Back to Journals » Clinical Epidemiology » Volume 14
Ischemic Heart Disease in Chronic Hepatitis B: A Danish Nationwide Cohort Study
Authors Lau FF , Bollerup S , Engsig F, Krarup H , Mygind LH, Hansen JB, Madsen LG , Thielsen P, Balslev U, Nielsen LN, Barfod TS, Clausen MR , Hobolth L, Laursen AL, Tarp B , Roege BT, Gerstoft J, Christensen PB, Weis N
Received 23 February 2022
Accepted for publication 19 May 2022
Published 18 July 2022 Volume 2022:14 Pages 879—888
DOI https://doi.org/10.2147/CLEP.S361910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Vera Ehrenstein
Frederik Faergemann Lau,1 Signe Bollerup,1 Frederik Engsig,1 Henrik Krarup,2 Lone Hagens Mygind,3 Jesper Bach Hansen,3 Lone Galmstrup Madsen,4,5 Peter Thielsen,6 Ulla Balslev,7 Lars Nørregaard Nielsen,8 Toke S Barfod,9 Mette Rye Clausen,10 Lise Hobolth,11 Alex Lund Laursen,12 Britta Tarp,13 Birgit T Roege,14 Jan Gerstoft,15 Peer Brehm Christensen,16,17 Nina Weis1,5
1Department of Infectious Diseases, Copenhagen University Hospital-Hvidovre, Hvidovre, Denmark; 2Department of Molecular Diagnostics, Aalborg University Hospital, Aalborg, Denmark; 3Department of Medical Gastroenterology, Aalborg University Hospital, Aalborg, Denmark; 4Department of Medical Gastroenterology, Zealand University Hospital, Koege, Denmark; 5Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; 6Department of Gastroenterology, Copenhagen University Hospital-Herlev, Herlev, Denmark; 7Department of Infectious Diseases, Copenhagen University Hospital-Herlev, Herlev, Denmark; 8Department of Lung- and Infectious Diseases, North Zealand Hospital-Hilleroed, Hilleroed, Denmark; 9Department of Internal Medicine and Infectious Diseases, Zealand University Hospital, Roskilde, Denmark; 10Department of Medical Gastroenterology and Hepatology, Copenhagen University Hospital-Rigshospitalet, Copenhagen, Denmark; 11Department of Gastroenterology, Copenhagen University Hospital-Hvidovre, Hvidovre, Denmark; 12Department of Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark; 13Diagnostic Center, Silkeborg Regional Hospital, Silkeborg, Denmark; 14Department of Internal Medicine, Kolding Hospital, Kolding, Denmark; 15Department of Infectious Diseases, Copenhagen University Hospital-Rigshospitalet, Copenhagen, Denmark; 16Department of Infectious Diseases, Odense University Hospital, Odense, Denmark; 17Clinical Institute, University of Southern Denmark, Odense, Denmark
Correspondence: Nina Weis, Department of Infectious Diseases, Copenhagen University Hospital – Hvidovre, Kettegaard Alle 30, Hvidovre, DK-2650, Denmark, Tel +45 3862 3514, Email [email protected]
Objective: Data on the risk of ischemic heart disease (IHD) in patients with chronic hepatitis B virus (CHB) are conflicting. Our objective was to address the rate of IHD in patients with CHB compared with individuals without CHB (control-persons) from the general population.
Study Design and Setting: We conducted a cohort study of prospectively obtained data from Danish nationwide registries. We produced cumulative incidence curves and calculated the unadjusted incidence rate ratio (IRR) of IHD in persons with and without CHB. The adjusted association between having CHB and developing IHD was examined using a cause-specific Cox regression model.
Results: In total, 6472 persons with CHB and 62,251 age- and sex-matched individuals from the general population were followed for 48,840 and 567,456 person-years, respectively, during which 103 (1,59%) with CHB and 1058 (1,70%) control-persons developed IHD. The crude IRR was 1.13 (95% CI: 0.91– 1.39). CHB did not have a statistically significant effect on the rate of IHD after adjusting for several confounding factors (adjusted hazard ratio: 0.96, 95% CI: 0.76– 1.21).
Conclusion: In this nationwide cohort study, we did not find any difference between rate of IHD in persons with CHB in comparison with the general population.
Keywords: hepatitis B virus, viral infection, atherosclerosis, coronary heart disease, ischemic heart disease, cardiovascular disease, general population
Introduction
The relation between chronic hepatitis B (CHB) and morbidity and mortality from complications such as cirrhosis and hepatocellular carcinoma is well established.1–5 While the epidemiological association between other infectious pathogens inducing chronic infection, eg, human immunodeficiency virus (HIV) and hepatitis C virus (HCV), and the development of atherosclerotic cardiovascular disease (CVD) is substantial,6,7 our current understanding of the effects of chronic hepatitis B virus (HBV) infection on the development of CVD is insufficient. The atherogenic effect of chronic infections is thought to result from inflammatory and immune-mediated pathogenic mechanisms.8 However, in terms of chronic HBV infection and CVD, results on the association are inconsistent. Few studies suggest a protective effect of HBV on the risk of CVD9,10 whereas other studies have shown no effect11–14 or increased risk of CVD.15 Depending on its nature, an association may have clinical implications for cardiovascular monitoring of CHB patients.
To address the risk of ischemic heart disease (IHD) in persons with CHB in comparison with the general population, we conducted a cohort study using data from multiple Danish nationwide registries.
Materials and Methods
Data Sources
The Danish Database for Hepatitis B and C (DANHEP)
DANHEP is a clinical database containing information about patients living with chronic hepatitis B or C in Denmark. Established on January 1, 2002, DANHEP is ongoing and open-ended. Healthcare services, including antiviral therapy, are publicly funded and free of charge to the individual in Denmark. Patient data are submitted provided that the patient is at least 16 years of age, has CHB (positive for HBsAg > 6 months) or chronic hepatitis C and has had at least one visit to a Danish hospital unit specialized in gastroenterology or infectious diseases. DANHEP contains individual information on each patient about demographics, HBV antigen and antibody status, hepatitis C virus (HCV), HIV and hepatitis D status, and liver stiffness measurement (LSM) with transient elastography value ((TE), since 2008). DANHEP has been described in detail elsewhere.16,17
The Danish Viral Hepatitis Laboratory Cohort (DANVIR)
DANVIR is a research database that includes a cohort of all patients that have been tested for HBV or HCV since 2000 at 1 of 14 of the 18 laboratories that perform testing for viral hepatitis DNA, RNA, antigens and antibodies in Denmark. It is estimated that the cohort includes more than 90% of the patients tested for HBV or HCV in Denmark.17
The Danish National Patient Registry (NPR)
Established in 1977, the NPR contains information about every contact of all individuals, identified through their unique personal identification number (PIN), with the Danish hospital service in regard to examinations, treatments, etc.18
The Danish Registry of Causes of Death (DRCD)
The DRCD contains information about cause(s) of death for nearly every deceased individual in Denmark since 1970. Individuals are identified through their unique PIN. Causes of death are recorded by a medical doctor and are classified using the ICD-10 system, previously the ICD-8 system.19
The Danish Civil Registration System (CRS)
The CRS is a national Danish registry containing demographic information, such as age, sex, immigration status, employment, and education, going back to 1968, on any individual with a Danish civil registration number. All citizens in Denmark are provided with this unique 10-digit number in both the CRS as well as most other Danish registries, permitting cross-linking of information between multiple registries.20
Study Population
The study population consisted of individuals aged 18 or older who were diagnosed with CHB between January 1, 2002 and January 1, 2017. Subjects were considered infected with CHB if they (i) were registered in DANHEP with at least one positive HBsAg measurement or (ii) were registered in DANVIR with two positive measurements of HBsAg 6 months or longer apart, or one positive HBsAg measurement as well as one negative measurement of IgM antibody to hepatitis B core antigen (anti-HBc IgM), or one positive measurement of HBsAg as well as a country of origin outside Northern Europe if there was no measurement of anti-HBc IgM.21 Each individual was assigned an index date corresponding to either (i) their date of inclusion in DANHEP, DANVIR or LPR or (ii) their 18th birthday, whichever came last.
For each person with CHB, 10 individuals without CHB (control-persons), matched by sex and age, were selected at random from the general population. Control-persons were assigned the same index date as their corresponding case. Individuals with a history of HIV or HCV infection, cerebrovascular ischemia or IHD prior to the study index date were excluded as these outcomes are related to atherogenesis and thus an increased risk of IHD.7,22 See Supplementary Material 1 for ICD-8 and ICD-10 codes used.
Outcome Measures
The outcome of interest was an event of IHD or death from IHD. The term IHD was considered substitutable with the terms coronary artery disease (CAD) and coronary heart disease (CHD).23 Any diagnosis of IHD would register as an event. Events of IHD were identified by ICD-8 and ICD-10 codes obtained from the NPR or DRCD. We defined IHD as ICD-10 codes I20-I25 and ICD-8 codes 41,009, 41,099, 41,109, 41,199, 41,209, 41,299, 41,309, 41,399, 41,409 and 41,499. The positive predictive value (PPV) in the NPR for I21-I23 codes has been estimated to be 98% (95% CI: 89.4–99.9).24 However, concerning I20-codes in the NPR, the PPV has been shown to be 42.0%.25 Thus, any I20-code that did not require ischemia to be documented was only regarded as valid if the individual, within 1 month from the date of diagnosis, was also registered with a procedure (SKS) code for either (i) coronary anastomosis, (ii) coronary bypass, (iii) coronary thromboendarterectomy or (iv) coronary recanalization. See Supplementary Material 1 for details on ICD-8, ICD-10 and SKS-codes used.
Variables
Comorbidity
We calculated the Charlson Comorbidity Index (CCI) score for each individual. Relevant ICD-10 diagnoses as described by Quan et al26 were obtained from the NPR (See Supplementary Material 2 for details). Comorbidity levels were dichotomized: none (CCI score = 0) and any (CCI score >0). Liver diseases were not scored on the CCI as they were considered potential mediators for IHD as cirrhosis may produce coagulation defects. The PPV of ICD-10 codes in the NPR used for calculating a CCI score is 98% (95% CI: 96.9.98.8).24
Employment
Information on employment status was obtained from the CRS, and subjects were categorized as either employed, unemployed, disability pensioner (individuals with a permanent disability limiting their ability to work), or other (eg, students and early retired individuals).
Alcohol
Subjects with an ICD-8 or ICD-10 diagnosis of alcohol abuse were identified; the information was obtained from the Danish National Registry of Mental or Behavioral Disorder. See Supplementary Material 1 for details on codes used.
Region of Origin
Country of origin was defined as mother’s country of birth. Information was obtained from the CRS. Countries of origin were subsequently categorized in regions through the United Nations area distribution.27,28
Type 2 Diabetes Mellitus (T2DM)
Any subject with a diagnosis code corresponding with T2DM in ICD-8 or ICD-10 in the NPR was included in this group. Moreover, any subject with a filled prescription of antidiabetic medication in the prescription registry, except from insulin, was included. For the relevant ICD-8, ICD-10 and prescription (ATC) codes, see Supplementary Material 1.
Cirrhosis
Subjects with an ICD-8 or ICD-10 diagnosis of liver cirrhosis were identified from the NPR. See Supplementary Material 1 for relevant codes.
Treatment
Information on antiviral treatment status was obtained from DANHEP. Treatment status was dichotomized according to whether the subject had ever received antiviral treatment.
Statistical Analysis
Demographics and characteristics of the study cohort were summarized using median and interquartile ranges for age, and frequency and percentage for categorical variables. Time at risk was calculated from index date until (i) diagnosis of IHD or death from IHD, both counted as an event, (ii) death from other cause was considered a competing risk, (iii) emigration, (iv) unknown status in CRS or (v) January 1, 2017, whichever came first led to censoring.
A significance level of 5% was used in all analyses. Statistical analyses were performed in STATA 16.1 and R 4.0.5.29,30
Main Analysis
In the adjusted analysis, factors considered possible confounders were age, sex, employment, region of origin, type 2 diabetes mellitus, comorbidity, and alcohol abuse. These were fitted as baseline variables in a Cox model. The proportional hazards assumption was assessed by plotting Schoenfeld residuals against time and comparing the observed curve against 50 simulated curves assuming proportional hazards. Age at baseline did not show proportional hazards and was stratified.
Cumulative incidence of IHD over time was determined using cumulative incidence functions with death from other causes considered competing risks. To assess if CHB has an effect on cumulative incidence of IHD after adjustment, we calculated sub-distribution hazards using a Fine–Gray model.
Sub-Analysis 1
To assess the effect of liver cirrhosis on the development of IHD, we adjusted the Cox model using time-updated cirrhosis status as an additional variable. Furthermore, we conducted a log-likelihood ratio test for interaction between cirrhosis and CHB.
Sub-Analysis 2
Since persons with CHB in Denmark predominantly are of foreign origin, lifestyle factors such as smoking habits might differ from those in persons with Danish origin. Further, when we assessed our data, persons with CHB were registered with a drug- or alcohol-related diagnosis more frequently than sex- and age-matched controls from the general population. Hence, we suspected that smoking rates among HBV-positive individuals of Danish origin may also be higher when compared to controls. Consequently, we suspected some residual confounding despite adjusting for region of origin and therefore conducted a separate cause-specific Cox analysis in which we excluded persons of Danish origin.
Sub-Analysis 3
In this analysis, we sought to assess the effect of being or having been treated with antiviral medication on the development of IHD we adjusted the Cox model using dichotomized treatment status as an additional variable.
Results
In total, 13,265 persons were identified and examined for eligibility. Out of these, a total of 6793 individuals were excluded: 6193 did not have an index date between January 1, 2002 and January 1, 2017 or were under 18 years of age (n=146) at the latter date; 40 had a prior diagnosis of ischemic cerebrovascular disease; 92 had a prior diagnosis of IHD; 109 had a prior diagnosis of HIV; 351 had a prior diagnosis of HCV; 8 persons died at date of diagnosis or before the age of 18. In total, 6472 persons with CHB and 63,607 control-persons were included in the analysis (Figure 1).
The median age of persons with CHB was 34.0 years and 51.6% were male. When compared to control-persons, CHB infected individuals were more likely to be unemployed (25.1% versus 7.9%), to be persons on disability pension (8.5% versus 3.8%), to not have Denmark as country of origin (31.0% versus 87.3%), to have comorbidities (8.7% versus 7.0%), to suffer from T2DM (4.8% versus 2.5%) and to have an alcohol-related diagnosis (5.2% versus 1.4%) (Table 1).
During the 571,095 person-years of follow-up (median 7.93 years), 48,840 years for cases and 523,065 years for control-persons, 103 with CHB experienced an event of IHD (1.6%), while 874 subjects in the control group experienced an event (1.4%). Further details on the entire study cohort are presented in Table 1.
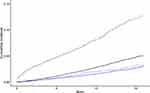
The cumulative incidence of IHD and death from other causes for persons with CHB and control-persons is plotted in Figure 2. Between these groups, the plotted cumulative incidence did not differ significantly during the 15-year follow-up period. However, the cumulative incidence of death from other causes was significantly larger for persons with CHB.
The estimated crude IRR of IHD events for individuals with CHB and control-persons was 1.13 (95% CI: 0.91–1.39). Table 2 shows the estimates for the effect of chronic HBV infection as well as other covariates on the rate of development of IHD and the cumulative incidence of IHD.
As seen in Table 2, CHB infection did not display a significant effect on the rate of development of IHD events with an estimated adjusted hazard ratio of 0.96 (95% CI: 0.75–1.22). Diabetes, male sex, not being employed, a country of origin in North Africa or Western Asia and comorbidity were found to be significantly associated with an increase in the rate of development of IHD. Further, chronic HBV infection did not display a significant effect on the cumulative incidence of IHD (subdistribution hazard ratio: 0.85, 95% CI: 0.63–1.07).
Adjusting for time-updated cirrhosis-status in sub-analysis 1, cirrhosis did not have a significant effect on the rate of development of IHD (HR: 0.40, 95% CI: 0.15–1.08). Furthermore, we did not find interaction between cirrhosis and CHB.
After exclusion of subjects of Danish origin in sub-analysis 2, the cause-specific Cox analysis of the correlation between CHB infection and IHD still did not yield a statistically significant result (HR: 0.60, 95% CI: 0.28–1.44).
When adjusting for antiviral treatment status in sub-analysis 3, antiviral treatment did not influence the development of IHD (HR: 0.38, 95% CI: 0.73–2.93).
Discussion
In this nationwide cohort study of prospectively collected data, we found no increased risk of IHD in persons with CHB. The difference in absolute risk of developing IHD between persons with CHB and age- and sex-matched individuals from the general population was not significant, although persons with CHB may have died from competing causes before a hypothetical event of IHD occurred. Furthermore, the cause-specific rate of development of IHD did not differ significantly between individuals with CHB and age- and sex-matched individuals from the general population. Predictably, we found male sex, comorbidity, T2DM, and not being employed to significantly increase the rate of development of IHD. Although the effect of cirrhosis was slightly protective on the development of IHD, this was not statistically significant. When assessing the interaction between cirrhosis and CHB infection, we found none. However, this finding should be interpreted carefully due to the low number of events of cirrhosis in persons with CHB infection (n = 258). Furthermore, being or ever having been treated with antiviral medication did not significantly affect the development of IHD. Although we cannot exclude the possibility of a false-negative result, we consider our estimates to be reliable given the size of our cohort.
Yet, our understanding of the potential underlying pathogenic effects of CHB infection on the process of atherogenesis and the development of IHD is limited. CHB infection induces a state of chronic inflammation and immune stimulation known to result in a range of liver complications.31,32 This has been proposed as the basis for a possible explanation of an association between chronic HBV infection and the development of IHD33 in light of existing associations between other infections as eg HIV and HCV and atherosclerosis.8,34 In contrast, findings contributing to the case for a potential protective effect of CHB infection on the development of IHD have also been suggested. One study found HBV seropositivity to be associated with lower mean C-reactive protein (CRP) levels,12 although contradicting findings of significantly elevated serum CRP levels in individuals with CHB also have been reported.35 Furthermore, liver cirrhosis (of any type) has been suggested to correlate with lower serum triglyceride levels36 and CHB infection has been linked to decreased cholesterol levels.37 Additionally, one study reported lower levels of fibrinogen, clotting factor II and clotting factor VII in HBsAg-positive subjects compared to HBsAg-negative subjects.38 A recent meta-analysis of 13 observational studies found that subjects with CHB infection had a lower risk of developing metabolic syndrome, a known cardiovascular risk factor, and further showed an inverse relation between CHB infection and elevated blood pressure, low HDL-cholesterol, and hypertriglyceridemia in certain subgroups.39
Previous studies evaluating the effect of chronic HBV infection on the development of CVD have observed conflicting results. One sizeable cohort study of Korean men found CHB infection to be associated with a lower hazard of myocardial infarction (HR: 0.74, 95% CI: 0.62–0.87) when adjusting for a number of cardiovascular risk factors.10 When further adjusting for liver dysfunction, the effect was more pronounced but only significant in CHB infected individuals with liver dysfunction (HR: 0.56, 95% CI: 0.45–0.7). Thus, the protective effect was ascribed to liver dysfunction with impaired coagulation status as a result of CHB infection. These findings are in trend with the slightly protective but non-significant effect of cirrhosis we showed (HR: 0.44, 95% CI: 0.18–1.07). In contrast to these findings, another large and more recent Chinese prospective cohort study reported an increased hazard of death from IHD in persons with CHB (HR: 1.31, 95% CI: 1.09–1.58).33 However, this effect was not present when the model was adjusted for whether subjects had had previous hepatitis of any kind or cirrhosis at baseline (HR: 1.25, 95% CI: 0.62–2.50). In contrast, one 2016 meta-analysis of three cross-sectional studies, one case–control study and one cohort study did not show a significantly increased risk of CAD in HBV-infected persons (pooled odds ratio (OR): 0.68, 95% CI: 0.4–1.13).40 Similarly, another 2018 review of the literature and meta-analysis of three case–control studies and six cohort studies did not demonstrate an increased risk of CHD in HBV-infected persons (risk ratio: 0.99, 95% CI: 0.76–1.22).41 However, both meta-analyses reported an either moderate or high degree of heterogeneity among the included studies. This can in part be ascribed to differences between the included studies regarding particularly study design and demographic characteristics, adjustment of effect variates, and criteria for outcome definitions; issues that seem to apply to studies extending beyond the ones were included in the meta-analyses.
Our results add to the overall trend of the current evidence suggesting that chronic HBV infection has no effect on the risk of IHD. The strengths of the present study include a large nationwide cohort with a long-term follow-up period, knowledge about co-infection with HIV and HCV, and data obtained for analysis from nationwide registries as made possible by a tax-funded health care system with equal accessibility. However, limitations such as effects of residual confounding may be present. We did not adjust for cardiovascular risk factors such as elevated body mass index (BMI) and elevated plasma triglyceride levels. However, BMI levels in CHB-infected individuals seem not to differ from that of the general population, and findings suggest CHB infection and plasma triglycerides levels to be inversely associated.39 Thus, we find these lifestyle factors unlikely to introduce substantial confounding bias. Lack of data on smoking status was a limitation. However, in sub-analysis 2, we indirectly adjusted for smoking status through excluding subjects of Danish origin as we did not expect significant differences in lifestyle risk factors between the remaining individuals with and without CHB infection born in endemic countries. In doing so, we found no increased risk of IHD in persons with CHB. However, smoking may yet contribute a residual confounding effect as the analysis lacked statistical power.
In conclusion, we found no increased risk of ischemic heart disease in persons with CHB compared with sex- and age-matched control-persons from the general population in this nationwide study with 15 years of follow-up. To the best of our knowledge, the present study is the first of its size to assess risk of ischemic heart disease in persons with CHB in comparison with the general population on a nationwide level in an HBV low-endemic country. Our findings indicate that strategy for prevention and management of ischemic heart disease in persons with CHB should be similar to that of the general population.
Data Sharing Statement
Data used in the present study cannot be shared due to Danish data protection rules and regulations.
Ethics Statement
The study has been approved by the Danish Data Protection Agency (j.nr. P-2019-829) as required by Danish Law. No further approvals were required.
Acknowledgments
We extend our gratitude to all the patients who participated in the DANHEP cohort study.
Funding
Signe Bollerup has received funding from Manufacturer Vilhelm Pedersen and Wife’s Foundation; Free research funds of University Hospital of Copenhagen, Amager and Hvidovre; Grant in memory of Carpenter Jørgen Holm and wife Elisa B. Hansen (1906–1948); Aase and Ejnar Danielsens Foundation and Scandinavian Society for Antimicrobial Chemotherapy Foundation (SLS-935536).
Disclosure
NW has been a clinical investigator, lecturer or member of advisory boards for AbbVie, GSK, MSD. Gilead and has received unrestricted grants for research from AbbVie and Gilead, with no relation to the present work. PBC has received unrestricted grants for research from AbbVie, Gilead, and MSD, not related to this study. FE reports personal fees from MSD, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61(10):1942–1956. doi:10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j
2. Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392(10161):2313–2324. doi:10.1016/S0140-6736(18)31865-8
3. Andersen E, Omland L, Jepsen P, et al. Risk of all-type cancer, hepatocellular carcinoma, non-Hodgkin lymphoma and pancreatic cancer in patients infected with hepatitis B virus. J Viral Hepat. 2015;22(10):828–834. doi:10.1111/jvh.12391
4. Amin J, Dore GJ, O’Connell DL, et al. Cancer incidence in people with hepatitis B or C infection: a large community-based linkage study. J Hepatol. 2006;45(2):197–203. doi:10.1016/j.jhep.2006.02.014
5. Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med. 2004;350(11):1118–1129. doi:10.1056/NEJMra031087
6. Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation. 2018;138(11):1100–1112. doi:10.1161/CIRCULATIONAHA.117.033369
7. Lee KK, Stelzle D, Bing R, et al. Global burden of atherosclerotic cardiovascular disease in people with hepatitis C virus infection: a systematic review, meta-analysis, and modelling study. Lancet Gastroenterol Hepatol. 2019;4(10):794–804. doi:10.1016/S2468-1253(19
8. Pothineni NVK, Subramany S, Kuriakose K, et al. Infections, atherosclerosis, and coronary heart disease. Eur Heart J. 2017;38(43):3195–3201. doi:10.1093/eurheartj/ehx362
9. Kumar A, Shariff M, Doshi R. Association between past hepatitis B infection and ischemic heart disease: an analysis from the 2007–2016 NHANES data. Am J Med Sci. 2020;360(4):372–377. doi:10.1016/j.amjms.2020.05.034
10. Sung J, Song YM, Choi YH, Ebrahim S, Davey Smith G. Hepatitis B virus seropositivity and the risk of stroke and myocardial infarction. Stroke. 2007;38(5):1436–1441. doi:10.1161/STROKEAHA.106.466268
11. Momiyama Y, Ohmori R, Kato R, Taniguchi H, Nakamura H, Ohsuzu F. Lack of any association between persistent hepatitis B or C virus infection and coronary artery disease. Atherosclerosis. 2005;181(1):211–213. doi:10.1016/j.atherosclerosis.2005.02.027
12. Tong DY, Wang XH, Xu CF, Yang YZ, Xiong SD. Hepatitis B virus infection and coronary atherosclerosis: results from a population with relatively high prevalence of hepatitis B virus. World J Gastroenterol. 2005;11(9):1292–1296. doi:10.3748/wjg.v11.i9.1292
13. Ghotaslou R, Aslanabadi N, Ghojazadeh M. Hepatitis B virus infection and the risk of coronary atherosclerosis. Ann Acad Med Singap. 2008;37(11):913–915.
14. Katoonizadeh A, Ghoroghi S, Sharafkhah M, et al. Chronic hepatitis B infection is not associated with increased risk of vascular mortality while having an association with metabolic syndrome. J Med Virol. 2016;88(7):1230–1237. doi:10.1002/jmv.24466
15. Ishizaka N, Ishizaka Y, Takahashi E, et al. Increased prevalence of carotid atherosclerosis in hepatitis B virus carriers. Circulation. 2002;105(9):1028–1030. doi:10.1161/hc0902.105718
16. Hansen N, Obel N, Christensen PB, et al. Predictors of antiviral treatment initiation in hepatitis C virus-infected patients: a Danish cohort study. J Viral Hepat. 2009;16(9):659–665. doi:10.1111/j.1365-2893.2009.01126.x
17. Omland LH, Jepsen P, Skinhøj P, et al. The impact of HIV-1 co-infection on long-term mortality in patients with hepatitis C: a population-based cohort study. HIV Med. 2009;10(2):65–71. doi:10.1111/j.1468-1293.2008.00652.x
18. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(7 Suppl):30–33. doi:10.1177/1403494811401482
19. Juel K, Helweg-Larsen K. The Danish registers of causes of death. Dan Med Bull. 1999;46(4):354–357.
20. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–25. doi:10.1177/1403494810387965
21. Hansen N, Hay G, Cowan S, et al. Hepatitis B prevalence in Denmark – an estimate based on nationwide registers and a national screening programme, as on 31 December 2007. Eurosurveillance. 2013;18(47):20637. doi:10.2807/1560-7917.ES2013.18.47.20637
22. Islam MF, Wu J, Jansson J, Wilson D. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13(8):453–468. doi:10.1111/j.1468-1293.2012.00996.x
23. Criteria I of M (US) C on SSCD. Ischemic Heart Disease. National Academies Press (US); 2010. Available from: https://www.ncbi.nlm.nih.gov/books/NBK209964/.
24. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11(1):83. doi:10.1186/1471-2288-11-83
25. Joensen AM, Jensen MK, Overvad K, et al. Predictive values of acute coronary syndrome discharge diagnoses differed in the Danish National Patient Registry. J Clin Epidemiol. 2009;62(2):188–194. doi:10.1016/j.jclinepi.2008.03.005
26. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi:10.1093/aje/kwq433
27. SDG Indicators — SDG Indicators. Available from: https://unstats.un.org/sdgs/indicators/regional-groups.
28. UNSD — Methodology. Available from: https://unstats.un.org/unsd/methodology/m49/.
29. Stata: Software for statistics and data science. Available from: https://www.stata.com/.
30. RStudio | Open source & professional software for data science teams. Available from: https://rstudio.com/.
31. Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA. 2018;319(17):1802–1813. doi:10.1001/jama.2018.3795
32. Yu Y, Gong R, Mu Y, et al. Hepatitis B virus induces a novel inflammation network involving three inflammatory factors, IL-29, IL-8, and cyclooxygenase-2. J Immunol. 2011;187(9):4844–4860. doi:10.4049/jimmunol.1100998
33. Si J, Yu C, Guo Y, et al. Chronic hepatitis B virus infection and total and cause-specific mortality: a prospective cohort study of 0.5 million people. BMJ Open. 2019;9(4):e027696. doi:10.1136/bmjopen-2018-027696
34. Epstein SE, Zhu J, Najafi AH, Burnett MS. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation. 2009;119(24):3133–3141. doi:10.1161/CIRCULATIONAHA.109.849455
35. Hao S, Wang Y, Gao G, Hepatitis LZ. B virus upregulates the expression of C-reactive protein both in vivo and in vitro. Ann Clin Lab Sci. 2017;47(4):432–435.
36. Marchesini G, Ronchi M, Forlani G, et al. Cardiovascular disease in cirrhosis–a point-prevalence study in relation to glucose tolerance. Am J Gastroenterol. 1999;94(3):655–662. doi:10.1111/j.1572-0241.1999.00931.x
37. Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: a cohort study. Hepatology. 2017;65(3):828–835. doi:10.1002/hep.28917
38. Meade TW, Stirling Y, Thompson SG, Ajdukiewicz A, Barbara JAJ, Chalmers DM. Carriers of hepatitis B surface antigen: possible association between low levels of clotting factors and protection against ischaemic heart disease. Thromb Res. 1987;45(5):709–713. doi:10.1016/0049-3848(87)90336-7
39. Razi B, Alizadeh S, Omidkhoda A, Imani D, Rezaei R. Association of chronic hepatitis B infection with metabolic syndrome and its components: meta-analysis of observational studies. Diabetes Metab Syndr. 2017;11(Suppl 2):S939–S947. doi:10.1016/j.dsx.2017.07.020
40. Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Hepatitis B virus infection and risk of coronary artery disease: a meta-analysis. Ann Transl Med. 2016;4(21):423. doi:10.21037/atm.2016.11.12
41. Wang Y, Xiong J, Niu M, Xu W, Xu K, Zhong H. Hepatitis B virus and the risk of coronary heart disease: a comprehensive systematic review and meta-analyses of observational studies. Int J Cardiol. 2018;265:204–209. doi:10.1016/j.ijcard.2018.04.059
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.