth muscle tumours with hyperintense area on T(1) weighted images: differentiation between leiomyosarcomas and leiomyomas. Br J Radiol. 2018;91(1084):20170767. doi:10.1259/bjr.20170767
62. Matsuura K, Inoue K, Hoshino E, et al. Utility of magnetic resonance imaging for differentiating malignant mesenchymal tumors of the uterus from T2-weighted hyperintense leiomyomas. Jpn J Radiol. 2022;40(4):385–395. doi:10.1007/s11604-021-01217-2
63. Tamai K, Koyama T, Saga T, et al. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2008;18(4):723–730. doi:10.1007/s00330-007-0787-7
64. Cornfeld D, Israel G, Martel M, Weinreb J, Schwartz P, McCarthy S. MRI appearance of mesenchymal tumors of the uterus. Eur J Radiol. 2010;74(1):241–249. doi:10.1016/j.ejrad.2009.03.005
65. Bi Q, Xiao Z, Lv F, Liu Y, Zou C, Shen Y. Utility of clinical parameters and multiparametric MRI as predictive factors for differentiating uterine sarcoma from atypical leiomyoma. Acad Radiol. 2018;25(8):993–1002. doi:10.1016/j.acra.2018.01.002
66. Rio G, Lima M, Gil R, Horta M, Cunha TM. T2 hyperintense myometrial tumors: can MRI features differentiate leiomyomas from leiomyosarcomas? Abdomi Radiol. 2019;44(10):3388–3397. doi:10.1007/s00261-019-02097-x
67. Lin G, Yang LY, Huang YT, et al. Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma / smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J Magn Reson Imaging. 2016;43(2):333–342. doi:10.1002/jmri.24998
68. Lakhman Y, Veeraraghavan H, Chaim J, et al. Differentiation of uterine leiomyosarcoma from atypical leiomyoma: diagnostic accuracy of qualitative MR imaging features and feasibility of texture analysis. Eur Radiol. 2017;27(7):2903–2915. doi:10.1007/s00330-016-4623-9
69. Malek M, Rahmani M, Seyyed Ebrahimi SM, et al. Investigating the diagnostic value of quantitative parameters based on T2-weighted and contrast-enhanced MRI with psoas muscle and outer myometrium as internal references for differentiating uterine sarcomas from leiomyomas at 3T MRI. Cancer Imaging. 2019;19(1):20. doi:10.1186/s40644-019-0206-8
70. Downes KA, Hart WR. Bizarre leiomyomas of the uterus: a comprehensive pathologic study of 24 cases with long-term follow-up. Am J Surg Pathol. 1997;21(11):1261–1270. doi:10.1097/00000478-199711000-00001
71. Bennett JA, Weigelt B, Chiang S, et al. Leiomyoma with bizarre nuclei: a morphological, immunohistochemical and molecular analysis of 31 cases. Mod Pathol. 2017;30(10):1476–1488. doi:10.1038/modpathol.2017.56
72. Mittal KR, Chen F, Wei JJ, et al. Molecular and immunohistochemical evidence for the origin of uterine leiomyosarcomas from associated leiomyoma and symplastic leiomyoma-like areas. Mod Pathol. 2009;22(10):1303–1311. doi:10.1038/modpathol.2009.96
73. Abeler VM, Røyne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54(3):355–364. doi:10.1111/j.1365-2559.2009.03231.x
74. Wang WL, Soslow R, Hensley M, et al. Histopathologic prognostic factors in stage I leiomyosarcoma of the uterus: a detailed analysis of 27 cases. Am J Surg Pathol. 2011;35(4):522–529. doi:10.1097/PAS.0b013e31820ca624
75. Xu X, Lin H, Wright JD, et al. Association between power morcellation and mortality in women with unexpected uterine cancer undergoing hysterectomy or myomectomy. J Clin Oncol. 2019;37(35):3412–3424. doi:10.1200/JCO.19.00562
76. Yildiz G, Mat E, Yildiz P, et al. The incidence of unexpected gynaecological malignancies in hysterectomies carried out for benign indications. J Obstet Gynaecol. 2021;41(2):298–304. doi:10.1080/01443615.2020.1833849
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.