Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Updated Perspectives on the Diagnosis and Management of Onychomycosis
Authors Falotico JM, Lipner SR
Received 4 August 2022
Accepted for publication 8 September 2022
Published 15 September 2022 Volume 2022:15 Pages 1933—1957
DOI https://doi.org/10.2147/CCID.S362635
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Julianne M Falotico,1 Shari R Lipner2
1Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA; 2Weill Cornell Medicine, Department of Dermatology, New York, NY, USA
Correspondence: Shari R Lipner, Weill Cornell Medicine, Department of Dermatology, 1305 York Avenue, NY, NY, 10021, USA, Tel +1 646-962-3376, Fax +1 646-962-0033, Email [email protected]
Abstract: Onychomycosis is the most common nail disease encountered in clinical practice and can cause pain, difficulty with ambulation, and psycho-social problems. A thorough history and physical examination, including dermoscopy, should be performed for each patient presenting with nail findings suggestive of onychomycosis. Several approaches are available for definitive diagnostic testing, including potassium hydroxide and microscopy, fungal culture, histopathology, polymerase chain reaction, or a combination of techniques. Confirmatory testing should be performed for each patient prior to initiating any antifungal therapies. There are several different therapeutic options available, including oral and topical medications as well as device-based treatments. Oral antifungals are generally recommended for moderate to severe onychomycosis and have higher cure rates, while topical antifungals are recommended for mild to moderate disease and have more favorable safety profiles. Oral terbinafine, itraconazole, and griseofulvin and topical ciclopirox 8% nail lacquer, efinaconazole 10% solution, and tavaborole 5% solution are approved by the Food and Drug Administration for treatment of onychomycosis in the United States and amorolfine 5% nail lacquer is approved in Europe. Laser treatment is approved in the United States for temporary increases in clear nail, but clinical results are suboptimal. Oral fluconazole is not approved in the United States for onychomycosis treatment, but is frequently used off-label with good efficacy. Several novel oral, topical, and over-the-counter therapies are currently under investigation. Physicians should consider the disease severity, infecting pathogen, medication safety, efficacy and cost, and patient age, comorbidities, medication history, and likelihood of compliance when determining management plans. Onychomycosis is a chronic disease with high recurrence rates and patients should be counseled on an appropriate plan to minimize recurrence risk following effective antifungal therapy.
Keywords: onychomycosis, nail disease, fungal nail infection, diagnosis, management, treatment, recurrence
Introduction
Onychomycosis is a fungal infection of the nail unit caused by dermatophytes, non-dermatophyte molds (NDM) and yeast.1,2 It is the most common nail infection encountered in clinical practice,3 with a worldwide prevalence of 5.5%, and an estimated prevalence of 2% to 14% in the United States (US),4 and 0.5% to 24% in Europe.5–8 Risk factors include prior dermatologic conditions, such as hyperhidrosis, tinea pedis, and psoriasis, as well as exogenous factors, including occlusive shoes, trauma, and poor nail grooming. Comorbidities, such as diabetes mellitus, immunosuppression, malignancy, venous insufficiency, peripheral arterial disease, obesity, and inflammatory bowel disease also increase risk.4 Altered foot biomechanics due to biomechanical malignments, congenital deformities, or neurological deficits can result in repetitive microtrauma during walking and increase the risk of infection and recurrence.9,10 Genetics may predispose to developing infection11,12 and transmission risk increases when members of the same household are infected.13 Onychomycosis may occur at any age, however prevalence increases with age,14,15 affecting roughly 50% of patients greater than 70 years old,16 and is rather rare in the pediatric population, with increased risk seen in children with Down’s syndrome or immunodeficiency.17
Onychomycosis, especially with secondary bacterial infections, can result in local pain and paresthesia, which pose significant psychosocial consequences. Limited dexterity and ambulation and difficulty finding comfortable fitting footwear can lead to social embarrassment and decreased self-esteem, which can be largely distressing, even when the infection is not severe.1,4 Patients also report stigmatization and dissatisfaction with the aesthetic appearance of their nails, and thus may avoid social interactions.18 In a systematic review of 30 studies evaluating the effect of onychomycosis and treatment on quality of life (QoL),19 women and patients with fingernail involvement had the poorest QoL scores. There were greater improvements in QoL with oral treatments versus topical therapies. Therefore, onychomycosis is more than just a cosmetic concern, and dermatologists and podiatrists should inquire about nail concerns during routine office visits in addition to evaluating all 20 nail units.1 In an analysis of the top 51 search engine hits for onychomycosis,20 overall readability was poor, with only one-third of websites meeting the acceptable seventh grade reading level for patients. Therefore, internet-based information about onychomycosis is lacking, highlighting the need for proper in-office patient education and counseling for this chronic and recurrent disease.
Pathogenic Organisms
The majority of onychomycosis cases are due to dermatophytes (60–90%), most commonly Trichophyton rubrum and T. mentagrophytes. Less common dermatophytes include T. verrucosum, T. violaceum, T. krajdenii, Epidermophyton floccosum, and Arthroderma spp., with infection due to Microsporum spp. being very rare. Cases secondary to dermatophyte infections are specifically referred to as tinea unguium.1,4,13 Infection with NDMs account for about 10% of cases worldwide, with the most common organisms being, Aspergillus spp., Fusarium spp., Acremonium spp., Scopulariopsis brevicaulis, Alternaria alternata, and Neoscytalidium spp.21–24 Yeast infections account for up to 10–20% of cases, with the most common pathogen being Candida spp., including C. albicans, C. krusei, C. parapsilosis, C. glabrata and C. tropicalis. Yeast infections are more frequent in the fingernails, especially if the hands are routinely submerged in water.4,25–27 Onychomycosis due to the yeast Kloeckera apiculata is uncommon but has been isolated in select cases.28,29 In children, infection with T. tonsurans may occur.13 Infections with two or more organisms can occur, and mixed dermatophyte-NDM infections account for estimated 3–11% of onychomycosis cases, which may potentially be more difficult to treat and more prone to recurrences.1,4 Importantly, prevalence and infecting pathogen can vary according to the population studied1 due to differences in geographic location, climates, and daily activities, including professions and personal habits. Specifically, prevalence in North America ranges from 8.7% to 13.8%14,25 and is predominately caused by dermatophytes due to immigration of dermatophytes from other parts of the world.30 Prevalence in Europe is broader at about 0.5–24%.5–8 In tropical and warmer climates, infection with NDM and yeasts are more common.31–33 Additionally, instead of acting planktonically (in suspension, independent, and free-floating), pathogens may merge into groups and form biofilms.4,34 Biofilms attach to surfaces, such as the nail plate, via an extracellular matrix,35 which protect fungi from host immune responses and give them increased virulence36 (Figure 1). Resultantly, biofilms may contribute to treatment resistance and difficulty controlling chronic infections.37,38
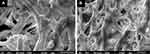 |
Figure 1 Scanning electron microscopy demonstrating mature fungal biofilms that were formed in 24-well plates. White arrows depict extracellular matrix covering and connecting the hyphae. (A) Trichophyton rubrum ATCC 28189. (B) Trichophyton mentagrophytes ATCC 11481. Reprinted from J Am Acad Dermatol, 1;80(4), Lipner SR, Scher RK, Onychomycosis: Clinical overview and diagnosis, 835–851, Copyright (2019), with permission from Elsevier.4 |
Clinical Presentation
History
Patients may fail to disclose nail conditions to their dermatologists or podiatrists due to the apprehension of discussing something that may seem trivial. Therefore, dermatologists and podiatrists must routinely inquire about nail problems as part of a complete history.1 Patients with onychomycosis typically complain of nail discoloration (most commonly yellow, white, or brown if the fungus is dense), nail separation, brittleness, and thickening.39 Symptoms may worsen progressively until the nail crumbles away,1 and in untreated cases, the skin may become sore and inflamed.40 Patients may report local pain and paresthesia in affected nails, trouble fitting into shoes, difficulty with ambulation, and social embarrassment.1,41 Medication use, including oral, topical, and over-the-counter therapies, should also be thoroughly reviewed with the patient, as diagnostic testing and treatment regimens can be impacted by current use of antifungals.4,42
Physical Examination
Onychomycosis may affect either fingernails or toenails or both,43 however, toenail involvement is much more common than fingernail involvement. For toenails, the great or second toenail is most frequently affected.44 Unless there has been antecedent trauma or the patient is immunosuppressed, it is unusual for a fingernail to be involved without concomitant toenail infection.1 Regardless, all 20 nails should be examined in clinical practice.24 Since it is common to have accompanying scale in the web spaces and/or plantar feet, dermatologists and podiatrists should perform a comprehensive physical examination including the hands and feet in addition to the nail units.4
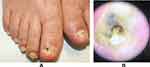
Common physical examination findings include yellow nail plate discoloration and thickening and subungual hyperkeratosis, which may cause nail plate onycholysis (Figure 2). In severe cases, there may be onychodystrophy with nail plate ridging, thickening, and crumbling, onychocryptosis, and nail loss. Trauma may result in red, black, or brown nail plate discoloration (hematoma).1,4 Dermatophytomas are fungal abscesses that present as orange and brown or white and yellow longitudinal streaks or patches in the subungual space. NDM infections can also result in nail matrix involvement, causing periungual inflammation and tenderness.24 A standardized composite score, such as The Scoring Clinical Index for Onychomycosis, can be used to assess overall disease severity and aid in the development of an individualized treatment plan.45
Dermoscopy
Dermoscopy can aid in differentiating between other nail diseases, including onychomycosis (Figure 2), psoriasis, pseudomonas colonization, and traumatic onycholysis.1,46 Typical dermatoscopic findings of onychomycosis are a jagged proximal margin of onycholysis and longitudinal striae, with vertical streaks of differing colors in the onycholytic area that resemble an aurora borealis.4,47 The subungual area may have a ruin-like appearance, representing hyperkeratosis and debris.48,49 Linear bands, multicolored or yellow/white patterns or streaks, subungual keratosis, reverse triangular or nonlongitudinal homogenous patterns, nail plate scales,50 and rarely, pseudo-Hutchinson sign or longitudinal patterns51 may be seen in fungal melanonychia. Importantly, dermoscopy should be used only as an aid and not for definitive diagnosis. Confirmatory testing should be performed prior to initiating both oral and topical treatments and is especially important considering that different nail pathologies can have similar clinical presentations.
Diagnostic Testing
History, clinical examination, and dermoscopy alone are not sufficient to make a definitive diagnosis of onychomycosis. Mycologic laboratory testing is necessary for diagnosis and is cost-effective.52 Laboratory confirmation is quick, easy to perform, and prevents treatment failures, inaccurate diagnoses, avoidable adverse effects, and possible drug–drug interactions.53,54 In 2013, the American Academy of Dermatology, as part of the ABIM Foundation’s Choosing Wisely campaign, recommended that confirmatory testing of onychomycosis be performed before prescribing oral antifungal therapy.55 In a retrospective analysis of 1774 patients with a diagnosis of onychomycosis, 2002–2018,56 only 39.3% underwent diagnostic testing, with a steady decrease in testing from 2007 onwards, except for an isolated spike in 2014. Therefore, diagnostic testing for onychomycosis is underperformed, with a need for increased physician education regarding the importance of confirmatory testing. Prior to any diagnostic testing, a three-to-six-month washout period of antifungal therapies, including all oral, topical, and over-the-counter medications, should be performed. Antifungals may be retained in the subungual debris and nail plate, which may be transported to culture media and can impede fungal growth for culture as well as affecting other diagnostic tests.4,57
There are multiple techniques available for diagnosing onychomycosis (Table 1). The specific method should be chosen after careful consideration of patient characteristics, clinician expertise, and cost, sensitivity, specificity, and turnaround time for the result.4 For potassium hydroxide (KOH) and microscopy, fungal culture, and polymerase chain reaction (PCR), the nail is cleaned with 70% isopropyl alcohol and soap and water to prevent contamination of the sample with the skin flora and/or environment. The affected nail plate is then clipped1,39 (Figure 3), with the precise location of sample collection determined by the clinical presentation.4 The subungual debris can be scraped onto a paper with a contrasting background to better visualize the quantity collected.58 Histopathology does not require similar preparation of the sample. Sampling the nail is a specialized technique learned and perfected during residency training and physician experience is an integral component of this process. Patient performed clippings are not appropriate for diagnostic testing.59
 |
Table 1 Summary of the Diagnostic Testing Methods |
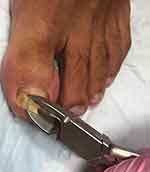 |
Figure 3 A nail clipper is used to clip the most proximal area of onycholysis. |
KOH and Microscopy
Fungal elements can be visualized with microscopic examination (positive direct microscopy) of nail scrapings treated with 5–40% KOH.40,53 The addition of KOH aids in dissolving larger keratinocytes, making them flatter and limiting reflection of cell borders. Light microscopy is used to look for the presence or absence of fungal elements.39,60 Although KOH is the most frequently used reagent, alternative reagents include sodium sulfide, sodium hydroxide, Parker blue black ink, or calcofluor white. Calcofluor white requires use of a fluorescent microscope for visualization.4,40 Enhancement of KOH with chlorazol black E can increase sensitivity and cost-effectiveness.61 Light microscopy with staining can be performed in the clinic in a matter of minutes; however, it lacks sensitivity, especially for detection of NDMs, cannot determine fungal viability or provide precise fungal identification, and is dependent on clinician expertise.4,62
Fungal Culture
Fungal culture is the only technique that can identify both the organism and determine its viability. In the laboratory, sabouraud dextrose agar with cycloheximide (to encourage dermatophyte growth) or without cycloheximide (to culture NDMs) is used.63 Chloramphenicol and gentamicin can be added to the agar to inhibit growth of bacterial contaminants.40 The culture is grown at 25–30°C for up to a month. Alternatively, dermatophyte test medium is a simple and rapid method that can be utilized to culture dermatophytes. Alkaline metabolites are released upon growth of dermatophytes, with the increase in pH resulting in a color change from yellow to red approximately 10 to 14 days later.64 There are high rates of false-negatives (up to 40%) with fungal cultures, which can be even lower when there is residual antifungal medication and/or partial treatments.1,40 Insufficient incubation time or temperature as well as the presence of more than one species in a sample may also contribute.64,65 There is higher culture yield when there is adequate quantity of subungual debris for evaluation.66
Histopathology
Histopathology is performed on nail plate clippings in 10% buffered formalin,4,53 with turnaround time for results within days, and is more sensitive than both KOH and fungal culture.67 It may also be more reliable in ascertaining whether the pathogen is permeating the nail plate or merely colonizing the nail.52 Hematoxylin and eosin staining is used to visualize fungal elements. Periodic acid-Schiff (PAS) or Grocott methenamine-silver staining enhance visualization of the hyphae,1 which are the preferred staining methods.68 Immunofluorescence, Fontana-Masson, and Mayer mucicarmine stains can also be used.69 Histopathology can identify yeast, spores, hyphae, and pseudohyphae,4 but cannot identify or determine viability of the causative organism.1 Histopathology can also differentiate between other nail conditions, such as nail psoriasis.
PCR
PCR is a newer technique that uses specific primers to amplify DNA segments in order to identify dermatophytes, NDMs and Candida spp.1,40,70 Common targets include a gene fragment of the fungal small ribosomal subunit 18s rRNA, the chitin synthase I gene, the topoisomerase II gene, or the internal transcribed spacer region of ribosomal DNA.70,71 Distinction between yeasts, dermatophytes, and molds is possible by using ≥2 restriction enzymes to digest amplicons.72 Results are available within 24 to 48 hours40 and PCR is three to four times less likely than fungal culture to yield false-negatives.73 Potential false-positive results with PCR are due to the stability of fungal DNA and possible contaminants.40 The test is expensive compared to culture or microscopy,1 however it is becoming more widely available and is covered by many insurance plans.4 A similar technique is real-time PCR, which quantitates relative transcript amounts40 and provides indirect information on fungal viability.74 A commercial PCR kit is another alternative, which is more accessible than regular PCR, is cost-effective, has high sensitivity and specificity for diagnosing onychomycosis, can reduce delays in diagnoses, and may be more accurate than fungal culture, especially in cases of NDM infections.75–77
Artificial Intelligence (AI)
A convolutional neural network is a type of deep-learning algorithm that resembles the organization of the visual cortex78 and has been used to diagnose a variety of dermatologic conditions, including psoriasis, melanoma, non-melanoma skin cancer, rosacea, and atopic dermatitis.79–84
AI has also been applied for diagnosis of onychomycosis.78,85,86 In a prospective study of 90 patients with onychodystrophy of the toenails,78 clinical photographs of the nails were taken by nonphysicians and evaluated clinically by five board-certified dermatologists (mean 5.6 years of experience), as well as by two board-certified dermatologists using dermoscopy and compared to AI to diagnose onychomycosis. KOH microscopy or fungal culture was used to confirm the diagnosis in all cases. The area under the curve (AUC) value of the AI [0.751; 95% confidence interval (CI), 0.646–0.856] was comparable to dermoscopy (0.755; 95% CI, 0.654–0.855) (Delong’s test; p = 0.952). The Youden index score (sensitivity+specificity-100%) of the AI (0.429) was also comparable to the mean Youden index score of the five board-certified dermatologists (0.230±0.176) (Wilcoxon rank-sum test; p = 0.667). In a study evaluating 1155 dermoscopic images (603 onychomycosis, 227 normal nails, 221 nail psoriasis, 104 traumatic onychodystrophy),85 the diagnostic performances between the AI and 54 dermatologists were compared. There was high specificity (>82%) of diagnosing onychomycosis for five dermoscopic nail plate patterns, including jagged edge, longitudinal striae, marble-like turbid areas, distal irregular termination, and cone-shaped keratosis. The Youden index of the AI (0.715) was significantly higher than mean Youden index of 54 dermatologists (0.427±0.188, 0.444±0.172) (p < 0.05). Not all diagnoses were confirmed by histology and the study included resident physicians. In a study comparing the diagnostic accuracy of AI to four dermatopathologists,87 199 PAS-stained nail tissue samples were evaluated, with 101 positive and 98 negative for onychomycosis, respectively. The AUC of the AI was 98.1% (CI 96.1–99.8%), with two senior dermatopathologists having higher AUC and specificity and two junior dermatopathologists having lower AUC and specificity than the AI. All dermatopathologists had higher sensitivity than the AI. Therefore, the use of AI on whole-slide images is statistically non-inferior regarding AUC and specificity compared with dermatopathologists using a conventional analogous setting with a microscope. Together, these studies show that AI may be an emerging tool that can be used to diagnose onychomycosis effectively and objectively. Prior to widespread adoption of new AI tools for onychomycosis diagnosis, more prospective studies incorporating AI into routine clinical workflow are necessary.
Treatment
Treatment for onychomycosis should be aimed at eliminating the fungal pathogen and restoring the normal state of the nail. This process can be quite slow, given that toenails and fingernails grow approximately 1 to 2 mm and 2 to 3 mm per month, respectively.88 It is important to educate patients that confirmation of the diagnosis is relatively quick compared to the amount of time that they had the condition, and that it is necessary to make sure of the diagnosis before starting treatment rather than treating empirically.53 Onychomycosis can mimic other benign conditions, including trauma, nail psoriasis, nail lichen planus, subungual exostosis, verruca, onychomatricoma, and bacterial infections, as well as malignant conditions, including squamous cell carcinoma and amelanotic melanoma.39 Empiric antifungal treatment in these cases increases the risk of unnecessary side effects, would not improve, and may even worsen, benign conditions, and may increase morbidity and mortality in cases of malignant conditions.53
There are several treatment options available for onychomycosis, including oral and topical antifungals, device-based therapies (ie, lasers), surgical nail avulsion, nail debridement, and combination therapies.40,88,89 Oral antifungals are generally recommended for moderate to severe onychomycosis and topical antifungals for mild to moderate disease2 (Tables 2 and 3). Nail debridement can decrease fungal load and can be used as an adjunct with topical therapies,40 while nail avulsion has limited use, primarily for cases of a painful and/or nongrowing single nail.90 Laser therapies are United States (US) Food and Drug Administration (FDA) approved for temporary increases in clear nail; however, cure rates are lower than for oral and topical antifungals with less rigorous endpoints required by the FDA for approval and definitive guidelines for use are currently lacking.1,2,88 Regardless, treatment plans should be individualized, with consideration of disease severity, infecting pathogen, medication cost, and patient comorbidities, medication history, and likelihood of compliance.88,89 Combination therapy should not be considered first-line treatment and should be reserved for patients with poor prognostic factors (ie, older age, immunosuppression, mixed infections) or for those who have failed monotherapy for onychomycosis.91 In a systematic review of race reporting in onychomycosis clinical trials, less than a fifth (17.5%; 32/182) of trials reported on race and/or ethnicity, with only one trial comparing treatment efficacy in different subgroups.92 Therefore, treatment recommendations garnered from these trials should be interpreted with caution in patients of color.
 |
Table 2 Summary of Commonly Used Oral Onychomycosis Medications |
 |
Table 3 Summary of FDA-Approved Topical Onychomycosis Medications |
Oral Therapies
Systemic medications are widely used for the treatment of onychomycosis due to their accessibility, high efficacy, and comparatively low cost.1,88 Oral medications reach the nail bed systemically and must penetrate the ventral nail plate.1,93 The amount of drug that can reach the infected site is therefore limited by low blood circulation to the nail bed.94 Oral medications may have poor penetration of biofilms95 and there is increased risk of serious side effects and drug–drug interactions.96
Currently, terbinafine, itraconazole, and griseofulvin are US FDA approved for onychomycosis treatment.88 Griseofulvin is rarely used because it requires longer treatment durations, with lower efficacy and higher risk of adverse events and recurrence compared to other oral antifungals.90 It is dosed at 375 up to 750 mg once daily for at least six months for toenails.2,97 Fluconazole is not US FDA approved of onychomycosis treatment but is frequently used off-label.40
Terbinafine
Terbinafine is an allylamine that inhibits squalene epoxidase, with broad-spectrum activity against dermatophytes and some activity against NDMs and Candida spp.98 It is dosed at 250 mg daily for 6 and 12 weeks for fingernails and toenail infections, respectively.88 The complete cure rates are 59% and 38% and the mycologic cure rates are 79% and 70% for fingernails and toenails, respectively.1 Potential side effects are mild and include headaches, rashes, and gastrointestinal symptoms. Rarely, hepatotoxicity and taste disturbances can occur.88,99 Although terbinafine-related hepatotoxicity is rare, patients are often hesitant to start terbinafine in fear of developing liver injury.100 In an analysis of 35 websites with information on terbinafine,100 only 51.4% provided accurate information on terbinafine-related hepatotoxicity and less than a quarter (20.0%) stated that hepatoxicity was rare. Few websites (11.4%) were authored by board-certified dermatologists. Therefore, accurate information regarding terbinafine-associated hepatotoxicity must be disseminated online in order to ease terbinafine fears and increase the likelihood of non-dermatologist providers and patients prescribing and accepting efficacious onychomycosis therapy, respectively.
According to the package insert, laboratory evaluation of liver function is recommended for all patients prior to initiating terbinafine therapy.101 Periodic laboratory monitoring during terbinafine treatment for adults is controversial.102 In a retrospective analysis of 944 adult patients taking a 12-week course of oral terbinafine for onychomycosis,103 only 2.4% and 2.8% of patients had abnormal monitoring liver function tests (LFT) and complete blood count (CBC) results, respectively, and were threefold more likely to be ≥65 years old versus the overall study population. In a retrospective analysis of 4309 adults and children taking terbinafine for dermatophyte infections, 2006–2016,104 grade 2 or higher monitoring ALT and AST elevations were found in only 0.2% and 0.07% of patients, respectively. Six patients (0.14%) discontinued treatment due to grade 1 to 3 terbinafine associated LFT abnormalities. In a retrospective review of the Taiwanese Longitudinal Health Insurance Database,105 12,376 patients took oral terbinafine with only two cases (0.016%) of drug-induced liver injury. Therefore, interval monitoring is unnecessary in healthy adults without preexisting hematologic and hepatic abnormalities but may be considered in older patients with comorbidities. There are fewer risk factors for terbinafine-induced hepatotoxicity in children, with no consensus regarding laboratory surveillance during treatment. In a retrospective analysis of 134 children prescribed terbinafine (average treatment course 8.2 weeks) for superficial fungal infections,106 0% and 1.7% had baseline and monitoring LFT elevations, respectively, and 3.9% and 4.4% had baseline and monitoring CBC elevations, respectively. All abnormal laboratory results were mild and resolved with treatment discontinuation or completion. In a retrospective review of 269 children prescribed terbinafine for onychomycosis,107 53.5% had laboratory monitoring of LFTs and/or CBC, with 23.6% before treatment and 70.8% before treatment and at 6 weeks. Most patients (87.5%) had normal laboratory results. Grade 1 abnormalities were noted in 8.3% and 4.2% of patients before or during therapy, respectively, with three patients discontinuing treatment. Therefore, monitoring tests in healthy children are also unnecessary. Baseline tests should be performed in both adults and pediatric patients to rule out pre-existing liver or hematologic diseases prior to initiating terbinafine treatment.
Terbinafine can be administered as pulse therapy as an off-label treatment option for onychomycosis and may be cost-effective and improve patient compliance.42,108 These pulse-dosed regimens have been studied in clinical trials but are not US FDA approved.88 In a meta-analysis evaluating different terbinafine treatment regimens for onychomycosis,109 a pulsed regimen of two cycles of 250 mg/day for four weeks on and four weeks off had similar mycologic cure rates to continuous terbinafine [risk ratio (RR) for intention to treat: 0.94 (95% CI: 0.74–1.19, p = 0.6, n = 2); RR for evaluable patients: 1.01 (95% CI: 0.84–1.22, p = 0.92, n = 2)]. Complete cure was also comparable to that of 12 weeks of continuous therapy (250 mg/day). In a systematic review of 30 studies comparing continuous and pulsed terbinafine regimens for treatment of onychomycosis,110 there were no significant differences in mycologic cure or likelihood of experiencing adverse events for terbinafine administered continuously (250 mg for 12 weeks) or pulsed (500 mg daily for one week per month, pulsed 3 times). Therefore, for patients that are hesitant to accept treatment with terbinafine, pulse treatment regimens can be considered since safety and efficacy are comparable to continuous treatments.
Itraconazole
Itraconazole is a triazole that inhibits lanosterol 14a-demethylase and is efficacious against dermatophytes, Candida spp. and NDMs.111,112 The dosing for toenails is 200 mg daily for 12 weeks and for fingernails is 200 mg twice daily for 1 week separated by 3 weeks of washout for 2 treatment pulses.88 The complete cure rates are 47% and 14% and the mycologic cure rates are 61% and 54% for fingernails and toenails, respectively. Potential side effects include headaches, upper respiratory tract infections, gastrointestinal symptoms, hypertriglyceridemia, elevated transaminases, and rarely, peripheral neuropathy and hepatitis.1,88 Drug–drug interactions are common and a thorough medication history should be performed prior to treatment initiation. Ventricular dysfunction, including congestive heart failure, is a contraindication to use.113
Booster Therapy
Additional doses of terbinafine or itraconazole can be used as booster or supplemental therapy after completion of the original full antifungal course.1,88 It may improve cure rates in onychomycosis patients with specific nail plate characteristics (lateral involvement, >2mm in thickness, >75% surface area involvement), slow growing nails, immunosuppression, and matrix involvement.114,115 The suggested dose is an additional four weeks of terbinafine or itraconazole six to nine months after the original initiation of antifungal treatment.116,117 However, booster therapy has not been studied in clinical trials, but given the low risk of additional oral therapy, it may be warranted in challenging cases.
Fluconazole
Fluconazole is a triazole that inhibits lanosterol 14a-demethylase. It is approved for onychomycosis treatment in Europe and China and is used off-label in the US, with efficacy against dermatophytes, Candida spp., and some NDMs.1,88 Due to a short residual concentration in the nails, longer treatment courses are necessary. Dosing for fingernails and toenails are 150 mg weekly for 6–9 months and 12–18 months, respectively.118,119 It may be challenging to remember to take fluconazole on a weekly rather than daily basis; therefore, patients can implement a reminder text or alarm notification system on their mobile devices to increase medication compliance.120 In a double blind, randomized study in 362 patients receiving fluconazole 150, 300, or 450 mg once weekly, complete cure rates were 37%, 46%, and 48%, respectively, for toenails at 12 months, with a low (4%) recurrence rate 6 months after treatment.121 The most common side effects include nausea, rash, headache, abdominal pain, and elevated LFTs. Rarely, liver injury or failure can occur, but is more common in immunosuppressed patients.122 Drug–drug interactions are also common, especially with warfarin and hypoglycemic agents.99 Fluconazole has advantages over itraconazole, including absorption that is non-dependent on gastric pH or food, once-weekly dosing, and ability to use in patients with comorbidities, including cardiac dysfunction.123 Prescribing patterns amongst dermatologists for fluconazole have trended upwards in recent years.124
In a 2017 Cochrane Review of 48 studies (10,200 participants) examining oral antifungal therapies for the treatment of toenail onychomycosis,125 terbinafine and the azoles were more effective in achieving clinical cure (RR 6.00, 95% CI 3.96–9.08; RR 22.18, 95% CI 12.63–38.95, respectively) and mycologic cure (RR 4.53, 95% CI 2.47–8.33; RR 5.86, 95% CI 3.23–10.62, respectively) compared to placebo. There was moderate quality evidence that terbinafine was likely more effective than the azoles for achieving clinical cure (RR 0.82, 95% CI 0.72–0.95) and mycologic cure (RR 0.77, 95% CI 0.68–0.88). There were no differences in recurrence rates (RR 1.11, 95% CI 0.68–1.79) or the risk of adverse events (RR 1.00, 95% CI 0.86–1.1) between terbinafine and the azoles, with the most common adverse events being headache, nausea, and viral infection in both groups.
Novel Oral Therapies
Recent attention has been given to novel oral therapies for use in onychomycosis treatment. Posaconazole is an extended-spectrum triazole that is approved in the US and Europe for oropharyngeal candidiasis and for prophylaxis of invasive fungal infections.126 In a phase IIb, randomized, multicenter study,126 218 adult patients with toenail onychomycosis received posaconazole (oral suspension) 100, 200, or 400 mg once daily for 24 weeks, posaconazole 400 mg once daily for 12 weeks, oral terbinafine 250 mg once daily for 12 weeks, or placebo for 24 weeks. All posaconazole groups had significantly greater proportions of patients achieving complete cure vs placebo at 48 weeks (p ≤ 0.012). Patients receiving posaconazole 200 or 400 mg for 24 weeks had higher complete cure rates (54.1%, 45.5%, respectively) vs terbinafine (37%), while complete cure was lower for patients taking posaconazole 400 mg for 12 weeks (20%) (p > 0.05, all). Mycologic cure at week 48 for patients taking posaconazole 200 mg and 400 mg for 24 weeks (70.3%, 78.8%, respectively) was similar to terbinafine (71.4%), while mycologic cure was lower for patients taking posaconazole 100 or 400 mg for 24 weeks (37.1%, 42.9% respectively). Posaconazole was well tolerated, with only 3.8% (7/182) of patients withdrawing from the study due to asymptomatic elevations in transaminases.
Fosravuconazole L-lysine ethanolate (F-RVCZ) is an azole prodrug of ravuconazole with improved bioavailability and hydrophilicity that is approved in Japan (100 mg/day for 3 months) for onychomycosis treatment.127 In a phase-III, multicenter, randomized, double-blind study,128 153 Japanese patients with toenail onychomycosis received either 100 mg F-RVCZ or placebo once daily for 12 weeks. At 48 weeks, the complete and mycologic cure rates were significantly higher in the F-RVCZ group (59.4%, 82.0%, respectively) vs placebo (5.8%, 20.0%, respectively) (p < 0.001, both). Adverse drug reactions were mild and reported in 23.8% of patients taking F-RVCZ.
Otesesconazole is a tetrazole that inhibits the lanosterol demethylase (CYP51) enzyme, which is required to produce the membrane lipid ergosterol necessary for fungal survival. It is not yet US FDA approved for onychomycosis treatment, but has been studied in recent clinical trials.129 In a phase II, multicenter, randomized, placebo-controlled, double-blind study,130 259 patients with toenail onychomycosis received either oteseconazole 300 mg or 600 mg once daily for 14 days, followed by a once-weekly dose for 10 or 22 weeks. At week 60, complete and mycologic cure rates were higher in the oteseconazole groups (41–45%, 65–75%, respectively) vs placebo (0%, 13%, respectively) (p < 0.001, all). All of these novel agents show promise as well-tolerated and efficacious onychomycosis treatment options but must be validated in phase III studies before widespread treatment recommendations can be made.
Topical Therapies
Topical medications for onychomycosis treatment are becoming increasingly popular because they may penetrate biofilms, have lower risks of drug–drug interactions given there is little to no systemic absorption of the medication, and do not require laboratory monitoring.88,96,131,132 Topical therapies must penetrate the nail plate to reach the transungual space where the fungus resides and applied concentrations must therefore be adequate to retain the minimal inhibitory concentration.1,93 There are barriers to penetration, including slow growth and thickness of the nail, a hard nail plate that functions as a concentrated hydrogel, an impermeable dense network of keratin fibers, and nails in diseased states (ie, thickened nail or nail plates that are detached from the nail bed). Nail keratin can also adversely affect antifungal activity.93,133–136 The newer topicals are costly and compliance may pose an issue due to lengthy treatment requirements (ie, 48 weeks for the toenails) and for patients with limited mobility and/or dexterity.1,88 Clear instructions outlining medication usage should be disseminated to patients in addition to counseling about common adverse effects.137 In a retrospective review of adverse events reported to the United States Food and Drug Administration Adverse Event Reporting database,137 the most common adverse reactions reported with ciclopirox 8% nail lacquer, efinaconazole 10% solution, and tavaborole 5% solution were drug ineffectiveness, with nail discoloration and application site erythema reported with all three drugs. Currently, ciclopirox 8% nail lacquer is US FDA approved for the treatment of fingernail and toenail onychomycosis and efinaconazole 10% solution and tavaborole 5% solution are approved for toenail onychomycosis.40 Amorolfine 5% nail lacquer is approved in Europe for onychomycosis treatment but is not available in the US.138
Ciclopirox
Ciclopirox 8% nail lacquer is a hydroxypyridone that chelates trivalent cations, resulting in inhibition of metal-dependent enzymes.139 It is effective against dermatophytes, Candida spp., and some NDMs and gram-positive and gram–negative bacteria.140 In two double-blind, placebo-controlled studies of 460 patients with onychomycosis of the great toenails without lunula involvement treated with ciclopirox 8% nail lacquer for 48 weeks, complete cure rates were between 5.5–8.5% and mycologic cure rates were between 29%-36%.141 A 2020 Cochrane Review found that ciclopirox 8% lacquer may be more effective than vehicle in achieving complete cure (RR 9.29, 95% CI 1.72–50.14) and mycologic cure (RR 3.15, 95% CI 1.93–5.12) based on two studies. There were little to no differences in adverse events observed (RR 1.61, 95% CI 0.89–2.9).142 Efficacy is increased with weekly nail clippings and monthly office debridement.143 Patients should be instructed to remove the lacquer with alcohol on a weekly basis.144 Side effects are localized and include periungual erythema, burning, and application site reactions.88
Efinaconazole
Efinaconazole 10% solution is a triazole that inhibits lanosterol 14a-demethylase, thereby disrupting ergosterol synthesis in the fungal cell membrane. It has activity against dermatophytes, Candida spp., and NDMs1 and is the preferred treatment in cases of dermatophytoma.2,145 In two multicenter, double-blind, randomized, vehicle-controlled phase III trials in 1665 patients (study 1: n = 870; study 2: n = 785) with DLSO of the toenail with 20–50% nail involvement,146 patients received efinaconazole or vehicle once daily for 48 weeks without debridement. Mycologic and complete cure rates were greater in efinaconazole (53.4–55.2%, 15.2–17.8%, respectively) vs vehicle (16.8–16.9%, 3.3–5.5%, respectively) groups (both p < 0.001). Adverse events were application site reactions and ingrown toenails, which were similar to vehicle treated patients. A 2020 Cochrane Review found that efinaconazole was more effective vs vehicle in achieving clinical cure (RR 3.07, 95% CI 2.08–4.53, 2 studies), complete cure (RR 3.54, 95% CI 2.24–5.60, 3 studies) and likely mycologic cure (RR 2.31, 95% CI 1.08–4.94, 3 studies). There was a slightly higher risk of adverse events with efinaconazole compared to placebo (RR 1.10, 95% CI 1.01–1.20, 3 studies).142
Efinaconazole should be applied to the affected toenails once daily for 48 weeks, including the skin around the nails (nail folds, hyponychium, and ventral surface of the nail plate) to increase medication delivery.147 In a study on human cadaver thumbnails painted with two coats of nail polish (three different brands),148 permeation of efinaconazole was 0.56% of the applied dose on day seven, with cumulative concentrations of efinaconazole in the receptor using Franz diffusion cells ranging from 13.6–16.1 μg/cm2 for polished nails versus 17.6 μg/cm2 for unpolished nails. There were no significant differences in efinaconazole levels between polished and unpolished nails at all time points. Therefore, removal of nail polish prior to application is unnecessary since it can penetrate nails coated with polish.148 However, the current formulation degrades nail polish.149,150 In a study on 13 female patients with distal lateral subungual onychomycosis (DLSO) of at least 1 great toenail (thickness ≤3 mm) treated with daily topical efinaconazole 10% solution for 52 weeks,151 average OSI scores in patients with concurrent nail polish use did not differ significantly from patients without nail polish use. Daily treatment with concurrent nail polish use was associated with diminished nail polish quality on average 60% of the time.
Tavaborole
Tavaborole 5% solution is a benzoxaborole that inhibits fungal aminoacyl transfer RNA synthetase and therefore protein synthesis,152 with broad-spectrum activity against dermatophytes, yeasts, and NDMs.1,88 In two multicenter, randomized, double-blind, vehicle-controlled trials, 1194 patients with onychomycosis affecting 20–60% of the toenail without dermatophytoma or lunula involvement were treated with tavaborole or vehicle once daily for 48 weeks, with mycologic and complete cure rates between 31.1–35.9% and 6.5–9.1%, respectively.153 A 2020 Cochrane review found that tavaborole is more effective than vehicle in achieving mycologic cure (RR 3.40, 95% CI 2.34–4.93) and likely complete cure (RR 7.40, 95% CI 2.71–20.24), but has a likely higher risk of adverse events (RR 3.82, 95% CI 1.65–8.85) based on two studies.142
Nail penetration of tavaborole is good due to its small size and hydrophilicity.154 In a prospective study,155 nails from cadaver donors were treated with 1, 2, or 4 coats of over-the-counter (OTC) or salon nail polish followed by tavaborole 5% topical solution applied to each nail once daily for 14 or 20 consecutive days. Tavaborole penetration through the nail was quantified using qualified liquid chromatography-tandem mass spectrometry and was compared to unpolished control nails. Mean cumulative tavaborole penetration on day 21 was higher for nails treated with 1 coat of OTC nail polish and tavaborole for 20 days (3526±1433 μg/cm2) vs unpolished nails (2661±1319 μg/cm2) (p > 0.05). Mean cumulative tavaborole penetration on day 15 for nails treated with tavaborole and 1 or 4 coats of salon nail polish (1227±974 μg/cm2, 1179 ± 554 μg/cm2, respectively) or 1 or 2 coats of OTC nail polish (1428±841 μg/cm2, 1493±1322 μg/cm2, respectively) for 14 days was higher vs control nails (566±318 μg/cm2), although significance was not assessed. Therefore, penetration is not significantly influenced by the application of cosmetic nail polish. Patients should be instructed to apply tavaborole daily for 48 weeks for toenail infections.40 Side effects are local and include erythema, dermatitis, and exfoliation.156
Amorolfine
Amorolfine 5% nail lacquer is a morpholine derivative that inhibits fungal enzymes 14-α reductase and 7,8 isomerase, thereby disrupting fungal sterol synthesis.157 It has activity against dermatophytes, molds, and some yeasts.158 In an open, randomized study of 456 patients with onychomycosis of the fingernails and/or toenails treated with amorolfine once or twice weekly for up to 6 months,159 complete cure was 46.0% and 54.2%, respectively, and mycologic cure was 70.6% and 76.1%, respectively, after three months of treatment. Mild local irritation occurred in 0.9% of patients. In a randomized, double-blind study of 80 patients with toenail onychomycosis applying daily 5% amorolfine,160 mycologic and complete cure rates were 60% and 38%, respectively. One patient reported mild burning sensation under the toenails. Amorolfine should be applied once or twice weekly to a cleaned nail plate and left for 3–5 minutes until dry. Organic solvents should be avoided when removing the lacquer.161 Since it penetrates the nail bed through the nail plate, concentrations remain for at least 14 days after application.162
Novel Topical Therapies
Novel topical formulations for onychomycosis as alternatives to traditional topical treatments have been of recent interest. In a phase III, multicenter, randomized, double-blind study,163 365 patients (ages 12–74) with 20–60% distal and lateral subungual onychomycosis of at least one toenail received a once daily application of topical terbinafine (MOB-015 formulation) or matching vehicle for 48 weeks. At week 52, mycologic and complete cure were higher in the MOB-015 (69.9%, 4.5%, respectively) vs vehicle (27.7%, 0%, respectively) groups (p < 0.001, p = 0.0195, respectively). Adverse events were mild to moderate and led to treatment discontinuation in 2.8% of patients in the MOB-015 group and 4.2% in the vehicle group. The formulation of the MOB-015 vehicle (urea, propylene glycol, lactic acid) may increase the opaqueness of the nail plate. These aesthetic color changes may interfere with analysis of complete cure and result in rates that were lower than expected.
Several other topical terbinafine formulations are still under investigation.1 Ciclopirox hydrolacquer (P-3051) is another novel topical therapy that is not approved for onychomycosis treatment in the US, but is approved and marketed in more than 40 countries and is commonly used in Europe. It is made with hydroxypropyl chitosan, which forms an invisible film on the nail surface, prohibits fungal invasion, and facilitations nail penetration.164 In a randomized, controlled, evaluator-blinded study,165 120 patients (ages 18–75) with toenail onychomycosis received a 48-week treatment course of once daily P-3051 or twice weekly amorolfine 5% nail lacquer. Complete and mycologic cure were higher in the P-3051 (35%, 100%, respectively) vs amorolfine (11.7%, 81.7%, respectively) groups (p < 0.001, both). No adverse events were serious or led to discontinuation in either group. Patient compliance is improved because P-3051 does not require nail filing before application and can easily be removed with water.165 A 2020 Cochrane review found moderate-quality evidence from two studies (490 participants) that P-3051 is probably more effective than amorolfine 5% or ciclopirox 8% lacquer in achieving complete cure (RR 2.43, 95% CI 1.32–4.48) but not mycologic cure (RR 1.08, 95% CI 0.85–1.37). There was no differences in the risk of adverse events (RR 0.60, 95% CI 0.19–1.92), with the most common being rash, burning, and erythema.142
Over-The-Counter Treatments
Recent attention has been given to natural remedies and OTC treatments for onychomycosis.166 Tea tree oil (TTO) is a volatile oil that is used in Australia, Europe, and North America for treatment of tinea pedis.167 In C. albicans, TTO has been shown to decrease glucose-induced acidification of media surrounding fungi and alter respiration and permeability of plasma membranes.168–170 In a randomized, multicenter, double-blind trial of 117 patients with DLSO of the toenails who applied either TTO 100% or clotrimazole solution 1% twice daily for 6 months,171 culture cure did not significantly differ between groups (18% vs 11%, respectively). Irritation and erythema occurred in 7.8% (5/64) of the TTO group. In a double-blind, placebo controlled study of 60 patients with DLSO who applied either cream containing TTO 5% and butenafine hydrochloride 2% (n = 40) or a control cream containing only TTO (n = 20) three times daily for eight weeks,172 complete cure was 80% in the active group vs 0% in the placebo group (p < 0.0001). Mild skin inflammation occurred in 10% of the active group patients.
Topical cough suppressants have also been used as home remedies for onychomycosis. The active ingredients are eucalyptus oil 1.2%, camphor 4.8%, and menthol 2.6%, and the inactive ingredients are nutmeg oil, cedarleaf oil, thymol, petrolatum, and turpentine oil.166 In a pilot study of 18 adult patients with toenail onychomycosis who applied topical cough suppressants (Vicks VapoRub; The Proctor & Gamble Company, Cincinnati, OH) once daily,173 27.8% (5/18) had mycologic cure, 22.2% (4/18) had complete clinical cure, 55.6% (10/18) had partial clinical cure, and 16.7% (3/18) had no clinical improvement. The average ratio of affected to total nail area decreased from 63% to 41% (p < 0.001) and there were no adverse events reported with treatment. Complete clinical cure was achieved in 83.3% (5/6) participants positive for either C. parapsilosis (n = 3) or T. mentagrophytes (n = 3), versus in none of the 12 participants with other organisms cultured (6 T. rubrum, 2 fungal elements, 1 Cryptococcus lamentii, 1 C. albicans, 1 Penicillium spp., 1 Fusarium spp.) (p < 0.001). In a single-site, prospective pilot study of 20 HIV-positive patients with onychomycosis who applied topical Vicks VapoRub (The Proctor & Gamble Company, Cincinnati, OH) daily,174 83% (15/18) had improvements in affected nails (median clearance 25%; range 6.3–87.5%) at week 24, with total resolution of infection in two participants. At 48 weeks, 53% (8/15) had stable or improved clearance of affected nails. No fungal organisms were identified in this study. There were no reported side effects.
Natural coniferous resin, derived from the Norway spruce tree (Picea abies) and mixed with boiled butter or animal fat, has been used for centuries to treat wounds and infections.166 In a prospective, randomized, controlled, investigator-blinded study,175 73 patients with toenail onychomycosis received either natural coniferous resin 30% once daily for 9 months, amorolfine lacquer 5% once weekly for 9 months, or 250 mg oral terbinafine once daily for 3 months. At 10 months, mycologic cure rates were 13% (95% CI, 0–28%), 8% (95% CI, 0–19%) and 56% (95% CI, 35–77), respectively (p ≤ 0.002). Patient compliance was 100% in the resin group, with no treatment related adverse events.
Ageratina pichinchensis (AP) extract has been used historically in Mexico for fungal infections176 and has shown efficacy in treating tinea pedis.177 In a double-blind, randomized, controlled trial,178 110 patients with toenail onychomycosis received six months of either AP lacquer (n = 55) or ciclopirox 8% lacquer (n = 55). Clinical effectiveness (completely normal nails) was 71.1% and 80.9%, respectively (p = 0.596), and mycologic cure was 59.1% and 63.8%, respectively (p = 0.328). There were no serious adverse events in either group.
Ozonized sunflower oil is a petroleum jelly–like material created by the reaction of ozone with sunflower plant (Helianthus annuus) oil179 and has clinical implications in Cuba for the treatment of impetigo and tinea pedis.180 In a single-blind, controlled, phase III study,180 400 patients with onychomycosis received either ozonized sunflower oil solution or ketoconazole cream 2% twice daily for three months. Improvement in nail condition and cure were seen in 9.5% and 90.5% of the sunflower oil group, respectively, and in 27.5% and 13.5% of the control group (p < 0.0001).
Limitations to these studies on natural or OTC therapies include their small number of participants and differences in trial design that prohibit comparison of results across studies or with other treatments.166 Many studies did not identify fungal organisms, calling into question whether these patients did indeed have onychomycosis. Although they appear to exhibit good tolerability and safety, more research is needed to demonstrate efficacy in larger randomized controlled trials. Physicians should inquire if patients are using or have tried any OTC or natural remedies for self-treatment of onychomycosis, as these compounds may have important pharmacological interactions with prescribed antifungals.
Devices
Laser Therapies
Lasers were approved by the US FDA in 2012 for temporary increases in clear nail. Short-pulsed and Q-switched 1064 nm Nd:YAG lasers have been approved, although others, including carbon dioxide and the diode 870, 930 nm laser, are in development.1 A 2020 Cochrane review found low-quality evidence from two studies (85 participants) that there are little or no differences in mycologic cure at 52 weeks between 1064-nm Nd:YAG laser and no/sham treatment (RR 1.04, 95% CI 0.59–1.85). It was uncertain whether there were differences in adverse events.142 In a review comparing improvement rates to laser therapies (n = 2 studies) versus those of FDA-approved oral and topical onychomycosis therapies (n = 21 studies),181 laser treatment was associated with lower mycologic cure rates (11%) vs oral and topical therapies (29–61%).181 In a retrospective review of 23 patients with onychomycosis treated with Nd:YAG 1064-nm laser and debridement,182 78% of patients had temporary improvement in target nail appearance, 46% had ≥50% decrease in nail involvement from baseline, 17% had <10% surface area involvement at last clinical assessment, and only 9% achieved clinical cure. Therefore, laser treatments may be beneficial for achieving temporary improvements in nail appearance but there is limited evidence that they can maintain clinical improvements or eradicate pathogenic fungi. Importantly, inclusion criteria, endpoints, and definitions of efficacy outcomes vastly differ between clinical trials for lasers and oral and topical therapies for onychomycosis, making comparisons across studies difficult and unreliable.88,183 Lasers are attractive in that there is minimal potential for systemic adverse events, given that they target the infecting fungi on the nail plate, and require a lower degree of patient compliance since they are administered by the physician.40 The laser should be delivered at a wavelength of 750–1300 nm in order to penetrate the nail and the pulse duration period should be shorter than the thermal relaxation time of the pathogen.40 There are important disadvantages to laser treatment, including the requirement for multiple treatment sessions (up to 19 months),181 the high expense of therapy, costing on average $400-$1200 per treatment session and usually not covered by insurance,184 and the significant transient pain and discomfort patients may experience during the procedure.185
Photodynamic Therapy
Photodynamic therapy (PDT) is another non-invasive device-based treatment that is US FDA approved for treatment of actinic keratoses and is used off-label for onychomycosis. It combines light-based modalities with photosensitizers, such as 5-aminolevulinic acid, methyl aminolevulinate or methylene blue.1,186,187 This results in the production of reactive oxygen species and free radicals, which exhibit antimicrobial properties in addition to being cytotoxic and inducing apoptosis in fungal cells.188,189 There have been few studies evaluating PDT for the treatment of onychomycosis. In a prospective, open, single center study of 30 patients with DLSO caused by T. rubrum treated with three PDT sessions,187 clinical cure rate at 18 months was 36.6%. In an open label, controlled study of 22 patients with mild/moderate (n = 11) or severe (n = 11) toenail onychomycosis treated with two sessions of PDT,186 mycologic cure rate was 100% in both groups and complete cure rates were 100% and 63.6%, respectively. PDT has limited utility for onychomycosis treatments because it requires numerous treatment sessions, pretreatment with urea or nail avulsion, and causes pain.88 More extensive randomized controlled trials in larger patient populations are required before PDT can be recommended for routine off-label use for onychomycosis treatment.
Plasma Therapy
Non-thermal plasma (NTP), or low-temperature plasma, is an emerging tool that has been studied for treatment of onychomycosis. NTP is created from short pulses (approximately 10 ns) of strong (approximately 20 kV/mm peak) electric fields, which results in generation of active chemical species, including ions, electrons, nitric oxide, ozone, and hydroxyl radicals. Given its small current and duration, there is limited tissue heating.190–192 In a study evaluating the efficacy of NTP in treating onychomycosis caused by C. albicans and T. mentagrophytes in an in-vitro toe/nail-plate model,190 12 minutes of NTP resulted in complete killing at doses of 39 and 15 kPulses, respectively. In a pilot study on 19 patients with DLSO affecting 25–50% of at least one great toenail treated with three weekly NPT sessions,191 overall clinical cure and mycologic cure were 53.8% and 15.4%, respectively. Singeing of the nail due to a faulty electrode was the only treatment-related adverse effect in one patient, with no long-term sequelae. In 40 patients with toenail onychomycosis treated with nail plate abrasion and refreshment (NPAR) alone (n = 12), NPT combined with NPAR (n = 17), or NPT combined with antimycotics (n = 11),193 mycologic cure was achieved in 85.7% of patients treated with NPAR and NPT combination therapy. Moderate clinical improvement was observed in 50% and 36.4% of patients treated with NPAR monotherapy and NPT/antimycotic combination therapy, respectively. Therefore, NPT is a non-invasive therapeutic tool that may synergistically improve onychomycosis treatment but requires validation in larger randomized controlled trials.
Microdrilling
Nail drilling regimens have been investigated to improve penetration of medications through the nail plate. In an open comparative study of 98 patients (106 infected nails) with toenail DLSO treated with nail drilling and combination (oral and topical terbinafine) therapy (group 1), nail drilling and topical terbinafine (group 2), and topical terbinafine only (group 3),194 group 1 and group 2 had significant improvement in percent clear nail (80.39%, 73.73%, respectively) compared to group 3 (48.52%) at 28 weeks (p < 0.001). Mycologic cure rate was higher in group 1 (47.1%) compared to group 2 (34.2%) and group 3 (8.0%). Overall, treatment was well tolerated; however, pain was higher in patients treated with nail drilling vs topical treatment only (p = 0.040). Therefore, antifungal treatments in combination with nail drilling may improve treatment efficacy.
Combination Treatments
Combination therapy for treatment of onychomycosis is attractive in that it has potential for drug synergy and prevention of antifungal resistance.195 However, it is not well studied and standardized treatment regimens have not been established. In a systematic review of 30 onychomycosis clinical or randomized controlled trials [15 medication only studies, 15 medication and procedural (laser, debridement, PDT) studies],91 significant clinical benefit of medication combination therapy vs monotherapy was observed in more than half (60%) of studies. However, trials were not robustly designed and lacked sufficient follow-up compared to pivotal monotherapy trials. Almost all (93.3%) procedural and medication combination therapy studies showed benefit over monotherapy studies; however, long-term follow-up was insufficient and efficacy was not upheld in severe cases of onychomycosis. Given the cost of combination treatment, potential for drug–drug interactions, and lack of robustly designed randomized controlled trials with long-term follow-up, combination therapy should be reserved as a second-line treatment option in patients with poor prognostic factors or resistant cases of onychomycosis.
Treatment in Children
There are no US FDA approved systemic therapies to treat onychomycosis in children, although terbinafine, itraconazole, and fluconazole are used-off label.196 In a retrospective review of 26 studies (18 case reports, 5 clinical trials, 3 retrospective analyses) evaluating systemic treatment of onychomycosis (terbinafine, itraconazole, griseofulvin, and fluconazole) in children, 1976–2011,197 the complete cure rate was 70.8%, with a safety profile similar to the adult population. Topical tavaborole 5% and efinaconazole 10% solution are US FDA approved for treating onychomycosis in children ages ≥6 years old. Topical ciclopirox 8% nail lacquer is US FDA approved for children ≥12 years old.198 There is no data on the efficacy of medical devices for treating pediatric onychomycosis.40
Oral Terbinafine
In 17 children receiving continuous oral terbinafine (dosage based on body weight) for 12 weeks,199 88.2% (15/17) achieved mycologic cure within one to five months after treatment discontinuation. In 14 children treated with terbinafine for two to five months,200 mycologic and complete cure were observed in 77% and 62% of patients, respectively. There were no serious or adverse side effects reported in either study.
Oral Itraconazole
In 18 children receiving itraconazole 200 mg once daily for 12 weeks,199 94.7% (18/19) achieved mycologic cure. In 27 children treated with itraconazole (16 continuously, 11 pulsed) for 2–4 months,200 mycologic and complete cure were observed in 84% and 76% of patients, respectively. One patient had mild elevations in transaminases, and one patient discontinued treatment due to ataxia. In one child with onychomycosis due to T. rubrum treated with continuous itraconazole therapy (5 mg/kg/day) for 12 weeks,201 mycologic cure and clinical cure were observed at 12 months follow-up. The capsule formulation of itraconazole may be difficult for children to swallow; it can be broken and the granules mixed with food, such as apple sauce or mashed potatoes. The oral suspension (10 mg/mL) should be administered under fasting conditions for better bioavailability.202,203
Oral Fluconazole
In two children with toenail onychomycosis treated with fluconazole 200 mg or 300 mg once weekly for 20 weeks,204 both achieved clinical cure.
Topical Ciclopirox
In a prospective, randomized, double-blind, vehicle-controlled study,205 37 children with toenail onychomycosis received either ciclopirox lacquer or vehicle lacquer daily for 32 weeks, with weekly removal of the lacquer and mechanical trimming. Children receiving vehicle treatment with poor responses (Investigator Global Assessment score >3 or positive fungal culture at week 8) were crossed over to the ciclopirox group at week 12. At 12 and 32 weeks, mycologic cure was higher in the ciclopirox group (70%, 77.1%, respectively) vs the vehicle group (20%, 22.0%, respectively) (p = 0.03). Nail plate discoloration was the only adverse event and was reversed after weekly use of acetone-free nail polish remover. No patients discontinued treatment.
Topical Efinaconazole
In a Phase 4, open-label, multicenter study in 62 children with mild-to-severe toenail DLSO treated with efinaconazole once daily for 48 weeks,206 mycologic and complete cure were 65% and 40%, respectively. Clinical efficacy, defined as affected target great toenail area <10%, was achieved in 50% of participants. Systemic exposure was low, with average area under the concentration-time curve of 11.4 ng*h/mL and maximum plasma concentration of 0.549 ng/mL. All treatment-emergent adverse events (TEAEs) were mild or moderate, with the most common being nasopharyngitis in 18 participants. No TEAEs resulted in study discontinuation.
Topical Tavaborole
In an open-label, single arm study of 55 children with toenail DLSO who applied tavaborole once daily (2 drops/great toenail, 1 drop/other toenails) for 48 weeks,207 mycologic and complete cure were achieved in 36.2% and 8.5% of patients, respectively, at 52 weeks. After 28 days of daily dosing, tavaborole was detected in plasma and was at steady state. TEAEs were reported in 55.6% of patients, with the most common being nasopharyngitis, contusion, sinusitis, and vomiting. No deaths or permanent discontinuations occurred due to TEAEs. Appendicitis was the only serious adverse event but was deemed unrelated to treatment.
Elderly Adults
In a single blind, randomized, prospective study evaluating the efficacy of continuous terbinafine (250 mg/day for 12 weeks) and pulse itraconazole (200 mg twice a day for 1 week x 3 pulses) in treating toenail onychomycosis in 101 elderly (≥60 years old) patients,116 mycologic cure and clinical efficacy at 18 months were 64.0% and 62.0%, respectively, for terbinafine, and 62.7% and 60.8%, respectively, for itraconazole, with no significant differences between the two groups. At six months, 26% (13/50) of terbinafine patients were given an 4 extra weeks of terbinafine therapy and 45% (23/51) of itraconazole patients were given an extra pulse since they had <50% reduction in affected nail plate area or <3mm outgrowth of unaffected nail plate. All adverse events were mild and transient. In a sub analysis of an open label, randomized, multicenter study of patients ≥65 years old with toenail onychomycosis treated with terbinafine (250 mg/day for 12 weeks) with or without toenail debridement,208 mycologic and complete cure at 48 weeks were 64% and 28%, respectively, in patients treated with terbinafine alone (n = 34) and 63.4% and 39%, respectively, in patients treated with terbinafine + debridement (n = 41). Nausea (4%), sinusitis (4%), arthralgia (2.7%), and hyperlipidemia (2.7%), were the most common adverse events. In a subgroup analysis of a multicenter (36 sites), open-label, single-arm study of 219 Japanese patients with severe (>50% clinical involvement) toenail onychomycosis receiving daily efinaconazole 10% solution for up to 72 weeks,209 treatment success rate (<10% clinical involvement of the target toenail) was 54.2% in the elderly (aged ≥65 years) and 59.4% in the non-elderly (aged <65 years). Overall, 6.4% of patients reported local site reactions, with all resolved or recovered during treatment or after discontinuation of the medication.
Elderly patients have specific risk factors that predispose to poor responses to antifungal therapy, including slow nail growth, recurrent nail dystrophy, and increased prevalence of diabetes mellitus and peripheral vascular disease.210 A large proportion of the elderly population is on multiple drug therapy secondary to numerous comorbidities. Therefore, topical therapy alone is the preferred method of treatment in this select group.2 If systemic treatment is required, oral terbinafine should be first line due to decreased risk of drug–drug interactions compared to the azoles.196
Pregnant and Lactating Women
Pregnant and lactating women are often excluded from onychomycosis clinical trials, precluding the ability to make treatment recommendations in this population. Oral itraconazole is pregnancy class C and oral fluconazole is pregnancy class D when >1 dose is consumed. Neither should be used during pregnancy.88,196 Oral itraconazole should be avoided for 2 months before planning pregnancy and oral fluconazole is secreted into breast milk and therefore should not be started until breastfeeding is complete.88 Oral terbinafine is pregnancy class B and is excreted into breast milk, with a ratio of terbinafine in milk to plasma of 7:1. Since there is limited data and therapy for onychomycosis is non-emergent, initiation of oral terbinafine should be avoided during pregnancy or while nursing.101 Topical ciclopirox is pregnancy category B but it is unclear whether it is excreted into breast milk and should therefore be deferred in people who are pregnant or lactating.141 Topical efinaconazole is pregnancy class C due to embryotoxicity in rats and therefore should not be used during pregnancy. It was found in milk of nursing rats receiving repeated subcutaneous doses, and although there is no data in human milk, efinaconazole should not be used during breastfeeding.88,146,211 Topical tavaborole is pregnancy category C, with no data in pregnant women or during lactation and should therefore be avoided in these patients.153
Prevention of Recurrence
After initial onychomycosis treatment, recurrences are reported at a rate of 20–25%, including relapse (same infection after incomplete cure) or reinfection (same infection after complete cure).10,212,213 Patients with genetic predispositions, positive family histories of onychomycosis, and those with immunosuppression are more susceptible to recurrences.214,215 Biofilms may also contribute by shielding fungal organisms.38 Patient education and pharmacological intervention are paramount to preventing recurrence.1 In a retrospective chart review (2010–2015) on 320 patients with complete cure treated with oral terbinafine or itraconazole for toenail onychomycosis who then used a topical antifungal for prophylaxis,216 the recurrence rate was significantly lower in patients with topical antifungal prophylaxis vs no prophylactic treatment following oral terbinafine (p < 0.001) but not itraconazole (p = 0.185). Overall, regardless of initial oral treatment, the use of topical antifungals as prophylaxis significantly decreased the likelihood of recurrence (p < 0.001). After steady-state levels of the antifungal agent have been achieved in the nail plate, twice weekly application of a topical antifungal can be used for prophylaxis,217 however, the ideal duration for prophylaxis is uncertain and may be a life-long requirement.88 Patients should also be counseled on lifestyle modifications to prevent recurrence, including keeping the feet cool and dry, avoiding occlusive footwear, discarding or treating infected footwear with topical antifungals, ultraviolet light, or ozone, discarding or treating infected socks by washing with hot water, trimming the nails short to avoid trauma, using flipflops in wet and public spaces, and promptly treating affected family members.215,216,218–220
Conclusion
Onychomycosis is the most common nail disease encountered in clinical practice and can significantly impair QoL, with patients experiencing pain, difficulty with ambulation, and social stigmata. Following history and physical examination, dermoscopy can be used to differentiate onychomycosis from other nail diseases, with definitive diagnosis made with KOH and microscopy, fungal culture, histopathology, PCR, or a combination of techniques. AI is a newly emerging tool for diagnosis that requires further validation before it can be used in clinical practice. Onychomycosis is highly treatable and a variety of therapies can be utilized, including oral and topical medications as well as device-based treatments. Systemic treatments have higher cure rates, while topical treatments have more favorable safety profiles. Device-based therapies require investigation with similar endpoints to those used in oral and topical medication clinical trials to make accurate comparisons. Patients should be counseled about recurrences and be started on a topical antifungal prophylaxis to minimize recurrence risk. Future research should focus on establishing robustly designed clinical trials in diverse patient populations to further investigate novel therapies and determine optimal and standardized guidelines for combination treatment.
Abbreviations
NDM, non-dermatophytes molds; US, United States; QoL, quality of life; KOH, potassium hydroxide; PAS, periodic acid-Schiff; PCR, polymerase chain reaction; AI, artificial intelligence; AUC, area under the curve; US, United States; FDA, Food and Drug Administration; LFT, liver function test; CBC, complete blood count; RR, risk ratio; F-RVCZ, Fosravuconazole L-lysine ethanolate; DLSO, distal lateral subungual onychomycosis; OTC, over the counter; TTO, tea tree oil; AP, Ageratina pichinchensis; PDT, photodynamic therapy; NTP, non-thermal plasma; TEAEs, treatment-emergent adverse events.
Funding
There is no funding to report.
Disclosure
Ms. Falotico has no conflicts of interest in this work. Dr. Lipner has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, and BelleTorus Corporation. The authors report no other conflicts of interest in this work.
References
1. Gupta A, Stec N, Summerbell R., et al. Onychomycosis: a review. J Eur Acad Dermatol Venereol. 2020;34(9):1972–1990. doi:10.1111/jdv.16394
2. Lipner SR, Joseph WS, Vlahovic TC, et al. Therapeutic Recommendations for the treatment of toenail onychomycosis in the US. J Drugs Dermatol. 2021;20(10):1076–1084. doi:10.36849/JDD.6291
3. Lipner SR, Hancock JE, Fleischer AB. The ambulatory care burden of nail conditions in the United States. J Dermatological Treatment. 2021;32(5):517–520. doi:10.1080/09546634.2019.1679337
4. Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80(4):835–851. doi:10.1016/j.jaad.2018.03.062
5. Sergeev A, Ivanov O, Sergeev Y, et al. Epidemiology of onychomycosis in modern Russia: incidence is growing. Mycoses. 2002;45(S2):56. doi:10.1111/j.1439-0507.2002.tb04771.x
6. Burzykowski T, Molenberghs G, Abeck D, et al. High prevalence of foot diseases in Europe: results of the Achilles Project. Mycoses. 2003;46(11‐12):496–505. doi:10.1046/j.0933-7407.2003.00933.x
7. Ioannidou D, Maraki S, Krasagakis S, et al. The epidemiology of onychomycoses in Crete, Greece, between 1992 and 2001. J Eur Acad Dermatol Venereol. 2006;20(2):170–174. doi:10.1111/j.1468-3083.2006.01412.x
8. Vélez A, Linares MJ, Fenández-Roldán JC, et al. Study of onychomycosis in Cordoba, Spain: prevailing fungi and pattern of infection. Mycopathologia. 1997;137(1):1–8. doi:10.1023/A:1006874303991
9. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australasian j Dermatol. 2002;43(2):105–112. doi:10.1046/j.1440-0960.2002.t01-1-00570.x
10. Scher R, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(s65):5–9. doi:10.1046/j.1365-2133.149.s65.5.x
11. Carrillo-Meléndrez H, Ortega-Hernández E, Granados J, et al. Role of HLA-DR alleles to increase genetic susceptibility to onychomycosis in nail psoriasis. Skin Appendage Disorders. 2016;2(1–2):22–25. doi:10.1159/000446444
12. Faergemann J, Correia O, Nowicki R, et al. Genetic predisposition–understanding underlying mechanisms of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19:17–19. doi:10.1111/j.1468-3083.2005.01283.x
13. Jazdarehee A, Malekafzali L, Lee J, et al. Transmission of onychomycosis and dermatophytosis between household members: a scoping review. J Fungi. 2022;8(1):60. doi:10.3390/jof8010060
14. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133(9):1172–1173. doi:10.1001/archderm.1997.03890450124022
15. Bunyaratavej S, Srinonprasert V, Kiratiwongwan R, et al. Onychomycosis in older adults: the age and associated factors affecting the complete cure rate. Australasian J Dermatol. 2022;63(1):74–80. doi:10.1111/ajd.13686
16. Thomas J, Jacobson G, Narkowicz C, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35(5):497–519. doi:10.1111/j.1365-2710.2009.01107.x
17. Solís‐Arias MP, García‐Romero MT. Onychomycosis in children. Int J Dermatol. 2017;56(2):123–130. doi:10.1111/ijd.13392
18. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5):702–704. doi:10.1016/S0190-9622(98)70199-9
19. Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: a systematic review. J Am Acad Dermatol. 2021;85(5):1227–1239. doi:10.1016/j.jaad.2020.05.143
20. Kang R, Lipner S. Evaluation of Onychomycosis Information on the Internet. J Drugs Dermatol. 2019;18(5):484–487.
21. Svejgaard E, Nilsson J. Onychomycosis in Denmark: prevalence of fungal nail infection in general practice. Mycoses. 2004;47(3‐4):131–135. doi:10.1111/j.1439-0507.2004.00968.x
22. Pérez‐Cantero A, Guarro J. Sarocladium and Acremonium infections: new faces of an old opportunistic fungus. Mycoses. 2020;63(11):1203–1214. doi:10.1111/myc.13169
23. Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol. 2010;28(2):151–159. doi:10.1016/j.clindermatol.2009.12.006
24. Gupta AK, Drummond-Main C, Cooper EA, et al. Systematic review of nondermatophyte mold onychomycosis: diagnosis, clinical types, epidemiology, and treatment. J Am Acad Dermatol. 2012;66(3):494–502. doi:10.1016/j.jaad.2011.02.038
25. Ghannoum M, Hajjeh R, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43(4):641–648. doi:10.1067/mjd.2000.107754
26. Jayatilake J, Tilakaratne W, Panagoda G. Candidal onychomycosis: a mini-review. Mycopathologia. 2009;168(4):165–173. doi:10.1007/s11046-009-9212-x
27. Gupta A, Gupta G, Jain H, et al. The prevalence of unsuspected onychomycosis and its causative organisms in a multicentre Canadian sample of 30 000 patients visiting physicians’ offices. J Eur Acad Dermatol Venereol. 2016;30(9):1567–1572. doi:10.1111/jdv.13677
28. Garcia-Martos P, Hernández-Molina JM, Galán F. Isolation of Hanseniaspora uvarum (Kloeckera apiculata) in humans. Mycopathologia. 1998;144(2):73–75. doi:10.1023/A:1006900909455
29. Sánchez-Cárdenas CD, Vega-Sánchez DC, González-Suárez TR, et al. Onychomycosis caused by kloeckera apiculata: a case report in a patient with multiple sclerosis. Skin Appendage Disorders. 2022;8(1):49–52. doi:10.1159/000518046
30. Gupta AK, Mays RR, Versteeg SG, et al. Global perspectives for the management of onychomycosis. Int J Dermatol. 2019;58(10):1118–1129. doi:10.1111/ijd.14346
31. Rafat Z, Hashemi S, Saboor-Yaraghi -A-A, et al. A systematic review and meta-analysis on the epidemiology, casual agents and demographic characteristics of onychomycosis in Iran. J Mycol Med. 2019;29(3):265–272. doi:10.1016/j.mycmed.2019.05.004
32. Raghavendra K, Yadav D, Kumar A, et al. The nondermatophyte molds: emerging as leading cause of onychomycosis in south-east Rajasthan. Indian Dermatol Online J. 2015;6(2):92. doi:10.4103/2229-5178.153010
33. Leelavathi M, Tzar M, Adawiah J. Common microorganisms causing onychomycosis in tropical climate. Sains Malaysiana. 2012;41(6):697–700.
34. Gupta AK, Foley KA. Evidence for biofilms in onychomycosis. Giornale italiano di dermatologia e venereologia. 2018;154(1):50–55. doi:10.23736/S0392-0488.18.06001-7
35. Ramage G, Mowat E, Jones B, et al. Our current understanding of fungal biofilms. Crit Rev Microbiol. 2009;35(4):340–355. doi:10.3109/10408410903241436
36. Percival SL, Emanuel C, Cutting KF, et al. Microbiology of the skin and the role of biofilms in infection. Int Wound J. 2012;9(1):14–32. doi:10.1111/j.1742-481X.2011.00836.x
37. Borghi E, Borgo F, Morace G. Fungal biofilms: update on resistance. Fungal Biofilms Related Infections. 2016;1:37–47.
38. Gupta AK, Daigle D, Carviel JL. The role of biofilms in onychomycosis. J Am Acad Dermatol. 2016;74(6):1241–1246. doi:10.1016/j.jaad.2016.01.008
39. Lipner SR, Scher RK. Onychomycosis: diagnosis and therapy. Med Mycol. 2015;2;28.
40. Gupta AK, Mays RR, Versteeg SG, et al. Update on current approaches to diagnosis and treatment of onychomycosis. Expert Rev Anti Infect Ther. 2018;16(12):929–938. doi:10.1080/14787210.2018.1544891
41. Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94(6):548.
42. Ricardo JW, Lipner SR. Safety of current therapies for onychomycosis. Expert Opin Drug Saf. 2020;19(11):1395–1408. doi:10.1080/14740338.2020.1829592
43. Bedaiwy MY, Metwally M-A-E-A, Elzawawy NA, et al. Epidemiology, causative agents and clinical features of onychomycosis in El-Gharbia governorate. Egypt J Botany. 2017;57(7):187–196. doi:10.21608/ejbo.2017.965.1080
44. Kaur R, Kashyap B, Bhalla P. Onychomycosis-epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26(2):108–116. doi:10.1016/S0255-0857(21)01924-1
45. Dubljanin E, Dzamic A, Vujcic I, et al. Correlation of clinical characteristics, by calculation of SCIO index, with the laboratory diagnosis of onychomycosis. Br J Microbiol. 2022;53(1):221–229. doi:10.1007/s42770-021-00676-z
46. Piraccini B, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27(4):509–513. doi:10.1111/j.1468-3083.2011.04323.x
47. Vlahovic TC. Onychomycosis: evaluation, treatment options, managing recurrence, and patient outcomes. Clin Podiatr Med Surg. 2016;33(3):305–318. doi:10.1016/j.cpm.2016.02.001
48. Jesús-Silva MA, Fernández-Martínez R, Roldán-Marín R, et al. Dermoscopic patterns in patients with a clinical diagnosis of onychomycosis—results of a prospective study including data of potassium hydroxide (KOH) and culture examination. Dermatol Practical Conceptual. 2015;5(2):39. doi:10.5826/dpc.0502a05
49. De Crignis G, Valgas N, Rezende P, et al. Dermatoscopy of onychomycosis. Int J Dermatol. 2014;53(2):e97–e99. doi:10.1111/ijd.12104
50. Finch J, Arenas R, Baran R. Fungal melanonychia. J Am Acad Dermatol. 2012;66(5):830–841. doi:10.1016/j.jaad.2010.11.018
51. Ohn J, Choe YS, Park J, et al. Dermoscopic patterns of fungal melanonychia: a comparative study with other causes of melanonychia. J Am Acad Dermatol. 2017;76(3):488–493. e2. doi:10.1016/j.jaad.2016.08.013
52. Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost-effective. J Cutan Med Surg. 2018;22(2):129–141. doi:10.1177/1203475417733461
53. Lipner SR, Scher RK. Onychomycosis–a small step for quality of care. Curr Med Res Opin. 2016;32(5):865–867. doi:10.1185/03007995.2016.1147026
54. Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA dermatol. 2016;152(7):847. doi:10.1001/jamadermatol.2016.0785
55. Dermatology AAo. Ten Things Physicians and Patients Should Question 2021; 2022. Available from: https://www.choosingwisely.org/societies/american-academy-of-dermatology/.
56. Geizhals S, Cooley V, Lipner SR. Diagnostic testing for onychomycosis: a retrospective study over 17 years. J Am Acad Dermatol. 2020;83(1):239–241. doi:10.1016/j.jaad.2019.12.019
57. Ghannoum M, Isham N, Catalano V. A second look at efficacy criteria for onychomycosis: clinical and mycological cure. Br J Dermatol. 2014;170(1):182–187. doi:10.1111/bjd.12594
58. Blank N, Lipner SR. A contrasting dark background for nail sampling. Cutis. 2021;108:341. doi:10.12788/cutis.0403
59. Lipner SR, Ghannoum M, Hinshaw MA, et al. Deferring nail mycological sampling during the covid-19 pandemic: recommendations from a multidisciplinary panel of nail specialists. Skin Appendage Disorders. 2022;8(3):241–244. doi:10.1159/000520628
60. Gupta AK, Simpson FC. Diagnosing onychomycosis. Clin Dermatol. 2013;31(5):540–543. doi:10.1016/j.clindermatol.2013.06.009
61. Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55(4):620–626. doi:10.1016/j.jaad.2006.03.033
62. Bombace F, Iovene MR, Galdiero M, et al. Non‐dermatophytic onychomycosis diagnostic criteria: an unresolved question. Mycoses. 2016;59(9):558–565. doi:10.1111/myc.12504
63. Baran R, de Berker DA, Holzberg M, et al. Baran and Dawber’s Diseases of the Nails and Their Management. John Wiley & Sons; 2012.
64. Mahoney JM, Bennet J, Olsen B. The diagnosis of onychomycosis. Dermatol Clin. 2003;21(3):463–467. doi:10.1016/S0733-8635(03)00035-4
65. Sidiq F, Hoostal M, Rogers SO. Rapid identification of fungi in culture-negative clinical blood and respiratory samples by DNA sequence analyses. BMC Res Notes. 2016;9(1):1–8. doi:10.1186/s13104-016-2097-0
66. Alberhasky RC. Laboratory diagnosis of onychomycosis. Clin Podiatr Med Surg. 2004;21(4):565–578. doi:10.1016/j.cpm.2004.06.001
67. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49(2):193–197. doi:10.1067/S0190-9622(03)01480-4
68. D’Hue Z, Perkins SM, Billings SD. GMS is superior to PAS for diagnosis of onychomycosis. J Cutan Pathol. 2008;35(8):745–747. doi:10.1111/j.1600-0560.2007.00890.x
69. Smith MB, McGinnis MR. Diagnostic Histopathology. Diagnosis and Treatment of Human Mycoses. Springer; 2008:37–51.
70. Verrier J, Monod M. Diagnosis of dermatophytosis using molecular biology. Mycopathologia. 2017;182(1):193–202. doi:10.1007/s11046-016-0038-z
71. Bock M, Maiwald M, Kappe R, et al. Polymerase chain reaction‐based detection of dermatophyte DNA with a fungus‐specific primer system: Nachweis von Dermatophyten‐DNA durch Polymerase‐Ketten‐Reaktion mit einem pilzspezifischen Primersystem. Mycoses. 1994;37(3‐4):79–84. doi:10.1111/j.1439-0507.1994.tb00781.x
72. Baek SC, Chae HJ, Houh D, et al. Detection and differentiation of causative fungi of onychomycosis using PCR amplification and restriction enzyme analysis. Int J Dermatol. 1998;37(9):682–686. doi:10.1046/j.1365-4362.1998.00517.x
73. Gupta AK, Nakrieko K-A. Onychomycosis infections: do polymerase chain reaction and culture reports agree? J Am Podiatr Med Assoc. 2017;107(4):280–286. doi:10.7547/15-136
74. Verrier J, Pronina M, Peter C, et al. Identification of infectious agents in onychomycoses by PCR-terminal restriction fragment length polymorphism. J Clin Microbiol. 2012;50(3):553–561. doi:10.1128/JCM.05164-11
75. Kondori N, Abrahamsson A-L, Ataollahy N, et al. Comparison of a new commercial test, Dermatophyte-PCR kit, with conventional methods for rapid detection and identification of Trichophyton rubrum in nail specimens. Med Mycol. 2010;48(7):1005–1008. doi:10.3109/13693781003743130
76. Petinataud D, Berger S, Ferdynus C, et al. Optimising the diagnostic strategy for onychomycosis from sample collection to fungal identification evaluation of a diagnostic kit for real‐time PCR. Mycoses. 2016;59(5):304–311. doi:10.1111/myc.12471
77. Gustafson E, Bakotic W, Bennett L, et al. DNA-based detection for onychomycosis correlates better to histopathology than does fungal culture. Dermatol Online J. 2019;25(7). doi:10.5070/D3257044800.
78. Kim YJ, Han SS, Yang HJ, et al. Prospective, comparative evaluation of a deep neural network and dermoscopy in the diagnosis of onychomycosis. PLoS One. 2020;15(6):e0234334. doi:10.1371/journal.pone.0234334
79. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. nature. 2017;542(7639):115–118. doi:10.1038/nature21056
80. Lu J, Kazmierczak E, Manton JH, et al. Automatic segmentation of scaling in 2-D psoriasis skin images. IEEE Trans Med Imaging. 2012;32(4):719–730. doi:10.1109/TMI.2012.2236349
81. Moreau A, Anderer P, Ross M, et al. Detection of nocturnal scratching movements in patients with atopic dermatitis using accelerometers and recurrent neural networks. IEEE j Biomedical Health Informatics. 2017;22(4):1011–1018. doi:10.1109/JBHI.2017.2710798
82. Phillips M, Marsden H, Jaffe W, et al. Assessment of accuracy of an artificial intelligence algorithm to detect melanoma in images of skin lesions. JAMA network open. 2019;2(10):e1913436–e1913436. doi:10.1001/jamanetworkopen.2019.13436
83. Brinker TJ, Hekler A, Enk AH, et al. Deep learning outperformed 136 of 157 dermatologists in a head-to-head dermoscopic melanoma image classification task. Eur J Cancer. 2019;113:47–54. doi:10.1016/j.ejca.2019.04.001
84. Fujisawa Y, Otomo Y, Ogata Y, et al. Deep‐learning‐based, computer‐aided classifier developed with a small dataset of clinical images surpasses board‐certified dermatologists in skin tumour diagnosis. Br J Dermatol. 2019;180(2):373–381. doi:10.1111/bjd.16924
85. Zhu X, Zheng B, Cai W, et al. Deep learning‐based diagnosis models for onychomycosis in dermoscopy. Mycoses. 2022;65(4):466–472. doi:10.1111/myc.13427
86. Han SS, Park GH, Lim W, et al. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS One. 2018;13(1):e0191493. doi:10.1371/journal.pone.0191493
87. Decroos F, Springenberg S, Lang T, et al. A deep learning approach for histopathological diagnosis of onychomycosis: not inferior to analogue diagnosis by histopathologists. Acta Derm Venereol. 2021;101(8):adv00532–adv00532. doi:10.2340/00015555-3893
88. Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80(4):853–867. doi:10.1016/j.jaad.2018.05.1260
89. Lipner SR. Pharmacotherapy for onychomycosis: new and emerging treatments. Expert Opin Pharmacother. 2019;20(6):725–735. doi:10.1080/14656566.2019.1571039
90. Ameen M, Lear J, Madan V, et al. British Association of Dermatologists’ guidelines for the management of onychomycosis 2014. Br J Dermatol. 2014;171(5):937–958. doi:10.1111/bjd.13358
91. Falotico JM, Lapides R, Lipner SR. Combination Therapy Should Be Reserved as Second-Line Treatment of Onychomycosis: a Systematic Review of Onychomycosis Clinical Trials. J Fungi. 2022;8(3):279. doi:10.3390/jof8030279
92. Chang MJ, Qiu Y, Lipner SR. Race reporting and representation in onychomycosis clinical trials: a systematic review. Mycoses. 2021;64(8):954–966. doi:10.1111/myc.13262
93. Vikas A, Rashmin P, Mrunali P, et al. Mechanistic insights of formulation approaches for the treatment of nail infection: conventional and novel drug delivery approaches. AAPS PharmSciTech. 2020;21(2):1–12. doi:10.1208/s12249-019-1591-9
94. Aslam R, Hussain T, Yousaf AM, et al. Onychomycosis: current understanding and strategies for enhancing drug delivery into human nail tissue. Curr Drug Res Rev Formerly. 2021;13(1):25–35. doi:10.2174/2589977512666200731171505
95. Burkharta CN, Burkhart CG, Gupta AK. Dermatophytoma: recalcitrance to treatment because of existence of fungal biofilm. J Am Acad Dermatol. 2002;47(4):629–631. doi:10.1067/mjd.2002.124699
96. Debruyne D, Coquerel A. Pharmacokinetics of antifungal agents in onychomycoses. Clin Pharmacokinet. 2001;40(6):441–472. doi:10.2165/00003088-200140060-00005
97. Valeant Pharmaceuticals North America, LLC. Gris-Peg (griseofulvin) ultramicrosize [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
98. Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. Part II. J Am Acad Dermatol. 1994;30(6):911–933. doi:10.1016/s0190-9622(94)70112-1
99. Wang Y, Lipner SR. Retrospective analysis of adverse events with systemic onychomycosis medications reported to the United States Food and Drug Administration. J Dermatological Treatment. 2021;32(7):783–787. doi:10.1080/09546634.2019.1708242
100. Ishack S, Miller RC, Lipner SR. Insights into the “fear factor” regarding terbinafine-associated hepatoxicity in an assessment of online information. J Am Acad Dermatol. 2022;86(6):e269–e271. doi:10.1016/j.jaad.2022.02.006
101. Novartis. Highlights of Prescribing Information 2012; 2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf.
102. Kramer O, Albrecht J. Clinical presentation of terbinafine‐induced severe liver injury and the value of laboratory monitoring: a Critically Appraised Topic. Br J Dermatol. 2017;177(5):1279–1284. doi:10.1111/bjd.15854
103. Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84(2):497–499. doi:10.1016/j.jaad.2020.04.172
104. Stolmeier DA, Stratman HB, McIntee TJ, et al. Utility of laboratory test result monitoring in patients taking oral terbinafine or griseofulvin for dermatophyte infections. JAMA dermatol. 2018;154(12):1409–1416. doi:10.1001/jamadermatol.2018.3578
105. Kao WY, Su CW, Huang YS, et al. Risk of oral antifungal agent‐induced liver injury in T aiwanese. Br J Clin Pharmacol. 2014;77(1):180–189. doi:10.1111/bcp.12178
106. Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85(4):1042–1044. doi:10.1016/j.jaad.2021.01.073
107. Patel D, Castelo-Soccio LA, Rubin AI, et al. Laboratory monitoring during systemic terbinafine therapy for pediatric onychomycosis. JAMA dermatol. 2017;153(12):1326–1327. doi:10.1001/jamadermatol.2017.4483
108. Warshaw EM. Evaluating costs for onychomycosis treatments: a practitioner’s perspective. J Am Podiatr Med Assoc. 2006;96(1):38–52. doi:10.7547/0960038
109. Gupta A, Paquet M, Simpson F, et al. Terbinafine in the treatment of dermatophyte toenail onychomycosis: a meta‐analysis of efficacy for continuous and intermittent regimens. J Eur Acad Dermatol Venereol. 2013;27(3):267–272. doi:10.1111/j.1468-3083.2012.04584.x
110. Gupta A, Stec N, Bamimore M, et al. The efficacy and safety of pulse vs. continuous therapy for dermatophyte toenail onychomycosis. J Eur Acad Dermatol Venereol. 2020;34(3):580–588. doi:10.1111/jdv.16101
111. Elewski BE. Mechanisms of action of systemic antifungal agents. J Am Acad Dermatol. 1993;28(5):S28–S34. doi:10.1016/S0190-9622(09)80305-8
112. Korting HC, Schöllmann C. The significance of itraconazole for treatment of fungal infections of skin, nails and mucous membranes. JDDG. 2009;7(1):11–19.
113. Elewski B, Pariser D, Rich P, et al. Current and emerging options in the treatment of onychomycosis. Int J Med. 2013;32(2):9–12.
114. Sigurgeirsson B, Paul C, Curran D, et al. Prognostic factors of mycological cure following treatment of onychomycosis with oral antifungal agents. Br j dermatol. 2002;147(6):1241–1243. doi:10.1046/j.1365-2133.2002.05035.x
115. Gupta A, Baran R, Summerbell R. Onychomycosis: strategies to improve efficacy and reduce recurrence. J Eur Acad Dermatol Venereol. 2002;16(6):579–586. doi:10.1046/j.1468-3083.2002.00589.x
116. Gupta AK, Konnikov N, Lynde CW. Single-blind, randomized, prospective study on terbinafine and itraconazole for treatment of dermatophyte toenail onychomycosis in the elderly. J Am Acad Dermatol. 2001;44(3):479–484. doi:10.1067/mjd.2001.110874
117. Gupta AK, Del Rosso JQ. An evaluation of intermittent therapies used to treat onychomycosis and other dermatomycoses with the oral antifungal agents. Int J Dermatol. 2000;39(6):401–411. doi:10.1046/j.1365-4362.2000.00964.x
118. Gupta AK, Drummond-Main C, Paquet M. Evidence-based optimal fluconazole dosing regimen for onychomycosis treatment. J Dermatological Treatment. 2013;24(1):75–80. doi:10.3109/09546634.2012.703308
119. Brown SJ. Efficacy of fluconazole for the treatment of onychomycosis. Ann Pharmacother. 2009;43(10):1684–1691. doi:10.1345/aph.1M165
120. Lin K, Lipner SR. Mobile phone reminders for onychomycosis medication adherence. J Am Acad Dermatol. 2019;80(5):e105–e107. doi:10.1016/j.jaad.2018.11.010
121. Scher RK, Breneman D, Rich P, et al. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38(6):S77–S86. doi:10.1016/S0190-9622(98)70490-6
122. Muńoz P, Moreno S, Berenguer J, et al. Fluconazole-related hepatotoxicity in patients with acquired immunodeficiency syndrome. Arch Intern Med. 1991;151(5):1020–1021. doi:10.1001/archinte.1991.00400050150032
123. Brammer K, Farrow PR, Faulkner J. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev Infect Dis. 1990;12(Supplement_3):S318–S326. doi:10.1093/clinids/12.Supplement_3.S318
124. Falotico JM, Wang Y, Rahman U, et al. Increased fluconazole usage by dermatologists in the Medicare provider utilization and payment database, 2014-2019. J Am Acad Dermatol. 2022;S0190(22):974.
125. Kreijkamp‐Kaspers S, Hawke K, Guo L, et al. Oral antifungal medication for toenail onychomycosis. Cochrane Database Sys Rev. 2017;2017(7). doi:10.1002/14651858.CD010031.pub2.
126. Elewski B, Pollak R, Ashton S, et al. A randomized, placebo‐and active‐controlled, parallel‐group, multicentre, investigator‐blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br J Dermatol. 2012;166(2):389–398. doi:10.1111/j.1365-2133.2011.10660.x
127. Noguchi H, Matsumoto T, Kimura U, et al. Fungal melanonychia caused by Candida parapsilosis successfully treated with oral fosravuconazole. J Dermatol. 2019;46(10):911–913. doi:10.1111/1346-8138.15024
128. Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L‐lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double‐blind, randomized phase III study. J Dermatol. 2018;45(10):1151–1159. doi:10.1111/1346-8138.14607
129. Gupta AK, Talukder M, Venkataraman M. Review of the alternative therapies for onychomycosis and superficial fungal infections: posaconazole, fosravuconazole, voriconazole, oteseconazole. Int J Dermatol. 2021. doi:10.1111/ijd.15999
130. Elewski B, Brand S, Degenhardt T, et al. A phase II, randomized, double‐blind, placebo‐controlled, dose‐ranging study to evaluate the efficacy and safety of VT‐1161 oral tablets in the treatment of patients with distal and lateral subungual onychomycosis of the toenail. Br J Dermatol. 2021;184(2):270–280. doi:10.1111/bjd.19224
131. Gupta AK, Versteeg SG, Shear NH. Common drug-drug interactions in antifungal treatments for superficial fungal infections. Expert Opin Drug Metab Toxicol. 2018;14(4):387–398. doi:10.1080/17425255.2018.1461834
132. Costa-Orlandi CB, Martinez LR, Bila NM, et al. Nitric Oxide-Releasing Nanoparticles Are Similar to Efinaconazole in Their Capacity to Eradicate Trichophyton rubrum Biofilms. Front Cell Infect Microbiol. 2021;1;665.
133. Davies-Strickleton H, Cook J, Hannam S, et al. Assessment of the nail penetration of antifungal agents, with different physico-chemical properties. PLoS One. 2020;15(2):e0229414. doi:10.1371/journal.pone.0229414
134. Thatai P, Sapra B. Transungual delivery: deliberations and creeds. Int J Cosmet Sci. 2014;36(5):398–411. doi:10.1111/ics.12142
135. Angelo T, Borgheti-Cardoso LN, Gelfuso GM, et al. Chemical and physical strategies in onychomycosis topical treatment: a review. Med Mycol. 2017;55(5):461–475. doi:10.1093/mmy/myw084
136. Dhamoon RK, Popli H, Gupta M. Novel drug delivery strategies for the treatment of onychomycosis. Pharmaceutical Nanotechnol. 2019;7(1):24–38. doi:10.2174/2211738507666190228104031
137. Wang Y, Lipner SR. Retrospective analysis of adverse events with topical onychomycosis medications reported to the United States Food and Drug Administration. Arch Dermatol Res. 2020;312(8):581–586. doi:10.1007/s00403-020-02044-7
138. Gupta AK, Uro M, Cooper EA. Onychomycosis therapy: past, present, future. J Drugs Dermatol. 2010;9(9):1109–1113.
139. Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3(2):350–358. doi:10.1016/j.celrep.2012.12.021
140. Bohn M, Kraemer KT. Dermatopharmacology of ciclopirox nail lacquer topical solution 8% in the treatment of onychomycosis. J Am Acad Dermatol. 2000;43(4):S57–S69. doi:10.1067/mjd.2000.109072
141. Dermik Laboratories. Penlac Nail Lacquer (Ciclopirox) Topical Solution, 8% [Package Insert]. Bridgewater, NJ: Dermik Laboratories; 2005. Available from: http://products.sanofi.us/penlac/penlac.html.
142. Foley K, Gupta AK, Versteeg S, et al. Topical and device‐based treatments for fungal infections of the toenails. Cochrane Database Sys Rev. 2020; 1. doi:10.1002/14651858.CD012093.pub2
143. Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 2000;43(4):S70–S80. doi:10.1067/mjd.2000.109071
144. Lipner SR, Ko D. Optimizing topical therapy for onychomycosis: the importance of patient education. Cutis. 2018;102(6):389–390.
145. Wang C, Cantrell W, Canavan T, et al. Successful treatment of dermatophytomas in 19 patients using efinaconazole 10% solution. Skin Appendage Disorders. 2019;5(5):304–308. doi:10.1159/000495042
146. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68(4):600–608. doi:10.1016/j.jaad.2012.10.013
147. Gupta AK, Cernea M. How effective is efinaconazole in the management of onychomycosis? Expert Opin Pharmacother. 2016;17(4):611–618. doi:10.1517/14656566.2016.1146687
148. Zeichner JA, Gold LS, Korotzer A. Penetration of (14C)-efinaconazole topical solution, 10%, does not appear to be influenced by nail Polish. J Clin Aesthet Dermatol. 2014;7(9):34.
149. Vlahovic TC, Coronado D, Chanda S, et al. Evaluation of the Appearance of Nail Polish Following Daily Treatment of Ex Vivo Human Fingernails With Topical Solutions of Tavaborole or Efinaconazole. J Drugs Dermatol. 2016;15(1):89–94.
150. Lipner SR, Scher RK. Efinaconazole 10% topical solution for the topical treatment of onychomycosis of the toenail. Expert Rev Clin Pharmacol. 2015;8(6):719–731. doi:10.1586/17512433.2015.1083418
151. Canavan TN, Bevans SL, Cantrell WC, et al. Single-center, prospective, blinded study comparing the efficacy and compatibility of efinaconazole 10% solution in treating onychomycosis with and without concurrent nail Polish use. Skin Appendage Disorders. 2019;5(1):9–12. doi:10.1159/000488369
152. Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. science. 2007;316(5832):1759–1761. doi:10.1126/science.1142189
153. Fougera Pharmaceuticals. Kerydin (Tavaborole) Topical Solution, 5% [Package Insert]. Melville, NY: Fougera Pharmaceuticals; 2005. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid51ae61072-bca0-43f0-a741-07bda2d50c87.
154. Kobayashi Y, Komatsu T, Sumi M, et al. In vitro permeation of several drugs through the human nail plate: relationship between physicochemical properties and nail permeability of drugs. Eur j Pharmaceutical Sci. 2004;21(4):471–477. doi:10.1016/j.ejps.2003.11.008
155. Tracey Vlahovic D, Zane LT. In vitro nail penetration of tavaborole topical solution, 5%, through nail Polish on ex vivo human fingernails. J Drugs Dermatol. 2015;14(7):675–678.
156. Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73(1):62–69. doi:10.1016/j.jaad.2015.04.010
157. Feng X, Xiong X, Ran Y. Efficacy and tolerability of amorolfine 5% nail lacquer in combination with systemic antifungal agents for onychomycosis: a meta‐analysis and systematic review. Dermatol Ther. 2017;30(3):e12457. doi:10.1111/dth.12457
158. Haria M, Bryson HM. A review of its pharmacological properties and therapeutic potential in the treatment of onychomycosis and other superficial fungal infections. J Med. 1995:58.
159. Reinel D. Topical treatment of onychomycosis with amorolfine 5% nail lacquer: comparative efficacy and tolerability of once and twice weekly use. Dermatology. 1992;184(Suppl. 1):21–24. doi:10.1159/000247612
160. Lauharanta J. Comparative efficacy and safety of amorolfine nail lacquer 2% versus 5% once weekly. Clin Exp Dermatol. 1992;17(s1):41–43. doi:10.1111/j.1365-2230.1992.tb00277.x
161. Tabara K, Szewczyk AE, Bienias W, et al. Amorolfine vs. ciclopirox–lacquers for the treatment of onychomycosis. Adv Dermatol Allergology. 2015;32(1):40–45. doi:10.5114/pdia.2014.40968
162. Mensing H, Polak‐Wyss A, Splanemann V. Determination of the subungual antifungal activity of amorolfine after 1 month’s treatment in patients with onychomycosis: comparison of two nail lacquer formulations. Clin Exp Dermatol. 1992;17:29–32. doi:10.1111/j.1365-2230.1992.tb00274.x
163. Gupta AK, Surprenant MS, Kempers SE, et al. Efficacy and safety of topical terbinafine 10% solution (MOB-015) in the treatment of mild to moderate distal subungual onychomycosis: a randomized, multicenter, double-blind, vehicle-controlled Phase 3 study. J Am Acad Dermatol. 2021;85(1):95–104. doi:10.1016/j.jaad.2020.06.055
164. Monti D, Herranz U, Dal BL, et al. Nail penetration and predicted mycological efficacy of an innovative hydrosoluble ciclopirox nail lacquer vs. a standard amorolfine lacquer in healthy subjects. J Eur Acad Dermatol Venereol. 2013;27(2):e153–e158. doi:10.1111/j.1468-3083.2012.04529.x
165. Iorizzo M, Hartmane I, Derveniece A, et al. Ciclopirox 8% HPCH nail lacquer in the treatment of mild-to-moderate onychomycosis: a randomized, double-blind amorolfine controlled study using a blinded evaluator. Skin Appendage Disorders. 2015;1(3):134–140. doi:10.1159/000441569
166. Halteh P, Scher RK, Lipner SR. Over-the-counter and natural remedies for onychomycosis: do they really work. Cutis. 2016;98(5):E16–e25.
167. Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19(1):50–62. doi:10.1128/CMR.19.1.50-62.2006
168. D’auria F, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13(4):377–383. doi:10.1179/joc.2001.13.4.377
169. Hammer K, Carson C, Riley T. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrobial Chemother. 2004;53(6):1081–1085. doi:10.1093/jac/dkh243
170. Hammer K, Carson C, Riley T. Melaleuca alternifolia (tea tree) oil inhibits germ tube formation by Candida albicans. Med Mycol. 2000;38(5):354–362. doi:10.1080/mmy.38.5.354.361
171. Buck DS, Nidorf DM, Addino JG. Comparison of two topical preparations for the treatment of onychomycosis: melaleuca alternifolia (tea tree) oil and clotrimazole. J Family Practice. 1994;38(6):601–606.
172. Syed T, Qureshi Z, Ali S, et al. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Trop Med Int Health. 1999;4(4):284–287. doi:10.1046/j.1365-3156.1999.00396.x
173. Derby R, Rohal P, Jackson C, et al. Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Family Med. 2011;24(1):69–74. doi:10.3122/jabfm.2011.01.100124
174. Snell M, Klebert M, Önen NF, et al. A novel treatment for onychomycosis in people living with HIV infection: vicks VapoRub™ is effective and safe. J Assoc Nurses AIDS Care. 2016;27(1):109–113. doi:10.1016/j.jana.2015.10.004
175. Auvinen T, Tiihonen R, Soini M, et al. Efficacy of topical resin lacquer, amorolfine and oral terbinafine for treating toenail onychomycosis: a prospective, randomized, controlled, investigator‐blinded, parallel‐group clinical trial. Br J Dermatol. 2015;173(4):940–948. doi:10.1111/bjd.13934
176. Argueta A, Cano Asseleih LM, Rodarte García ME. Atlas de las plantas de la medicina tradicional mexicana. 1994: (C QK 99. M6. A84 1994).
177. Romero-Cerecero O, Rojas G, Navarro V, et al. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: an explorative pilot study controlled with ketoconazole. Planta Med. 2006;72(14):1257–1261. doi:10.1055/s-2006-951694
178. Romero-Cerecero O, Zamilpa A, Jiménez-Ferrer JE, et al. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. A comparative study with ciclopirox. Planta Med. 2008;74(12):1430–1435. doi:10.1055/s-2008-1081338
179. Bocci V. Biological and clinical effects of ozone. Has ozone therapy a future in medicine? Br J Biomed Sci. 1999;56(4):270.
180. Menéndez S, Falcón L, Maqueira Y. Therapeutic efficacy of topical OLEOZON® in patients suffering from onychomycosis. Mycoses. 2011;54(5):e272–e277. doi:10.1111/j.1439-0507.2010.01898.x
181. Gupta A, Versteeg S. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31(7):1111–1118. doi:10.1111/jdv.14212
182. Gupta AK, Paquet M. A retrospective chart review of the clinical efficacy of Nd: YAG 1064-nm laser for toenail onychomycosis. J Dermatological Treatment. 2015;26(4):376–378. doi:10.3109/09546634.2014.975671
183. Hay R. Therapy of skin, hair and nail fungal infections. J Fungi. 2018;4(3):99. doi:10.3390/jof4030099
184. Hollmig ST, Rahman Z, Henderson MT, et al. Lack of efficacy with 1064-nm neodymium: yttrium-aluminum-garnet laser for the treatment of onychomycosis: a randomized, controlled trial. J Am Acad Dermatol. 2014;70(5):911–917. doi:10.1016/j.jaad.2013.12.024
185. Carney C, Cantrell W, Warner J, et al. Treatment of onychomycosis using a submillisecond 1064-nm neodymium: yttrium-aluminum-garnet laser. J Am Acad Dermatol. 2013;69(4):578–582. doi:10.1016/j.jaad.2013.04.054
186. Souza LWF, Souza SVT, Botelho A. Distal and lateral toenail onychomycosis caused by Trichophyton rubrum: treatment with photodynamic therapy based on methylene blue dye. An Bras Dermatol. 2014;89:184–186. doi:10.1590/abd1806-4841.20142197
187. Sotiriou E, Koussidou-Eremonti T, Chaidemenos G, et al. Photodynamic therapy for distal and lateral subungual toenail onychomycosis caused by Trichophyton rubrum: preliminary results of a single-centre open trial. Acta Derm Venereol. 2010;90(2):216. doi:10.2340/00015555-0811
188. Xu Z-L, Xu J, Zhuo F, et al. Effects of laser irradiation on Trichophyton rubrum growth and ultrastructure. Chin Med J. 2012;125(20):3697–3700.
189. Fonda-Pascual P, Moreno-Arrones OM, Alegre-Sanchez A, et al. In situ production of ROS in the skin by photodynamic therapy as a powerful tool in clinical dermatology. Methods. 2016;109:190–202. doi:10.1016/j.ymeth.2016.07.008
190. Bulson JM, Liveris D, Derkatch I, et al. Non‐thermal atmospheric plasma treatment of onychomycosis in an in vitro human nail model. Mycoses. 2020;63(2):225–232. doi:10.1111/myc.13030
191. Lipner S, Friedman G, Scher R. Pilot study to evaluate a plasma device for the treatment of onychomycosis. Clin Exp Dermatol. 2017;42(3):295–298. doi:10.1111/ced.12973
192. Ouf SA, El-Adly AA, Mohamed -A-AH. Inhibitory effect of silver nanoparticles mediated by atmospheric pressure air cold plasma jet against dermatophyte fungi. J Med Microbiol. 2015;64(10):1151–1161. doi:10.1099/jmm.0.000133
193. Lux J, Dobiáš R, Kuklová I, et al. Inactivation of dermatophytes causing onychomycosis and Its therapy using non-thermal plasma. J Fungi. 2020;6(4):214. doi:10.3390/jof6040214
194. Shemer A, Gupta A, Amichai B, et al. An open comparative study of nail drilling as adjunctive treatment for toenail onychomycosis. J Dermatological Treatment. 2016;27(5):480–483. doi:10.3109/09546634.2016.1151856
195. Polak A. The past, present and future of antimycotic combination therapy. Mycoses. 1999;42(5‐6):355–370. doi:10.1046/j.1439-0507.1999.00475.x
196. Kaul S, Yadav S, Dogra S. Treatment of dermatophytosis in elderly, children, and pregnant women. Indian Dermatol Online J. 2017;8(5):310. doi:10.4103/idoj.IDOJ_169_17
197. Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013;30(3):294–302. doi:10.1111/pde.12048
198. Gupta AK, Venkataraman M, Shear NH, et al. Onychomycosis in children–review on treatment and management strategies. J Dermatological Treatment. 2020;5:1–12.
199. Ginter‐Hanselmayer G, Weger W, Smolle J. Onychomycosis: a new emerging infectious disease in childhood population and adolescents. Report on treatment experience with terbinafine and itraconazole in 36 patients. J Eur Acad Dermatol Venereol. 2008;22(4):470–475. doi:10.1111/j.1468-3083.2007.02498.x
200. Heikkilä H, Stubb S. Onychomycosis in children: treatment results of forty-seven patients. Acta Derm Venereol. 2002;82(6):484–485. doi:10.1080/000155502762064764
201. Gupta AK, Nolting S, de Prost Y, et al. The use of itraconazole to treat cutaneous fungal infections in children. Dermatology. 1999;199(3):248–252. doi:10.1159/000018256
202. Gupta AK, Mays RR, Versteeg SG, et al. Onychomycosis in children: safety and efficacy of antifungal agents. Pediatr Dermatol. 2018;35(5):552–559. doi:10.1111/pde.13561
203. Gupta AK, Chang P, Del Rosso JQ, et al. PHARMACOLOGY AND THERAPEUTICS: onychomycosis in Children: prevalence and Management. Pediatr Dermatol. 1998;15(6):464–471. doi:10.1046/j.1525-1470.1998.1998015464.x
204. Assaf RR, Elewski BE. Intermittent fluconazole dosing in patients with onychomycosis: results of a pilot study. J Am Acad Dermatol. 1996;35(2):216–219. doi:10.1016/S0190-9622(96)90327-8
205. Friedlander SF, Chan YC, Chan YH, et al. Onychomycosis does not always require systemic treatment for cure: a trial using topical therapy. Pediatr Dermatol. 2013;30(3):316–322. doi:10.1111/pde.12064
206. Eichenfield LF, Elewski B, Sugarman JL, et al. Efinaconazole 10% topical solution for the treatment of onychomycosis in pediatric patients: open-label phase 4 study. J Am Acad Dermatol. 2021;84(4):1140–1142. doi:10.1016/j.jaad.2020.06.1004
207. Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18(2):190–195.
208. Tavakkol A, Fellman S, Kianifard F. Safety and efficacy of oral terbinafine in the treatment of onychomycosis: analysis of the elderly subgroup in improving results in ONychomycosis-concomitant lamisil® and debridement (IRON-CLAD), an open-label, randomized trial. Am J Geriatr Pharmacother. 2006;4(1):1–13. doi:10.1016/j.amjopharm.2005.12.012
209. Iozumi K, Abe M, Ito Y, et al. Efficacy of long‐term treatment with efinaconazole 10% solution in patients with onychomycosis, including severe cases: a multicenter, single‐arm study. J Dermatol. 2019;46(8):641–651. doi:10.1111/1346-8138.14935
210. Loo DS. Onychomycosis in the elderly. Drugs Aging. 2007;24(4):293–302. doi:10.2165/00002512-200724040-00003
211. Valeant Pharmaceuticals. Jublia (Efinaconazole) Topical Solution, 10% [Package Insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2014. Available from http://www.valeant.com/Portals/25/Pdf/PI/Jublia-PI.pdf.
212. Piraccini BM, Sisti A, Tosti A. Long-term follow-up of toenail onychomycosis caused by dermatophytes after successful treatment with systemic antifungal agents. J Am Acad Dermatol. 2010;62(3):411–414. doi:10.1016/j.jaad.2009.04.062
213. Gupta AK, Simpson FC. New therapeutic options for onychomycosis. Expert Opin Pharmacother. 2012;13(8):1131–1142. doi:10.1517/14656566.2012.681779
214. Tosti A, Piraccini B, Stinchi C, et al. Relapses of onychomycosis after successful treatment with systemic antifungals: a three-year follow-up. Dermatology. 1998;197(2):162–166. doi:10.1159/000017990
215. Tosti A, Elewski BE. Onychomycosis: practical approaches to minimize relapse and recurrence. Skin Appendage Disorders. 2016;2(1–2):83–87. doi:10.1159/000448056
216. Shemer A, Gupta AK, Kamshov A, et al. Topical antifungal treatment prevents recurrence of toenail onychomycosis following cure. Dermatol Ther. 2017;30(5):e12545. doi:10.1111/dth.12545
217. Gupta AK, Elewski BE, Rosen T, et al. Onychomycosis: strategies to minimize recurrence. J Drugs Dermatol. 2016;15(3):279–282.
218. Feuilhade de Chauvin M. A study on the decontamination of insoles colonized by Trichophyton rubrum: effect of terbinafine spray powder 1% and terbinafine spray solution 1%. J Eur Acad Dermatol Venereol. 2012;26(7):875–878. doi:10.1111/j.1468-3083.2011.04176.x
219. Gupta AK, Brintnell WC. Sanitization of contaminated footwear from onychomycosis patients using ozone gas: a novel adjunct therapy for treating onychomycosis and tinea pedis? J Cutan Med Surg. 2013;17(4):243–249. doi:10.2310/7750.2012.12068
220. Hammer TR, Mucha H, Hoefer D. Infection risk by dermatophytes during storage and after domestic laundry and their temperature-dependent inactivation. Mycopathologia. 2011;171(1):43–49. doi:10.1007/s11046-010-9347-9
221. Velasquez-Agudelo V, Cardona-Arias JA. Meta-analysis of the utility of culture, biopsy, and direct KOH examination for the diagnosis of onychomycosis. BMC Infect Dis. 2017;17(1):1–11. doi:10.1186/s12879-017-2258-3
222. Lim SS, Ohn J, Mun J-H. Diagnosis of onychomycosis: from conventional techniques and dermoscopy to artificial intelligence. Front Med. 2021;8:637216. doi:10.3389/fmed.2021.637216
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.