Back to Journals » International Journal of Nanomedicine » Volume 17
Nanotechnology: A Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis
Authors Dessale M, Mengistu G , Mengist HM
Received 20 June 2022
Accepted for publication 22 August 2022
Published 26 August 2022 Volume 2022:17 Pages 3735—3749
DOI https://doi.org/10.2147/IJN.S378074
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Mesfin Dessale, Getachew Mengistu, Hylemariam Mihiretie Mengist
Department of Medical Laboratory Sciences, Debre Markos University, Debre Markos, Amhara, Ethiopia
Correspondence: Hylemariam Mihiretie Mengist, Email [email protected]
Abstract: Cancer remains the most devastating disease and the major cause of mortality worldwide. Although early diagnosis and treatment are the key approach in fighting against cancer, the available conventional diagnostic and therapeutic methods are not efficient. Besides, ineffective cancer cell selectivity and toxicity of traditional chemotherapy remain the most significant challenge. These limitations entail the need for the development of both safe and effective cancer diagnosis and treatment options. Due to its robust application, nanotechnology could be a promising method for in-vivo imaging and detection of cancer cells and cancer biomarkers. Nanotechnology could provide a quick, safe, cost-effective, and efficient method for cancer management. It also provides simultaneous diagnosis and treatment of cancer using nano-theragnostic particles that facilitate early detection and selective destruction of cancer cells. Updated and recent discussions are important for selecting the best cancer diagnosis, treatment, and management options, and new insights on designing effective protocols are utmost important. This review discusses the application of nanotechnology in cancer diagnosis, therapeutics, and theragnosis and provides future perspectives in the field.
Keywords: cancer, nanotechnology, diagnosis, treatment, theragnostics
Introduction
Cancer is a very complex disease that develops through a multistep process which includes resistance to cell death (apoptosis), uncontrolled cell growth, alterations in cellular signaling, tissue invasion, metastasis, and angiogenesis.1 Cancer generally begins as a localized tumor and can spread (metastasize) to distant sites in the body, making the management difficult. Cancer morbidity and mortality are on the rise across the world. According to global cancer incidence, mortality and prevalence (GLOBOCAN) 2018 data, the number of new cancer cases was expected to exceed 18.1 million, with 9.6 million cancer-related deaths.2 The World Health Organization (WHO) estimated more than 19.3 million new cases and 10 million deaths from cancer in 2020.3 It is projected that 30 million people will die of cancer each year after 2030.4
Factors that enhance the emergence of cancer include rising pollution, radiation, sedentary life, unbalanced diet, infection with oncogenic microorganisms, and other variables (eg, heredity) which are also becoming common in developing countries. Any of these variables can cause a damage in host cells’ deoxyribose nucleic acid (DNA) genes known as oncogenes that lead to cancer. Individual cells that have achieved immortality and are capable of replicating at incredible rates surpass all healthy functional cells resulting in death.5–9
Nanotechnology is the field that deals with atomic, molecular, and supramolecular levels of molecules (1–100 nm) to understand the properties that can be exploited for human well-being. Nanotechnology uses nanoscale principles and methods to know biosystems, and it is being emerged with modern biology and medicine to generate more nanoscale materials that can be used in biological systems.10,11
Nanoparticles are used in medical applications because of their unique properties such as quantum properties, a surface-to-mass ratio much larger than that of other particles, and an adsorption capacity to transport other compounds such as probes, proteins, and drugs. The composition of nanoparticles can be varied, just as starting materials can be biological lipids, dextran, lactic acid, phospholipids, chitosan, or chemicals such as silica, carbon, metals, and different polymers.12–16
Imaging techniques and morphological study of tissues (histopathology) or cells (cytology) are currently used to aid in the early detection of cancer. Imaging technologies enable to see tissue alterations for the detection of cancer cells. However, due to the time demanding nature of these methods, cancer cells can have time to replicate and invade tissue. Furthermore, existing imaging technologies are unable to discriminate between benign and malignant tumors.17 Besides, cytology and histopathology cannot be used to diagnose cancer at an early stage in a reliable and independent manner. As a result, developing reliable methods that can detect cancer at an early stage is necessary.18–21
Nanotechnology-based diagnostic technologies are being developed as promising tools for cancer diagnosis that are real-time, convenient, and cost-effective.22 Nanoparticles are being used to capture cancer biomarkers such as exosomes, circulating tumor cells, circulating tumor DNA, and cancer associated proteins for effective cancer diagnosis.20,23,24 The high surface area-to-volume ratio of nanoparticles in comparison to bulk materials is a key benefit for using them for cancer diagnosis.20,24–28 This characteristic allows for the dense coating of nanoparticle surfaces with antibodies, small molecules, peptides, aptamers, and other moieties to detect specific cancer molecules.28–31 Multivalent effects can also be achieved by exposing cancer cells to a variety of binding ligands, which can increase an assay’s specificity and sensitivity.32–34
Currently, chemotherapy, surgery, radiation, and a combination of these treatments are the most common methods of cancer treatment. However, these methods have significant drawbacks including, but not limited to, non-specificity and toxicity.35–38 The goal of modern medication is to optimize the pharmacological efficacy of drugs and minimize possible side effects. To avoid any unwanted responses, the drug’s local concentration at cancer sites must be high, while its concentration in other tissues must be low.39 The application of nanotechnology in cancer treatment holds the potential to overcome the constraints of the conventional methods. Using nanotechnology, the amount of drug required to provide a therapeutic impact can be considerably lowered, and the drug concentration on the cancer site can be boosted without having any negative effects on healthy cells.40–43
Several nanoparticle-based drug delivery systems such as nano-discs, high-density lipoprotein (HDL) nanostructures, gold nanoparticles, and viral nanoparticles have demonstrated promising outcomes in cancer therapy. Auspicious progress has been achieved in understanding the biological characteristics of cancer to improve the usage of nanoparticles by overcoming biological barriers and distinguishing between malignant and healthy tissues. Nano-drugs offer a lot of potential in cancer therapy because of their unique qualities such as limiting damage to healthy cells, overcoming multidrug resistance (MDR), and improving anti-cancer drug solubility.19
Nanoparticles are used in medical applications because of their unique properties and due to their adsorption capacity to transport other compounds such as probes, proteins, and drugs.44 The composition of nanoparticles can be varied, just as starting materials can be biological lipids,45 dextran,46 lactic acid,47 phospholipids,48 chitosan,49 or chemicals such as silica,50 carbon,51 metals,52 and different polymers.12 Updating the available literature, summarizing new discussions, and adding new insights are very crucial to select the best options for cancer diagnosis and treatment. The main goal of this review is to summarize current advances in nanotechnology-based cancer diagnosis, therapeutics, and theragnostics. Further, current challenges and future perspectives are also discussed which could contribute to future studies working in the field.
Application of Nanotechnology for Cancer Diagnosis and Treatment
The unique features of nanoparticles led to their adoption in a variety of applications. Nanoparticles have a variety of shapes, sizes, and structures.53 Nanoparticles have several auspicious advantages that they are multifunctional, they can deliver hydrophobic compounds, they can target disease cells actively and passively, they have the capacity to prolong the drug’s circulation time, they can boost the entrance and accumulation of drugs in tumor sites, they overcome drug resistance, they promote the safety and tolerability of drugs, and they assist the improvement of other technologies.54,55
Nanotechnology for Cancer Diagnosis
Although nanotechnology has yet to be used in clinical cancer detection, it is already applied in a range of medical tests and screens, such as gold nanoparticles in home pregnancy tests.56 Nanoparticles are being used to capture cancer biomarkers including cancer-associated proteins,57,58 circulating tumor DNA (ctDNA),59,60 circulating tumor cells (CTC), and exosomes.61,62 Antibodies, small molecules, peptides, aptamers, and other compounds can be thickly coated on nanoparticle surfaces because of this high surface area-to-volume ratio. Multivalent effects can be obtained by presenting multiple binding ligands to cancer cells, which can improve the specificity and sensitivity of diagnosis.29
Nanotechnology for the Detection of Cancer Biomarkers
A cancer biomarker is a detectable biological molecule present in the blood, various tissues, and body fluids including saliva and urine that indicates the presence of cancer cells in the body.19 Proteins (released proteins or cell surface proteins),63 carbohydrates,64 or nucleic acids (ct DNA, micro-RNA, etc.)65 are all examples of cancer biomarkers that are secreted by the body or cancer cells during carcinogenesis.66 Measurement of cancer biomarker levels aids in the early detection of cancer or tumor recurrence as well as the monitoring of therapeutic efficacy.
Nanotechnology provides excellent selectivity and sensitivity, as well as the capacity to assess numerous targets at the same time. Nanoparticles/nanomaterials can be used to improve biosensors and offer more precise targeting.67 Furthermore, the introduction of nanoparticles increases the surface-to-volume ratio of biosensors, making them more sensitive to the demands of certain biomolecular diagnostics.68 The three most frequent nanoparticle probes employed in cancer diagnosis are quantum dots (QDs), gold nanoparticles (AuNPs), and polymer dots (PDs).69 Different cancer markers can be used in nanotechnology. The following markers are among the examples.
Protein Detection
CEA for colorectal cancer,70 AFP for liver cancer,71 PSA for prostate cancer,72,73 and CA-125 for ovarian cancer74 are among the protein markers that have received approval for cancer detection. The identification of these traits can be aided by specific interactions with antibodies, antibody fragments, or aptamers.75 After then, the interaction event will be turned into a measurable signal.76 QDs have unique features such as high quantum yield and molar extinction coefficient, wide absorption, excellent photobleaching resistance, and outstanding degradation resistance.77,78 QD-based biosensors have been utilized to detect cancer biomarkers in recent investigations.79 QDs conjugated with aptamers were applied detect CA-125 biomarker for ovarian cancer74 while QD-embedded silica nanoparticles were used to detect PSA antigens with lateral flow technique.80 QDs were also used to detect CEA81–83 and AFP antigens.84,85 Generally, a sandwich-type test, which includes a biomarker, a capture antibody, a second capture antibody, and a secondary antibody that binds to the capture antibody, is a typical approach for detecting protein biomarkers.18 Various approaches, like staining and fluorescence, can be used to visualize the secondary antibody.86 Aptamers, which are single-stranded DNA (ssDNA) or RNA sequences that can be extracted via ligand systematic evolution and exponential enrichment (SELEX), can also be attached to nanoparticles. Aptamers can attach to their targets such as ions, bacteria, peptides, viruses, phospholipids, and even complete cells) with high affinity and strong binding specificity.87
Circulating Tumor DNA Detection
Tumor-derived DNA fragments (about 100–200 base pairs long) circulate in the bloodstream as circulating tumor DNA (ctDNA). Cancer-specific genetic abnormalities can be detected using ctDNA, which can be secreted from primary tumors or circulating tumor cells (CTCs).19,88,89 The detection of genetic abnormalities in ctDNA can aid in the detection of cancer even before any symptoms appear.90 To detect cancer-related genetic abnormalities, highly specific hybridization with nucleic acid probes having complementary sequences can be utilized. For the detection of a single exon in the BRCA1 gene in breast cancer, a DNA silver nanocluster (NC) fluorescent probe was designed. This probe significantly raised the limit of detection (LOD) under optimal conditions. Large deletion mutations in BRCA1 were discovered using nanocluster fluorescence produced by recognition hybridization.91
MicroRNA Detection
Micro-RNAs (miRNAs) are naturally occurring ssRNAs with 20–22 nucleotide sequences long and have the capacity to bind with mRNA and repress its translation. A single miRNA can regulate many gene expressions, and a single mRNA can be targeted with many miRNAs.92 These genetically encoded regulatory molecules support gene expression that controls cell proliferation, growth, and apoptosis. The normal cellular function will be compromised as the synthesis of miRNAs is dysregulated, which finally leads to cancer. Oncogenic viruses cause cancer by affecting the regulation of miRNAs in different ways including upregulating the miRNA of the host to activate the proliferation of virally infected cells, encoding viral miRNAs that target the host cell mRNA, and minimizing the host miRNA that has a role in tumor suppression.92 Detection of miRNA has a good advantage in that circulating miRNAs are more stable. Moreover, a simple blood sample can be used because RNAse enzyme activity does not affect these miRNAs.93 Different tissue or organ-specific miRNA markers can be used for early diagnosis, prognosis, and therapy monitoring.94
DNA Methylation Detection
The genome methylation landscape (Methylscape) has recently been identified as a common feature of most types of malignancies, suggesting that it could be used as a cancer biomarker. In a study, the authors used DNA-gold affinity and DNA solvation to find differences between cancer and normal genomes, and then, they could develop simple, rapid, selective, and sensitive electrochemical or colorimetric one-step assays to diagnose cancer.95
Extracellular Vesicle Detection
Extracellular vehicles (EVs) are circulating vesicles (30nm–1μm in size) that package molecular information from mother cells, such as miRNA, DNA, protein, and mRNA, and allow the detection of tumor cells at molecular state that are usually difficult to access. Scientists created a unique magnetic nanopore capture approach to isolate specific subsets of EVs from plasma in a recent work.19
Nanotechnology for Detection of Cancer Cells
Detection of Circulating Tumor Cells
Metastasis is responsible for approximately 90% of solid tumor mortality. During metastatic dissemination, a cancer cell from the primary tumor first invades the surrounding tissue, then enters the blood and lymph systems’ microvasculature (intravasation), followed by survival and translocation through the bloodstream to micro-vessels in distant tissues. Finally, these cells exit the bloodstream (extravasation) and survive in the distant microenvironment, which provides a suitable foreign microenvironment for the development of secondary tumors. The early diagnosis of metastatic cancer cells in the bloodstream, CTCs, can have a significant impact on cancer prognosis and diagnosis.96
CTCs have been examined extensively as part of a liquid biopsy due to their potential applications. CTC detection is a minimally invasive method for understanding the molecular organization of tumors. Despite this, CTCs have a low abundance and variability, posing technological obstacles for CTC isolation and characterization. Researchers have focused on the use of nanotechnologies for sensitive detection of CTCs in recent years; these technologies can help characterize cells and molecules, resulting in a wide range of clinical applications, such as disease detection at an early stage and evaluation of treatment response and disease progression.19
Nanomaterials have an important advantage for CTC detection in that they have a large surface-to-volume ratio, which allows for the adsorption of high-efficiency targeting ligands that can recognize specific molecules on cancer cells. As a result, CTC isolation has high specificity and recovery, and the sensitivity of detection is improved.19
Different types of nanomaterials have been described for CTC detection, including magnetic nanoparticles (MNPs), AuNPs, QDs, nanowires, nanopillars, silicon nanopillars, carbon nanotubes, dendrimers, graphene oxide, and polymers. These nanomaterials have been shown to improve the sensitivity and specificity of CTC collection devices, with the potential to aid cancer detection and prognosis97 (Table 1).
 |
Table 1 Nanotechnology for Detection of Cancer Cells |
Detection of Cell Surface Proteins
Binding of nanoparticle probes coupled with moieties (proteins, short peptides, antibodies, oligonucleotide aptamers) to surface markers on cancer cells and those entering cells and detecting genetic content is the major method for detecting cancer cells. The first and most crucial step in detecting cancer cells, such as CTCs, is to capture or isolate them. Although physical features of cells such as size, deformability, and density are sometimes exploited, cell capture is primarily dependent on the affinity of cell surface chemicals for CTCs as measured by antibodies or aptamers. The major targets are CTCs’ unique surface proteins (eg, EpCAM). EpCAM can be employed as a cell surface biomarker because it has been shown in numerous studies to be significantly expressed on CTCs from a variety of human cancers. As a result, anti-EpCAM compounds are frequently used in CTC screening.19
mRNA-Based Detection
Nanoparticles have also been produced to detect intracellular nucleic acids in addition to external nucleic acids. Seferos et al98 showed that transfection agents and cellular “nanoflares” can be used to detect mRNA in living cells using novel gold nanoparticle probes modified by oligonucleotides hybridized to complements tagged with a fluorophore. Nanoflares overcome many obstacles in the development of effective and sensitive intracellular probes. Nanoflares are effective for detecting intracellular mRNA because they have a high orientation, dense oligonucleotide coating, and they may penetrate cells without a harmful transfection agent.98 Figure 1 describes the application of nanoparticles in cancer diagnosis.
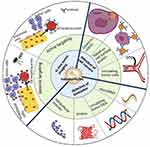 |
Figure 1 Schematic illustration of nanotechnology applications in cancer diagnosis. Nanotechnology-based diagnostic technologies are being developed as promising tools for cancer diagnosis and detection that are real-time, convenient, and cost-effective. Adapted from Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. Journal of Hematology & Oncology. 2019;12(1):137. https://creativecommons.org/licenses/by/4.0/.146 |
Nanotechnology for Cancer Therapy
Due to their unique properties and suitable features, nanoparticles are ideal means of delivering treatment. For drug delivery, the size of the nanoparticles should be less than 0.1 µm. The composition of nanoparticles can be varied, just as starting materials can be biological lipids, dextran, lactic acid, phospholipids, chitosan, or chemicals such as silica, carbon, metals, and different polymers. The interactions of biological components of nanoparticles are different from those of non-biological components. Biodegradable nanoparticles are needed in drug delivery to transport the drug and release it at the target site.12
Surgery, chemotherapy, and radiation are currently available and frequently used cancer therapy methods. However, they all not only kill cancer cells but also normal cells, and this is the main and foremost drawback of current cancer therapy. Nanoparticles are non-toxic, chemically stable, and biocompatible materials in nature, and this nature enables them to be selected as an efficient drug delivery tool.39
Recently, nanotechnology and nanoparticles have attracted great interest in cancer therapeutics as they can provide improved and targeted drug delivery systems to overcome the drawbacks of conventional chemotherapy.99 Limitations of chemotherapy include inefficient drug delivery at the target site due to physical and biochemical barriers and drug resistance at the tumor level because of cellular and non-cellular mechanisms that limit drug action resulting in recurrence and high mortality.
Poorly developed vascularized tumor regions and tumor microenvironment are the main mechanisms that impede drug access to tumor tissue and reduce drug efficiency. Similarly, increased interstitial pressure and decreased microvascular pressure may also delay the extravasation of drug molecules.100 To overcome these limitations, the development of nanoscale delivery vehicles for anticancer drugs with nanoparticles could address the pharmacokinetic disadvantages of traditional chemotherapy.99
The main goals of nanotechnology in drug delivery include specification of drug delivery, reducing toxicity along with maintaining therapeutic effects, safety, biocompatibility, and improving the production of new facial drugs. The fundamental knowledge required before selecting suitable carriers as a drug delivery system includes drug uptake and release, stability and shelf life of drug and carrier, biocompatibility, bio-distribution of the drug and carrier, and mode of action of the drug.12
Viral nanoparticles are naturally biocompatible and encode their building instructions using the host cell’s resources. Their packaging abilities are promising when it comes to delivering chemotherapy to tumors. PEGylation, in turn, can be applied to enhance viral survival from the immune system, and its natural host specificity can be exploited in fighting tumors.39
Virus-like particles (VLPs) are constructs that can be engineered for just such purposes without concern for the actual viral infection of the whole organism.101 The cowpea mosaic virus has recently become of interest because it does not contain nucleic acid material. It is empty, which means that its use should not have any harmful effects like the virus strain does, and therefore it can be safely introduced into a living organism.
It has been found that injection or inhalation of this VLP into a tumor microenvironment can induce an immune response that releases large amounts of neutrophils. These recruit cytokines and T lymphocytes to invade and attack metastatic cancer cells. This yields a result ranging from delayed metastasis to the complete elimination of cancer cells. This method does not require the insertion of an anti-cancer drug. Rather, it simply uses our natural defense mechanisms against cancer cells. Prospects are high and under-development, although the extensive study has not yet been conducted.39
Nano-diamonds are microscopic carbon-based agents that have numerous biological applications because of their extremely high biocompatibility as compared to other nanoparticles similar in size. They can be used as biomarkers and tracers that mark cancer or loaded with doxorubicin and deliver it to metastatic tumor cells. Chan and his research group102 have used nano-diamonds to not only target cancer cells but also to deliver doxorubicin directly into the mitochondria once inside. This kills the cell by eliminating its energy source, consequent inhibition of growth, reproduction, and normal cell function. Cancer cells that are typically resistant to doxorubicin (eg, MCF-7) can still be targeted by nano-diamonds by specifically targeting the mitochondria rather than the whole cell in general.102
Nanotechnology has paved a new insight into cancer immunotherapy. The multifunctional nature of nanoparticles enables an improved way to deliver different antigens, nucleic acids, and antibodies.103 Nanotechnology has been used to load synthetic or natural molecules that have a crucial role in restoring the immune system’s anti-tumor function. Small molecular inhibitors, antibodies, and proteins that target programmed cell death protein 1 (PD 1)104 and cytotoxic T-lymphocyte associated protein 4 (CTLA 4)105 have shown promising results in recent years.106 Nanoparticles can be used as vehicles for these immunotherapeutic agents such as cancer vaccines, cytokines, and adoptive cellular therapies to fight cancer.
DNA and RNA-based cancer treatment with gene therapy becomes a promising tool in recent days. However, challenges such as solubility, permeability, lack of target specificity, and stability faced this technique. Moreover, nucleic acid is an extremely negatively charged and hydrophilic structure that makes it be easily digested with enzymes and cleared with renal elimination. Nanotechnology has become a good tool to address these challenges.107,108 Organic nanoparticles including lipid-based nanoparticles, prodrug-based nanoparticles, and lipid-polymer hybrid nanocarriers and inorganic nanoparticles like QDs, AuNPs, and silica-based nanoparticles have been used to deliver siRNAs.100,109 Combination therapy (chemotherapy combined with wild-type p53) encapsulated with nanoparticles also showed a better result.110 CRISPR/Cas9 gene-editing system delivered with nanoparticles has also shown promising advancement in cancer therapy in recent years.111
Drug Targeting Mechanism in Cancer Nanotechnology
There are two models of drug targeting mechanisms: passive targeting and active targeting. In passive targeting, the reason behind enhanced permeability and retention (EPR) based drug targeting is the rapidly growing leaky vascularization and defective lymphatic drainage that contributes to the retention of nanoparticles and submicron particles in tumors. Nanoscale drug carriers, which include liposomes, dendrimers, polymeric micelles, polymer-drug conjugates, and inorganic nanoparticles, are being extensively studied in drug delivery for this specific approach in cancer chemotherapy.112 These nanoparticles pass through hyper-permeable blood vessels and, due to their small size (typically between 1 nm and 200 nm), preferentially accumulate at the tumor site through their EPR effect.113
The basis of the active targeting strategy involves the interaction of a ligand-loaded drug carrier with surface-exposed receptors on the target cells, which aids in their accumulation in a tumor and also aids in their intracellular accumulation through receptor-mediated endocytosis.114 Tumor cells are normally overexpressed with one or more types of specific receptors that can act as target sites for active targeting by ligand-functionalized nanoparticles. Due to this reason, an active targeting approach uses cellular targets to recognize the tumor and endothelial cells,115,116 as illustrated in Figure 2.
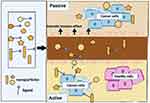 |
Figure 2 Passive and active drug targeting mechanism. Adapted from Jin C, Wang K, Oppong-Gyebi A, Hu J. Application of Nanotechnology in Cancer Diagnosis and Therapy - A Mini-Review. International Journal of Medical Sciences. 2020;17:2964–2973. https://creativecommons.org/licenses/by/4.0/.147 |
The Concept of Theragnosis
The term “theragnosis” refers to the integration of imaging/diagnostic and therapeutic capabilities on a single platform, with the imaging/diagnostic agent and therapeutic agent being delivered in a single dose with an integrated system to diagnose, treat, and monitor therapeutic response at the same time.117,118 Theragnostic nanoparticles are multifunctional nano-systems that combine diagnostic and therapeutic properties into a single biocompatible and biodegradable nanoparticle. They are well designed for more specific disease management.119 Theragnostic nanoparticles need to be safe for human beings and have the following capabilities: (1) accumulate in target(s) sites selectively and rapidly; (2) indicate morphological and biochemical features of the disease; (3) effectively deliver an appropriate amount of drug(s) required with no harmful effect on healthy organs; and (4) eliminated from the body within hours or biodegraded into non-toxic by-products.119
The diagnostic and therapeutic abilities of nanoparticles can be combined in a single system for theragnosis (illustrated in Figure 3). These multifunctional nanoparticles are expected to propel medication research to new heights while minimizing risks and costs. Nanoparticles with hydrophilic and hydrophobic surfaces can now be manufactured using new polymerization and emulsifying processes, allowing them to be loaded with a variety of active compounds (ie, a contrasting agent of hydrophobic nature while a therapeutic agent of hydrophilic nature and vice versa).115
Cancer theragnosis is a new concept in cancer nanotechnology that is proving to be very useful for both clinicians and patients. Nanotechnology’s main goal is to enable nanoparticle-based agents to deliver payload (radioisotopes, medicines, genes, etc.) efficiently and selectively while avoiding systemic toxicity, and to accurately monitor non-invasively delivered therapeutic efficacy over time.120
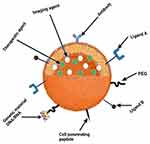 |
Figure 3 A schematic representation of theragnostic nanoparticles (diagnostic and therapeutic agents in a single NP). Adapted from Alshehri S, Imam SS, Rizwanullah M, Akhter S, Mahdi W, Kazi M, et al. Progress of cancer nanotechnology as diagnostics, therapeutics, and theranostics nanomedicine: preclinical promise and translational challenges. Pharmaceutics. 2020;13(1):24. https://creativecommons.org/licenses/by/4.0/.148 |
Nanotechnology has established itself as a possible aid in cancer treatment due to its wide range of applications. Gold nanoparticles, QDs, carbon nanoparticles, silver nanoparticles, and Chitosan-based nanoparticles (CNPs) have all been created into multimodal theragnostic nanoparticles with targeting, imaging, and therapeutic characteristics. Theragnostic nanoparticles (similar to nanoparticles), are administered to tumor sites through active and passive targeting.121
Theragnostic nanoparticles conjugated with a specific ligand of cancer cells can be targeted actively, whereas theragnostic nanoparticles are passively targeted by extravasation of nanoparticles from a leakier tumor blood artery and accumulating at the tumor location via the EPR effect. Cancer theragnostic nanoparticles can be generated by encapsulating anti-cancer drugs with nanoparticles that are labeled with noninvasive imaging modalities such as MRI, CT, and PET.121
Non-invasive imaging methods in cancer diagnosis aid in early detection, targeted drug delivery, and cancer therapy monitoring. Chemotherapy, photo thermal therapy, and siRNA/miRNA therapy are a few examples of cancer therapies being extensively studied with theragnostic nanoparticles.122–128
Furthermore, theragnostic nanoparticles could aid in the assessment of a patient’s optimal anti-cancer treatment dosage and tumor growth monitoring.129 A rush of research activity in the creation of theragnosis nanoparticles has led to the use of novel optical imaging modalities such as fluorescence resonance energy transfer (FRET) to monitor the drug release behavior of nanoparticles at selected tumor sites. As a result, continued progress in the development of cancer theragnostic nanoparticles will pave the way for a new era in cancer diagnosis and treatment.127
The designed theragnostic nanocarriers have the possibility to be targeted, non-targeted, or stimuli based. A targeting ligand is added to the targeted theragnostic carriers, which can bind to overexpressed receptors in tumor cells. Antibodies can also be used to target cancer cells although their immunogenicity and big size make them difficult to penetrate into tumor cells.130 Because of their smaller size and lesser immunogenicity, peptides are useful as theragnostic carriers. The enhanced permeability effect is the main principle of non-targeted theragnostic carriers. The tumor microenvironment, which includes hypoxia and low extracellular pH, are the main advantages of stimuli-based theragnostic nanocarriers.131
Discussion and Future Perspective
Nanotechnology is the best approach to deal with the diagnosis and treatment of cancer. Besides, theragnosis has become an emerging novel approach for the safe and efficient diagnosis and treatment of malignancies.132 It offers a number of benefits, including improved detection, targeted drug delivery to tumor cells, and lessened fatal effects on healthy tissues.133 These theragnostic methods combine targeted drug delivery with diagnostic imaging techniques. Additionally, by utilizing specialized probes, imaging approaches can assess the efficacy of drugs during the drug development process, maximizing the choice of imaging tools and agents in addition to choosing the best combination for various therapeutic applications.134,135 Nanotechnology has shown a devastating improvement in aiding conventional therapy such as photothermal therapy.121,136 Although significant progress has been seen in nanotechnology-based cancer diagnostics and treatment, only a few cases have progressed to clinical trials.137,138 To apply nanotechnology in cancer diagnosis and therapeutic practice, many hurdles must be overcome.
Reliability is the primary difficulty in nanotechnology-based cancer diagnosis. To apply nano-based technologies in diagnostic centers, it is mandatory to obtain a consistent result. NP-based detection signals can be influenced by a variety of circumstances such as nonspecific NP probe binding, aggregation, and unsuitable detection.139 Reliability and reproducibility of assays must be considered and thoroughly examined with big clinical sample pools in clinical trial application. In this regard, continuous effort is necessary to overcome these hurdles.
The second difficulty is to produce nanoprobes in large amounts that are highly sensitive, repeatable, and have long-term storage stability at an affordable price. Most of the today’s nanoprobes are made in labs under highly optimized settings; yet, producing these probes in batches remains a significant difficulty. The detection efficiencies vary substantially due to the composition, size, shape, charge, and surface coating of nanoprobes. The synthesis procedures and nanoprobe functionalization must be simplified to reduce batch-to-batch variations. Furthermore, nanoprobes tend to aggregate during storage. It is also necessary to evaluate the cost-effectiveness of developing a nanotechnology-based platform.140
The third difficulty is to create NP-based devices that are very sensitive, easy to handle and use, and cost-effective. Although many NP-based tests were developed in academic laboratories, many of them are unsuitable for clinical use. The successful development of nanoparticle-based point-of-care devices will substantially simplify the clinical application of nanotechnology in cancer diagnostics.19
The fourth challenge is the potential for nanoparticle toxicity due to systemic administration. This problem is largely about in-vivo NP-based imaging. The potential toxicity of new nanoparticle probes should be examined before they are used in-vivo imaging. The toxicity of nanoparticles is influenced by their shape, size, charge, surface chemistry, targeted ligands, and composition. The biodistribution, biodegradability, and pharmacokinetic features of nanoparticles should also be taken into account.19
Cancer biomarkers can be used for the diagnosis of cancer at early time. Nonetheless, various obstacles have hampered the use of biomarkers, including low biomarker concentrations in body fluids, variation in biomarker abundance and timing within patients, and the difficulty of conducting prospective research.141
Nanotechnology has been used extensively in a wide range of cancer diagnosis and therapeutic investigations, and it may provide a good alternative to cancer diagnosis and treatment soon.142,143 Although much research has been done in the field of cancer nanotechnology to date, there is still a remaining scrutiny to fully understand the scenario. Despite the difficulties, the promise of nanomaterials in cancer diagnosis and therapy is undeniable, and continued progress in the field of nanomaterials would give a significant cancer detection and treatment method. Furthermore, the synthesis of a successful multimodal nanoparticle must be highlighted, as this might provide cancer with a double punch in terms of rapid diagnosis and effective therapy.143
The future of nanotechnology depends on the development of multi-functional nanoplatforms that combine therapeutic capabilities with multimodal imaging capabilities. To overcome the challenges of cancer heterogeneity and adaptation, such coordination of detection capability with therapeutic interventions is required.144 The nanocarriers’ small size is a significant benefit since they can quickly permeate the tumor vasculature and be kept due to the EPR effect.145 Cancer nanotechnology will undoubtedly deliver a robust, effective, reliable, and safe cancer detection and treatment solution soon.
Although nanotechnology is a promising approach for tackling cancer through effective drug delivery and early diagnosis, there are still several hurdles to deal with. More controlled and longitudinal studies are quite helpful to tackle the limitations of nanotechnology-based cancer diagnosis and treatment. Efforts making reliable application of nanotechnology are utmost important; thus, multidisciplinary and comprehensive studies are required. Nanotechnology-based applications in cancer management should also be affordable and accessible to developing countries.
Concluding Remarks
Many efforts have been in place in the last decade to create nanotechnology-based cancer diagnosis and treatment. Compared to currently existing cancer diagnostics in the clinical settings, several NP-based assays improved selectivity and sensitivity or provided whole new capabilities that could not be attained by the traditional methods. Nanotechnology, with its high sensitivity, specificity, and multiplexed measuring capabilities, has tremendous potential for improving cancer detection and therapy, resulting in a higher cancer patient survival rate. One of the greatest advantages is the combined application of diagnosis and treatment in a single platform (theragnosis). Overall, nano-oncology has opened an unlimited number of possibilities for finding and designing drugs and drug delivery systems for cancer treatment. In contrast to the outstanding advantages of nanotechnology in cancer management, the technology has its own drawbacks, mainly possible toxicity, resource intensiveness, unproved reliability and applicability. Therefore, continuous effort is required to improve these limitations to apply the platform in clinical decision-making.
Acknowledgments
We are thankful to all who supported us while writing this review. We duly acknowledge the authors of the articles where the figures were adapted from.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Reichert JM, Wenger JB. Development trends for new cancer therapeutics and vaccines. Drug Discov Today. 2008;13(1–2):30–37. doi:10.1016/j.drudis.2007.09.003
2. Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Erratum: global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70(4):313.
3. Hyuna Sung JF, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. American Cancer Society; 2021:40.
4. The Lancet. GLOBOCAN 2018: Counting the Toll of Cancer. The Lancet; 2018:985.
5. Anand P, Kunnumakara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097–2116. doi:10.1007/s11095-008-9661-9
6. Sargazi S, Moudi M, Kooshkaki O, Mirinejad S, Saravani R. Hydro-alcoholic extract of Achillea wilhelmsii C. Koch reduces the expression of cell death-associated genes while inducing DNA damage in HeLa cervical cancer cells. Iran J Med Sci. 2020;45(5):359. doi:10.30476/ijms.2020.72657.0
7. Sargazi S, Mukhtar M, Rahdar A, Barani M, Pandey S, Díez-Pascual AM. Active targeted nanoparticles for delivery of poly (ADP-ribose) polymerase (PARP) inhibitors: a preliminary review. Int J Mol Sci. 2021;22(19):10319. doi:10.3390/ijms221910319
8. Sargazi S, Kooshkaki O, Reza JZ, et al. Mild antagonistic effect of valproic acid in combination with AZD2461 in MCF-7 breast cancer cells. Med J Islam Repub Iran. 2019;33:29. doi:10.34171/mjiri.33.29
9. Sargazi S, Saravani R, Reza JZ, et al. Induction of apoptosis and modulation of homologous recombination DNA repair pathway in prostate cancer cells by the combination of AZD2461 and valproic acid. Excli J. 2019;18:485. doi:10.17179/excli2019-1098
10. Roco MC, Bainbridge WS. The new world of discovery, invention, and innovation: convergence of knowledge, technology, and society. J Nanoparticle Res. 2013;15(9):1–17. doi:10.1007/s11051-013-1946-1
11. Surendiran A, Sandhiya S, Pradhan S, Adithan C. Novel applications of nanotechnology in medicine. Indian J Med Res. 2009;130(6):689–701.
12. Jani P, Subramanian S, Korde A, Rathod L, Sawant KK. Theranostic nanocarriers in cancer: dual capabilities on a single platform. In: Functional Bionanomaterials. Springer; 2020:293–312.
13. Nikolova M, Slavchov R, Nikolova G. Nanotechnology in medicine. In: Drug Discovery and Evaluation: Methods in Clinical Pharmacology. Springer; 2020:533–546.
14. Saxena SK, Nyodu R, Kumar S, Maurya VK. Current advances in nanotechnology and medicine. In: NanoBioMedicine. Springer; 2020:3–16.
15. Mukherjee A, Bhattacharyya S. Nanotechnology in medicine. In: Biotechnology Business-Concept to Delivery. Springer; 2020:57–64.
16. Guo D, Xie G, Luo J. Mechanical properties of nanoparticles: basics and applications. J Phys D: Appl Phys. 2013;47(1):013001. doi:10.1088/0022-3727/47/1/013001
17. Choi Y-E, Kwak J-W, Park JW. Nanotechnology for early cancer detection. Sensors. 2010;10(1):428–455. doi:10.3390/s100100428
18. Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, Mirkin CA. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem Rev. 2015;115(19):10530–10574. doi:10.1021/acs.chemrev.5b00321
19. Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12(1):1–13. doi:10.1186/s13045-019-0833-3
20. Chen T, Ren L, Liu X, et al. DNA nanotechnology for cancer diagnosis and therapy. Int J Mol Sci. 2018;19(6):1671. doi:10.3390/ijms19061671
21. Sim S, Wong NK. Nanotechnology and its use in imaging and drug delivery. Biomed Rep. 2021;14(5):1–9. doi:10.3892/br.2021.1418
22. Wu X, Xiao T, Luo Z, et al. A micro-/nano-chip and quantum dots-based 3D cytosensor for quantitative analysis of circulating tumor cells. J Nanobiotechnology. 2018;16(1):1–9. doi:10.1186/s12951-017-0328-8
23. Burz C, Pop -V-V, Buiga R, et al. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget. 2018;9(36):24561. doi:10.18632/oncotarget.25337
24. Song S, Qin Y, He Y, Huang Q, Fan C, Chen H-Y. Functional nanoprobes for ultrasensitive detection of biomolecules. Chem Soc Rev. 2010;39(11):4234–4243. doi:10.1039/c000682n
25. Couvreur P. Nanoparticles in drug delivery: past, present and future. Adv Drug Deliv Rev. 2013;65(1):21–23. doi:10.1016/j.addr.2012.04.010
26. Lee BK, Yun YH, Park K. Smart nanoparticles for drug delivery: boundaries and opportunities. Chem Eng Sci. 2015;125:158–164. doi:10.1016/j.ces.2014.06.042
27. Sivasankar M, Kumar BP. Role of nanoparticles in drug delivery system. Int J Res Pharma Biomed Sci. 2010;1(2):41–66.
28. Gagliardi A, Giuliano E, Venkateswararao E, et al. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front Pharmacol. 2021;12:601626. doi:10.3389/fphar.2021.601626
29. Kumar B, Singh S, Skvortsova I, Kumar V. Promising targets in anti-cancer drug development: recent updates. Curr Med Chem. 2017;24(42):4729–4752. doi:10.2174/0929867324666170331123648
30. Siddique S, Chow JC. Gold nanoparticles for drug delivery and cancer therapy. Appl Sci. 2020;10(11):3824. doi:10.3390/app10113824
31. Rizvi SA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharma J. 2018;26(1):64–70. doi:10.1016/j.jsps.2017.10.012
32. Ye F, Zhao Y, El-Sayed R, Muhammed M, Hassan M. Advances in nanotechnology for cancer biomarkers. Nano Today. 2018;18:103–123. doi:10.1016/j.nantod.2017.12.008
33. Cryer AM, Thorley AJ. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacol Ther. 2019;198:189–205. doi:10.1016/j.pharmthera.2019.02.010
34. Chen X-J, Zhang X-Q, Liu Q, Zhang J, Zhou G. Nanotechnology: a promising method for oral cancer detection and diagnosis. J Nanobiotechnology. 2018;16(1):1–17. doi:10.1186/s12951-018-0378-6
35. Sargazi S, Laraib U, Barani M, et al. Recent trends in the mesoporous silica nanoparticles with rode-like morphology for cancer theranostics: a review. J Mol Struct. 2022;1261:132922. doi:10.1016/j.molstruc.2022.132922
36. Sargazi S, Laraib U, Er S, et al. Application of green gold nanoparticles in cancer therapy and diagnosis. Nanomaterials. 2022;12(7):1102. doi:10.3390/nano12071102
37. Kianfar E, Aramideh Khouy R, Nosrati A. Protein nanoparticles in drug delivery: animal protein, plant proteins and protein cages, albumin nanoparticles. J Nanobiotechnology. 2021;19(1):1–32. doi:10.1186/s12951-020-00755-7
38. Ahmed A, Sarwar S, Hu Y, et al. Surface-modified polymeric nanoparticles for drug delivery to cancer cells. Expert Opin Drug Deliv. 2021;18(1):1–24. doi:10.1080/17425247.2020.1822321
39. Ho BN, Pfeffer CM, Singh AT. Update on nanotechnology-based drug delivery systems in cancer treatment. Anticancer Res. 2017;37(11):5975–5981. doi:10.21873/anticanres.12044
40. Vasir JK, Reddy MK, Labhasetwar VD. Nanosystems in drug targeting: opportunities and challenges. Curr Nanosci. 2005;1(1):47–64. doi:10.2174/1573413052953110
41. Kianfar E. Magnetic nanoparticles in targeted drug delivery: a review. J Superconduct Novel Magnet. 2021;34(7):1709–1735.
42. Mikušová V, Mikuš P. Advances in chitosan-based nanoparticles for drug delivery. Int J Mol Sci. 2021;22(17):9652. doi:10.3390/ijms22179652
43. Mollazadeh S, Mackiewicz M, Yazdimamaghani M. Recent advances in the redox-responsive drug delivery nanoplatforms: a chemical structure and physical property perspective. Mater Sci Engine C. 2021;118:111536. doi:10.1016/j.msec.2020.111536
44. Sarvarian P, Samadi P, Gholipour E, et al. Application of emerging plant-derived nanoparticles as a novel approach for nano-drug delivery systems. Immunol Invest. 2022;51(4):1039–1059. doi:10.1080/08820139.2021.1891094
45. Bukhari SI, Imam SS, Ahmad MZ, et al. Recent progress in lipid nanoparticles for cancer theranostics: opportunity and challenges. Pharmaceutics. 2021;13(6):840. doi:10.3390/pharmaceutics13060840
46. Curcio M, Paolì A, Cirillo G, et al. Combining dextran conjugates with stimuli-responsive and folate-targeting activity: a new class of multifunctional nanoparticles for cancer therapy. Nanomaterials. 2021;11(5):1108. doi:10.3390/nano11051108
47. Li S, Lui K-H, Li X, et al. pH-triggered poly (ethylene glycol)–poly (lactic acid/glycolic acid)/croconaine nanoparticles-assisted multiplexed photoacoustic imaging and enhanced photothermal cancer therapy. Appl Bio Mater. 2021;4(5):4152–4164. doi:10.1021/acsabm.0c01578
48. Rahimnejad M, Rabiee N, Ahmadi S, et al. Emerging phospholipid nanobiomaterials for biomedical applications to lab-on-a-chip, drug delivery, and cellular engineering. Appl Bio Mater. 2021;4(12):8110–8128. doi:10.1021/acsabm.1c00932
49. Ahmad MZ, Rizwanullah M, Ahmad J, et al. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int J Polym Mater Polym Biomater. 2022;71(8):602–623. doi:10.1080/00914037.2020.1869737
50. Mochizuki C, Nakamura J, Nakamura M. Development of non-porous silica nanoparticles towards cancer photo-theranostics. Biomedicines. 2021;9(1):73. doi:10.3390/biomedicines9010073
51. Murugesan R, Raman S. Recent trends in carbon nanotubes based prostate cancer therapy: a biomedical hybrid for diagnosis and treatment. Curr Drug Deliv. 2022;19(2):229–237. doi:10.2174/1567201818666210224101456
52. Clasky AJ, Watchorn JD, Chen PZ, Gu FX. From prevention to diagnosis and treatment: biomedical applications of metal nanoparticle-hydrogel composites. Acta Biomaterialia. 2021;122:1–25. doi:10.1016/j.actbio.2020.12.030
53. Machado S, Pacheco J, Nouws H, Albergaria JT, Delerue-Matos C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci Total Environ. 2015;533:76–81. doi:10.1016/j.scitotenv.2015.06.091
54. Yang Q, Jones SW, Parker CL, Zamboni WC, Bear JE, Lai SK. Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Mol Pharm. 2014;11(4):1250–1258. doi:10.1021/mp400703d
55. Zeineldin R, Syoufjy J. Cancer nanotechnology: opportunities for prevention, diagnosis, and therapy. Cancer Nanotechnol. 2017;2017:3–12.
56. Zhou W, Gao X, Liu D, Chen X. Gold nanoparticles for in vitro diagnostics. Chem Rev. 2015;115(19):10575–10636. doi:10.1021/acs.chemrev.5b00100
57. Gardner L, Kostarelos K, Mallick P, Dive C, Hadjidemetriou M. Nano-omics: nanotechnology-based multidimensional harvesting of the blood-circulating cancerome. In: Nature Reviews Clinical Oncology. Nature Publishing Group; 2022:1–11.
58. Zeeshan F. Nanotechnology in pulmonary disease diagnosis. In: Advanced Drug Delivery Strategies for Targeting Chronic Inflammatory Lung Diseases. Springer; 2022:195–205.
59. Kumar J, Basak S, Kalkal A, Packirisamy G. Recent advances in nanotechnology and microfluidic-based approaches for isolation and detection of circulating tumor cells (CTCs). Nano Struct Nano Objects. 2022;31:100886. doi:10.1016/j.nanoso.2022.100886
60. Wu N, Aquilina M, Qian B-Z, et al.. The application of nanotechnology for quantification of circulating tumour DNA in liquid biopsies: a systematic review. IEEE Rev Biomed Eng. 2022;1. doi:10.1109/RBME.2022.3159389
61. Denis JA, Lacorte J-M. Detection of RAS mutations in circulating tumor cells: applications in colorectal cancer and prospects. Ann Biol Clin. 2017;75:607–618.
62. Hanif S, Muhammad P, Niu Z, et al. Nanotechnology‐based strategies for early diagnosis of central nervous system disorders. Adv NanoBiomed Res. 2021;1(10):2100008. doi:10.1002/anbr.202100008
63. Borrebaeck CA. Precision diagnostics: moving towards protein biomarker signatures of clinical utility in cancer. Nat Rev Cancer. 2017;17(3):199–204. doi:10.1038/nrc.2016.153
64. Taqui S, Daniels LB. Putting it into perspective: multimarker panels for cardiovascular disease risk assessment. Biomark Med. 2013;7(2):317–327. doi:10.2217/bmm.13.15
65. Ma H, Liu J, Ali MM, et al. Nucleic acid aptamers in cancer research, diagnosis and therapy. Chem Soc Rev. 2015;44(5):1240–1256. doi:10.1039/C4CS00357H
66. Ponomaryova A, Rykova E, Cherdyntseva N, et al. P90: dynamic changes of circulating microRNA expression in response to the lung cancer combined therapy. Eur J Cancer Supplements. 2015;13(1):43–44. doi:10.1016/j.ejcsup.2015.08.077
67. Sharifi M, Avadi MR, Attar F, et al. Cancer diagnosis using nanomaterials based electrochemical nanobiosensors. Biosens Bioelectron. 2019;126:773–784. doi:10.1016/j.bios.2018.11.026
68. Doria G, Conde J, Veigas B, et al. Noble metal nanoparticles for biosensing applications. Sensors. 2012;12(2):1657–1687. doi:10.3390/s120201657
69. Zhang H, Lv J, Jia Z. Efficient fluorescence resonance energy transfer between quantum dots and gold nanoparticles based on porous silicon photonic crystal for DNA detection. Sensors. 2017;17(5):1078. doi:10.3390/s17051078
70. Campos-da-Paz M, Dórea JG, Galdino AS, Lacava ZG, de Fatima Menezes Almeida Santos M. Carcinoembryonic antigen (CEA) and hepatic metastasis in colorectal cancer: update on biomarker for clinical and biotechnological approaches. Recent Pat Biotechnol. 2018;12(4):269–279. doi:10.2174/1872208312666180731104244
71. Tzartzeva K, Singal AG. Testing for AFP in combination with ultrasound improves early liver cancer detection. Expert Rev Gastroenterol Hepatol. 2018;12(10):947–949. doi:10.1080/17474124.2018.1512855
72. Ilic D, Djulbegovic M, Jung JH, et al.. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362. doi:10.1136/bmj.k3519
73. Moradi A, Srinivasan S, Clements J, Batra J. Beyond the biomarker role: prostate-specific antigen (PSA) in the prostate cancer microenvironment. Cancer Metastasis Rev. 2019;38(3):333–346. doi:10.1007/s10555-019-09815-3
74. Razmi N, Hasanzadeh M. Current advancement on diagnosis of ovarian cancer using biosensing of CA 125 biomarker: analytical approaches. Trends Analytical Chem. 2018;108:1–12. doi:10.1016/j.trac.2018.08.017
75. Laraib U, Sargazi S, Rahdar A, Khatami M, Pandey S. Nanotechnology-based approaches for effective detection of tumor markers: a comprehensive state-of-The-art review. Int J Biol Macromol. 2022;195:356–383. doi:10.1016/j.ijbiomac.2021.12.052
76. Chang P-Y, Kuo Y-B, Wu T-L, et al. Association and prognostic value of serum inflammation markers in patients with leukoplakia and oral cavity cancer. Clin Chem Lab Med. 2013;51(6):1291–1300. doi:10.1515/cclm-2012-0504
77. Freeman R, Willner I. Optical molecular sensing with semiconductor quantum dots (QDs). Chem Soc Rev. 2012;41(10):4067–4085. doi:10.1039/c2cs15357b
78. Fatima I, Rahdar A, Sargazi S, Barani M, Hassanisaadi M, Thakur VK. Quantum dots: synthesis, antibody conjugation, and HER2-receptor targeting for breast cancer therapy. J Funct Biomater. 2021;12(4):75. doi:10.3390/jfb12040075
79. Tabish TA, Hayat H, Abbas A, Narayan RJ. Graphene quantum dot–based electrochemical biosensing for early cancer detection. Curr Opin Electrochem. 2021;30:100786. doi:10.1016/j.coelec.2021.100786
80. Bock S, Kim H-M, Kim J, et al. Lateral flow immunoassay with quantum-dot-embedded silica nanoparticles for prostate-specific antigen detection. Nanomaterials. 2021;12(1):33. doi:10.3390/nano12010033
81. Zheng X, Mo G, He Y, et al. An electrochemiluminescence immunosensor based on ZnSe@ ZnS QDs composite for CEA detection in human serum. J Electroanal Chem. 2019;844:132–141. doi:10.1016/j.jelechem.2019.05.025
82. Wei X-H, Qiao X, Fan J, et al. A label-free ECL aptasensor for sensitive detection of carcinoembryonic antigen based on CdS QDs@ MOF and TEOA@ Au as bi-coreactants of Ru (bpy) 32+. Microchem J. 2022;173:106910. doi:10.1016/j.microc.2021.106910
83. Xiang W, Lv Q, Shi H, Xie B, Gao L. Aptamer-based biosensor for detecting carcinoembryonic antigen. Talanta. 2020;214:120716. doi:10.1016/j.talanta.2020.120716
84. Kal-Koshvandi AT. Recent advances in optical biosensors for the detection of cancer biomarker α-fetoprotein (AFP). Trends Analytical Chem. 2020;128:115920. doi:10.1016/j.trac.2020.115920
85. Mohammadinejad A, Oskuee RK, Eivazzadeh-Keihan R, et al. Development of biosensors for detection of alpha-fetoprotein: as a major biomarker for hepatocellular carcinoma. Trends Analytical Chem. 2020;130:115961. doi:10.1016/j.trac.2020.115961
86. Anagnostou V, Smith KN, Forde PM, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non–small cell lung cancer. Cancer Discov. 2017;7(3):264–276. doi:10.1158/2159-8290.CD-16-0828
87. Tong R, Coyle VJ, Tang L, Barger AM, Fan TM, Cheng J. Polylactide nanoparticles containing stably incorporated cyanine dyes for in vitro and in vivo imaging applications. Microsc Res Tech. 2010;73(9):901–909. doi:10.1002/jemt.20824
88. Filipska M, Rosell R. Mutated circulating tumor DNA as a liquid biopsy in lung cancer detection and treatment. Mol Oncol. 2021;15(6):1667–1682. doi:10.1002/1878-0261.12983
89. Kurtz DM, Soo J, Co Ting Keh L, et al. Enhanced detection of minimal residual disease by targeted sequencing of phased variants in circulating tumor DNA. Nat Biotechnol. 2021;39(12):1537–1547. doi:10.1038/s41587-021-00981-w
90. Tan SJ, Yeo T, Sukhatme SA, Kong SL, Lim W-T, Lim CT. Personalized treatment through detection and monitoring of genetic aberrations in single circulating tumor cells. In: Isolation and Molecular Characterization of Circulating Tumor Cells. Springer; 2017:255–273.
91. Borghei Y-S, Hosseini M, Ganjali MR. Detection of large deletion in human BRCA1 gene in human breast carcinoma MCF-7 cells by using DNA-silver nanoclusters. Methods Appl Fluoresc. 2017;6(1):015001. doi:10.1088/2050-6120/aa8988
92. Fiammengo R. Can nanotechnology improve cancer diagnosis through miRNA detection? Biomark Med. 2017;11(1):69–86. doi:10.2217/bmm-2016-0195
93. Larrea E, Sole C, Manterola L, et al. New concepts in cancer biomarkers: circulating miRNAs in liquid biopsies. Int J Mol Sci. 2016;17(5):627. doi:10.3390/ijms17050627
94. Cui M, Wang H, Yao X, et al.. Circulating microRNAs in cancer: potential and challenge. Front Genet. 2019;10. doi:10.3389/fgene.2019.00626
95. Sina AAI, Carrascosa LG, Liang Z, et al. Epigenetically reprogrammed methylation landscape drives the DNA self-assembly and serves as a universal cancer biomarker. Nat Commun. 2018;9(1):1–13. doi:10.1038/s41467-018-07214-w
96. Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi:10.1016/j.cell.2006.11.001
97. Huang Q, Wang F-B, Yuan C-H, et al. Gelatin nanoparticle-coated silicon beads for density-selective capture and release of heterogeneous circulating tumor cells with high purity. Theranostics. 2018;8(6):1624. doi:10.7150/thno.23531
98. Choi CHJ, Hao L, Narayan SP, Auyeung E, Mirkin CA. Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proc Natl Acad Sci. 2013;110(19):7625–7630. doi:10.1073/pnas.1305804110
99. Prasad R, Pandey R, Varma R, Barman I. Polymer based nanoparticles for drug delivery systems and cancer therapeutics. In: Natural Polymers for Drug Delivery Oxfordshire. Wallingford UK: CABI; 2016:53–70.
100. Er S, Laraib U, Arshad R, et al. Amino acids, peptides, and proteins: implications for nanotechnological applications in biosensing and drug/gene delivery. Nanomaterials. 2021;11(11):3002. doi:10.3390/nano11113002
101. Ma Y, Nolte RJ, Cornelissen JJ. Virus-based nanocarriers for drug delivery. Adv Drug Deliv Rev. 2012;64(9):811–825. doi:10.1016/j.addr.2012.01.005
102. Chan MS, Liu LS, Leung HM, Lo PK. Cancer-cell-specific mitochondria-targeted drug delivery by dual-ligand-functionalized nanodiamonds circumvent drug resistance. ACS Appl Mater Interfaces. 2017;9(13):11780–11789. doi:10.1021/acsami.6b15954
103. Smith DM, Simon JK, Baker JR. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. doi:10.1038/nri3488
104. Bu J, Nair A, Iida M, et al. An avidity-based PD-L1 antagonist using nanoparticle-antibody conjugates for enhanced immunotherapy. Nano Lett. 2020;20(7):4901–4909. doi:10.1021/acs.nanolett.0c00953
105. Sanaei M-J, Pourbagheri-Sigaroodi A, Kaveh V, Sheikholeslami SA, Salari S, Bashash D. The application of nano-medicine to overcome the challenges related to immune checkpoint blockades in cancer immunotherapy: recent advances and opportunities. Crit Rev Oncol Hematol. 2021;157:103160. doi:10.1016/j.critrevonc.2020.103160
106. Shang N, Figini M, Shangguan J, et al. Dendritic cells based immunotherapy. Am J Cancer Res. 2017;7(10):2091.
107. Jin J-O, Kim G, Hwang J, Han KH, Kwak M, Lee PC. Nucleic acid nanotechnology for cancer treatment. Biochim Biophys Acta. 2020;1874(1):188377. doi:10.1016/j.bbcan.2020.188377
108. Sabir F, Zeeshan M, Laraib U, et al. DNA based and stimuli-responsive smart nanocarrier for diagnosis and treatment of cancer: applications and challenges. Cancers. 2021;13(14):3396. doi:10.3390/cancers13143396
109. Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36(12):4158–4171. doi:10.1093/nar/gkn342
110. Lu X, Wang -Q-Q, Xu F-J, Tang G-P, Yang W-T. A cationic prodrug/therapeutic gene nanocomplex for the synergistic treatment of tumors. Biomaterials. 2011;32(21):4849–4856. doi:10.1016/j.biomaterials.2011.03.022
111. Aghamiri S, Talaei S, Ghavidel AA, et al. Nanoparticles-mediated CRISPR/Cas9 delivery: recent advances in cancer treatment. J Drug Deliv Sci Technol. 2020;56:101533. doi:10.1016/j.jddst.2020.101533
112. Akhter MH, Rizwanullah M, Ahmad J, Ahsan MJ, Mujtaba MA, Amin S. Nanocarriers in advanced drug targeting: setting novel paradigm in cancer therapeutics. Artif Cells, Nanomed Biotechnol. 2018;46(5):873–884. doi:10.1080/21691401.2017.1366333
113. Liao J, Jia Y, Wu Y, et al. Physical‐, chemical‐, and biological‐responsive nanomedicine for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(1):e1581. doi:10.1002/wnan.1581
114. Hejmady S, Pradhan R, Alexander A, et al. Recent advances in targeted nanomedicine as promising antitumor therapeutics. Drug Discov Today. 2020;25(12):2227–2244. doi:10.1016/j.drudis.2020.09.031
115. Zhang H, Dong S, Li Z, et al. Biointerface engineering nanoplatforms for cancer-targeted drug delivery. Asian J Pharma Sci. 2020;15(4):397–415. doi:10.1016/j.ajps.2019.11.004
116. Jin C, Wang K, Oppong-Gyebi A, Hu J. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17(18):2964. doi:10.7150/ijms.49801
117. Silva CO, Pinho JO, Lopes JM, Almeida AJ, Gaspar MM, Reis C. Current trends in cancer nanotheranostics: metallic, polymeric, and lipid-based systems. Pharmaceutics. 2019;11(1):22. doi:10.3390/pharmaceutics11010022
118. Jain T, Kumar S, Dutta P. Theranostics: a way of modern medical diagnostics and the role of chitosan. J Mol Genet Med. 2015;9(159):1747–1862.
119. Jokerst JV, Gambhir SS. Molecular imaging with theranostic nanoparticles. Acc Chem Res. 2011;44(10):1050–1060. doi:10.1021/ar200106e
120. Shim MS, Kim CS, Ahn Y-C, Chen Z, Kwon YJ. Combined multimodal optical imaging and targeted gene silencing using stimuli-transforming nanotheragnostics. J Am Chem Soc. 2010;132(24):8316–8324. doi:10.1021/ja100580y
121. Chaturvedi VK, Singh A, Singh VK, Singh MP. Cancer nanotechnology: a new revolution for cancer diagnosis and therapy. Curr Drug Metab. 2019;20(6):416–429. doi:10.2174/1389200219666180918111528
122. Wang K, Na M-H, Hoffman AS, et al. In situ dose amplification by apoptosis-targeted drug delivery. J Control Release. 2011;154(3):214–217. doi:10.1016/j.jconrel.2011.06.043
123. Chen H, Kim S, Li L, Wang S, Park K, Cheng J-X. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Förster resonance energy transfer imaging. Proc Natl Acad Sci. 2008;105(18):6596–6601. doi:10.1073/pnas.0707046105
124. Kwon KC, Jo E, Kwon YW, et al. Superparamagnetic gold nanoparticles synthesized on protein particle scaffolds for cancer theragnosis. Adv Mater. 2017;29(38):1701146. doi:10.1002/adma.201701146
125. Ryu JH, Lee S, Son S, et al. Theranostic nanoparticles for future personalized medicine. J Control Release. 2014;190:477–484. doi:10.1016/j.jconrel.2014.04.027
126. Huh MS, Lee S-Y, Park S, et al. Tumor-homing glycol chitosan/polyethylenimine nanoparticles for the systemic delivery of siRNA in tumor-bearing mice. J Control Release. 2010;144(2):134–143. doi:10.1016/j.jconrel.2010.02.023
127. Caldorera-Moore ME, Liechty WB, Peppas NA. Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Acc Chem Res. 2011;44(10):1061–1070. doi:10.1021/ar2001777
128. Arshad R, Fatima I, Sargazi S, et al. Novel perspectives towards RNA-based nano-theranostic approaches for cancer management. Nanomaterials. 2021;11(12):3330. doi:10.3390/nano11123330
129. Oliveira S, van Rooy I, Kranenburg O, Storm G, Schiffelers RM. Fusogenic peptides enhance endosomal escape improving siRNA-induced silencing of oncogenes. Int J Pharm. 2007;331(2):211–214. doi:10.1016/j.ijpharm.2006.11.050
130. Alshehri S, Imam SS, Rizwanullah M, et al. Progress of cancer nanotechnology as diagnostics, therapeutics, and theranostics nanomedicine: preclinical promise and translational challenges. Pharmaceutics. 2021;13(1):24. doi:10.3390/pharmaceutics13010024
131. Bhujwalla ZM, Kakkad S, Chen Z, et al. Theranostics and metabolotheranostics for precision medicine in oncology. J Magn Reson. 2018;291:141–151. doi:10.1016/j.jmr.2018.03.004
132. Fan Z, Fu PP, Yu H, Ray PC. Theranostic nanomedicine for cancer detection and treatment. J Food Drug Analysis. 2014;22(1):3–17. doi:10.1016/j.jfda.2014.01.001
133. Satija S, Mehta M, Sharma M, et al. Vesicular drug delivery systems as theranostics in COVID-19. Future Sci. 2020;2020:1607–1609.
134. d’Angelo M, Castelli V, Benedetti E, et al. Theranostic nanomedicine for malignant gliomas. Front Bioengine Biotechnol. 2019;7:325. doi:10.3389/fbioe.2019.00325
135. Tan YY, Yap PK, Lim GLX, et al. Perspectives and advancements in the design of nanomaterials for targeted cancer theranostics. Chem Biol Interact. 2020;329:109221. doi:10.1016/j.cbi.2020.109221
136. Fernandes N, Rodrigues CF, Moreira AF, Correia IJ. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater Sci. 2020;8(11):2990–3020. doi:10.1039/d0bm00222d
137. Muthu MS, Feng -S-S. Theranostic liposomes for cancer diagnosis and treatment: current development and pre-clinical success. Expert Opin Drug Deliv. 2013;10(2):151–155. doi:10.1517/17425247.2013.729576
138. Aghebati‐Maleki A, Dolati S, Ahmadi M, et al. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235(3):1962–1972. doi:10.1002/jcp.29126
139. Lin Y-W, Huang -C-C, Chang H-T. Gold nanoparticle probes for the detection of mercury, lead and copper ions. Analyst. 2011;136(5):863–871. doi:10.1039/C0AN00652A
140. Kim H-M, Jeong S, Hahm E, et al. Large scale synthesis of surface-enhanced Raman scattering nanoprobes with high reproducibility and long-term stability. J Industr Engine Chem. 2016;33:22–27. doi:10.1016/j.jiec.2015.09.035
141. Hull L, Farrell D, Grodzinski P. Highlights of recent developments and trends in cancer nanotechnology research—view from NCI alliance for nanotechnology in cancer. Biotechnol Adv. 2014;32(4):666–678. doi:10.1016/j.biotechadv.2013.08.003
142. Barani M, Hosseinikhah SM, Rahdar A, et al. Nanotechnology in bladder cancer: diagnosis and treatment. Cancers. 2021;13(9):2214. doi:10.3390/cancers13092214
143. Barani M, Bilal M, Sabir F, Rahdar A, Kyzas GZ. Nanotechnology in ovarian cancer: diagnosis and treatment. Life Sci. 2021;266:118914. doi:10.1016/j.lfs.2020.118914
144. Roma-Rodrigues C, Pombo I, Raposo L, Pedrosa P, Fernandes AR, Baptista PV. Nanotheranostics targeting the tumor microenvironment. Front Bioengine Biotechnol. 2019;7:197.
145. Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug Chem. 2011;22(10):1879–1903. doi:10.1021/bc200151q
146. Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12(1):137.
147. Jin C, Wang K, Oppong-Gyebi A, Hu J. Application of nanotechnology in cancer diagnosis and therapy - a mini-review. Int J Med Sci. 2020;17:2964–2973.
148. Alshehri S, Imam SS, Rizwanullah M, et al. Progress of cancer nanotechnology as diagnostics, therapeutics, and theranostics nanomedicine: preclinical promise and translational challenges. Pharmaceutics. 2020;13(1):24.
149. Hong W, Lee S, Chang HJ, Lee ES, Cho Y. Multifunctional magnetic nanowires: a novel breakthrough for ultrasensitive detection and isolation of rare cancer cells from non-metastatic early breast cancer patients using small volumes of blood. Biomaterials. 2016;106:78–86. doi:10.1016/j.biomaterials.2016.08.020
150. Pang X, Cui C, Su M, Wang Y, Wei Q, Tan W. Construction of self-powered cytosensing device based on ZnO nanodisks@ g-C3N4 quantum dots and application in the detection of CCRF-CEM cells. Nano Energy. 2018;46:101–109. doi:10.1016/j.nanoen.2018.01.018
151. Wu C, Schneider T, Zeigler M, et al. Bioconjugation of ultrabright semiconducting polymer dots for specific cellular targeting. J Am Chem Soc. 2010;132(43):15410–15417. doi:10.1021/ja107196s
152. Zhu Y, Chandra P, Shim Y-B. Ultrasensitive and selective electrochemical diagnosis of breast cancer based on a hydrazine–Au nanoparticle–aptamer bioconjugate. Anal Chem. 2013;85(2):1058–1064. doi:10.1021/ac302923k
153. Zhang Y, Chen B, He M, Yang B, Zhang J, Hu B. Immunomagnetic separation combined with inductively coupled plasma mass spectrometry for the detection of tumor cells using gold nanoparticle labeling. Anal Chem. 2014;86(16):8082–8089. doi:10.1021/ac500964s
154. Liu Y, Zhu F, Dan W, Fu Y, Liu S. Construction of carbon nanotube based nanoarchitectures for selective impedimetric detection of cancer cells in whole blood. Analyst. 2014;139(20):5086–5092. doi:10.1039/C4AN00758A
155. Shen J, Li K, Cheng L, Liu Z, Lee S-T, Liu J. Specific detection and simultaneously localized photothermal treatment of cancer cells using layer-by-layer assembled multifunctional nanoparticles. ACS Appl Mater Interfaces. 2014;6(9):6443–6452. doi:10.1021/am405924g
156. Sun N, Li X, Wang Z, et al. A multiscale TiO2 nanorod array for ultrasensitive capture of circulating tumor cells. ACS Appl Mater Interfaces. 2016;8(20):12638–12643. doi:10.1021/acsami.6b02178
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
