Back to Journals » Psoriasis: Targets and Therapy » Volume 12
Comorbid Psoriasis and Metabolic Syndrome: Clinical Implications and Optimal Management
Authors De Brandt E, Hillary T
Received 7 March 2022
Accepted for publication 30 April 2022
Published 25 May 2022 Volume 2022:12 Pages 113—126
DOI https://doi.org/10.2147/PTT.S293107
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Uwe Wollina
Eveline De Brandt, Tom Hillary
Department of Dermatology, University Hospitals Leuven, Leuven, Belgium
Correspondence: Tom Hillary, Department of Dermatology, University Hospitals Leuven, Herestraat 49, Leuven, 3000, Belgium, Tel +3216337950 Email [email protected]
Purpose: To review the literature on guidance on the follow-up of psoriasis and its comorbidities and to provide practical recommendations.
Patients and Methods: A PubMed search was conducted using MeSH terms and free text keywords related to “psoriasis”, “obesity”, “hypertension”, “diabetes”, “dyslipidemia”, “metabolic syndrome” and “Psoriatic arthritis”. The search was conducted between September 2021 and January 2022. References of selected articles were scanned to identify additional articles.
Results: Recommendations on the follow-up of hypertension, obesity, dyslipidemia, type 2 diabetes, metabolic syndrome, psoriatic arthritis, non-alcoholic fatty liver disease and inflammatory bowel disease in psoriasis patients were extracted from the included articles. These data are presented in summary tables for both adults and children. A practical and feasible approach for each comorbidity is discussed.
Conclusion: Awareness among dermatologists for relevant psoriasis-associated comorbidities is crucial. The dermatologist should function as gatekeeper and screen for comorbidities, in order to make timely referrals when indicated.
Keywords: psoriasis, comorbidities, metabolic syndrome, psoriatic arthritis, screening, atherosclerosis, liver disease, cardiovascular disease
Introduction
Chronic plaque psoriasis is an inflammatory skin disease affecting 2% to 3% of the general adult population.1 Psoriasis symptoms can occur at any age, however there are 2 peaks of onset: the first at age 20 to 30 years and the second at age 50 to 60 years.2 Together with psoriatic arthritis, it comprises the psoriatic disease spectrum. However, it is a multimorbid condition: comorbidities including diabetes, dyslipidemia, arterial hypertension or metabolic associated fatty liver disease (MAFLD) (formerly known as nonalcoholic fatty liver disease (NAFLD)) have been described to be thoroughly associated with psoriatic disease. Since 1981, several morbidities (abdominal obesity, high triglyceride level, low HDL, high blood pressure, high fasting blood sugar) have been clustered into the concept of Metabolic Syndrome (MetS).3 The prevalence of MetS in psoriasis patients ranges from 20% to 50%, this is up to three times higher compared to the general population.4–6 Furthermore, obesity, dyslipidemia, and diabetes are associated with greater psoriasis disease severity and reduced response to treatment.7–9 The pathogenesis of MetS is multifactorial, with a combination of nutritional, environmental, and genetic factors. Chronic low-grade inflammation together with visceral adipose tissue, adipocyte dysfunction, and insulin resistance plays a major role in the progression of the syndrome by impairing lipid and glucose homeostasis in insulin-sensitive tissues, such as the liver, muscle, and adipocytes.10 To date, the exact mechanism that links MetS to psoriasis is not fully understood.
More than one decade ago, the concept of the psoriatic march has been proposed.11 In this concept, it was suggested that psoriatic inflammatory cells predispose to cardiovascular disease, hence defining psoriasis as an independent cardiovascular risk factor.12,13
In recent years, the understanding of the underlying pathophysiology of psoriasis has increased exponentially, and IL-23 has been identified as the culprit for the pathogenic Th17-cells, which lead to the cutaneous psoriatic changes.14 These insights have subsequently led to multiple new treatment targets. Of interest, IL-17A, a cytokine released by TH17 cells, appears to play a central role in the pathophysiology/development of atherosclerotic plaques.15 Furthermore, as in MAFLD, the IL23-Th17 axis has been identified as a crucial immunological pathway.16,17 In addition, diabetes mellitus (DM), hypertension, dyslipidemia, obesity and insulin resistance share an upregulation of inflammatory cytokines like IFN-γ, IL-23 and TNF-α.18
Dermatologists should play an important role in the early recognition and assessment of psoriasis associated comorbidities. Reassuringly, a recent survey among dermatologists in Belgium showed high awareness of these comorbidities, which is a prerequisite for screening.19
In this review, we present the existing literature on recommendations for the screening and prevention of psoriasis-associated comorbidities in patients of all ages. We aim to provide a practical overview and recommendation for implementation in daily practice.
Materials and Methods
A PubMed search was conducted using MeSH terms and free text keywords related to “psoriasis”, “psoriatic arthritis”, “obesity”, “hypertension”, “diabetes”, “dyslipidemia”, and “metabolic syndrome”. Searches were limited to publications in the English language. After screening the title and abstract, relevant articles were included for full text assessment with no time frame. The search was conducted between September 2021 and January 2022. References of selected articles were scanned to identify additional articles.
Results
Arterial Hypertension/Cardiovascular Disease
Adults
A strong association between psoriasis and cardiovascular disease has been found in multiple studies.16,18 Arterial hypertension (aHT) is a leading risk factor for cardiovascular morbidity and mortality.20 aHT is defined as a blood pressure (BP) above 130/80 mmHg according to the ACC/AHA guidelines and 140/90 mmHg according to the ESC/ESH, NICE, and ISH guidelines.21 Given that psoriasis patients have an inherently increased risk of developing aHT, dermatologists need to give special consideration to this comorbidity when following up and treating psoriasis patients.16
Current evidence does not support restrictions on the use of antihypertensive medication in patients with psoriasis.22 However, it has been reported that long-term regular use of β-blockers may increase the risk of developing psoriasis.23 Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated preferably with amlodipine.22
Psoriasis cohort studies have shown a significantly lower risk of myocardial infarction in patients taking TNF inhibitors, oral agents (like methotrexate) and phototherapy in comparison to control groups treated with topical agents.24,25 Table 1 shows some of the potential side effects of anti-psoriatic drugs on cardiovascular risk factors. We recommend regular monitoring of the blood pressure as part of the dermatological follow-up of the psoriasis patient. Furthermore, we emphasize the importance of patient education about psoriasis-related cardiovascular risks and promoting a healthy lifestyle (smoking cessation, exercise and dietary changes are encouraged to achieve and maintain a normal BMI).26
 |
Table 1 Potential Side Effects of Anti-Psoriatic Drugs on Cardiovascular Risk Factors |
Children
Only one retrospective study supports the association between pediatric psoriasis and hypertension.27 The American Academy of Pediatrics (AAP) expert panel guidelines recommend annual screening starting at the age of three. They state that primary prevention is best practiced at an early age.28 When aHT is recorded or multiple risk factors are present for cardiovascular disease (CVD) (eg obesity, family history, DM, …), a referral to the general practitioner (GP) or cardiologist is indicated.
Dyslipidemia
Adults
Dyslipidemia refers to the persistent elevation of serum cholesterol and/or triglycerides. Keye et al found in a case–control study that psoriasis patients hold a higher risk of dyslipidemia than controls without psoriasis.29 In 2018, the American Heart Association and the American College of Cardiology categorized psoriasis as an atherosclerotic CVD risk-enhancing condition, favouring early intervention with a statin.30 Elmets et al recommend to screen patients with moderate-to-severe psoriasis periodically with fasting lipid tests (fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).22 He also states that non-fasting screening is preferable to not screening at all. Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their GP for further management.31
We recommend annual monitoring of non-fasting lipid panel. We consider more frequent screening useful in patients treated with lipid-altering medication, or with uncontrolled moderate-to-severe psoriasis.
Children
According to international guidelines, having psoriasis does not warrant additional lipid screening beyond the general age-related recommendations for all children. Of interest, both the National Heart, Lung and Blood Institute and the AAP support universal screening for high blood lipids in all children, regardless of risk factors, between the ages of 9 and 11 years old and again at 17 and 21 years old.28
Outside of the specified age ranges, screening is also recommended in the presence of any additional cardiovascular risk factors (family history of cardiovascular disease (CVD), hypertension, smoking, obesity, HDL <40 mg/dL, Type 1 or 2 DM, renal disease, nephrotic syndrome, post-orthotopic heart transplant, Kawasaki disease with current or regressed aneurysms, HIV, chronic inflammatory disease).32 Healthcare providers should be mindful of LDL-cholesterol variations in teens between 12 and 17 years old.33
We recommend annual monitoring of non-fasting lipid panel in children or adolescents with uncontrolled psoriasis. We consider more frequent screening in patients treated with lipid-altering medication.
Obesity
Adults
Similar to psoriasis, an upregulation of Th17 and Th1 helper cells has been shown in patients with obesity. Therefore, the co-occurring of both conditions could exacerbate each other.34 For recommendations on obesity management, we refer to the MetS section.
Children
Boccardi et al found an association between new-onset psoriasis and pre-existing overweight, especially in boys and children under the age of 10.35 Children with obesity are more likely to have severe psoriasis compared to normal-weight psoriatic children.36 Joint American Academy of Dermatology-National Psoriasis Foundation guidelines state that these children should be routinely assessed for obesity by either their dermatologist or primary care provider.37
Metabolic Syndrome
Adults
Metabolic syndrome (MetS) is a descriptive term for the co-occurrence (of at least three) of obesity, hypertension, hypertriglyceridemia, hypercholesterolemia and insulin resistance in an individual. MetS is associated with increased cardiovascular morbidity and mortality.38 The prevalence of MetS is up to three times higher in patients with psoriasis compared to the general population, and this may impact the treatment of choice in certain patients.4–6 For example, apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.31
Furthermore, attention for possible drug interactions between MetS treatment and psoriasis treatment is required: the combination of cyclosporine and statins has a higher risk of rhabdomyolysis than statins alone. Additionally, awareness of drug-induced comorbidities (eg cyclosporine-induced hypertension and dyslipidemia) is warranted.39
In recent studies, the use of pioglitazone has been recommended in patients with metabolic syndrome because of its beneficial effect on weight and glucose levels. There is limited evidence that pioglitazone may also have a positive effect on psoriasis.40
The AAD guidelines encourage an annual measurement of the waist circumference by the dermatologist or primary care provider in patients with moderate-to-severe psoriasis. Additionally, these guidelines state that blood pressure measurement, fasting serum glucose, hemoglobin A1c and fasting lipid levels should be performed by the GP annually.31 We refer to screening recommendations on the separate components of MetS. In addition, we promote educating psoriasis patients about the increased risk of MetS, its association with cardiovascular disease and preventive measures.
Children
In recent years, the incidence of metabolic syndrome in children has increased significantly.41
Similar to adults with psoriasis, studies have suggested an association between the incidence of psoriasis and obesity in children. This association is even more significant in children with severe psoriasis, including adjustment for previous or active treatment with systemic immunosuppressants (including systemic corticosteroids).42
Type 2 Diabetes
Adults
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia induced by defects in insulin production or action. Chronic hyperglycemia is associated with long-term organ damage.43 Since many diabetes-related complications can be prevented, early diagnosis and treatment are essential.
Type 2 diabetes was reported to be significantly more prevalent in a cohort with mild-moderate psoriasis (37.4%) versus a healthy control group (16%; aOR 3.137, 95% CI 2.675–3.68, P = 0.00001). This association became even more apparent in patients with severe psoriasis (41%) compared with healthy controls (16%; OR 3.77, 95% CI 2.60–5.47, P = 0.00001).44
These data suggest that severe psoriasis should be considered as an important risk factor for developing type 2 diabetes. We recommend annual screening for abnormal blood sugar (with HbA1c levels and fasting glucose) in all patients with moderate-to-severe psoriasis. Consider more frequent testing in patients with uncontrolled moderate-to-severe psoriasis. Patients with psoriasis who develop diabetes mellitus should be referred to their general practitioner for further assessment, lifestyle advice, treatment and follow-up.22
Children
Similarly, the risk of developing insulin resistance in children with psoriasis is increased in comparison with unaffected children. Two studies by Augustin et al found that the prevalence of insulin resistance in children with psoriasis is twice as high compared to a normal population.2,45 According to the American Diabetes Association, regular screening by measuring fasting serum glucose is advised every 3 years starting at 12 years in high-risk patients or 10 years in all obese pediatric patients. Risk factors (not including psoriasis) can be seen in Table 2.33,37,46 Since an association has been found between insulin resistance and psoriasis in children, it was suggested that psoriasis could be considered as an independent risk factor for developing type 2 diabetes mellitus.
 |
Table 2 Risk Factors for Developing Type 2 Diabetes in Children (Left Panel) and Adults (Right Panel) |
Psoriatic Arthritis
Adults
Psoriatic arthritis (PsA) is one of the most common comorbidities in psoriasis patients. It is classified as a seronegative spondyloarthritis, with predominantly peripheral involvement. Symptoms of arthritis, enthesitis or dactylitis predominate the clinical presentation. Alinaghi et al reported a pooled PsA prevalence of 19.7% in patients with psoriasis. In adults, the prevalence of PsA was 21.6%, by contrast the prevalence was much lower in children and/or adolescents with psoriasis at 3.3%. Interestingly, there seems to be a correlation between disease severity and risk of PsA: the prevalence of PsA was reported to be significantly higher in the group with moderate-to-severe psoriasis compared to the group with mild psoriasis (24.6% vs 15.8%).47
Adult patients tend to develop cutaneous manifestations of psoriasis first, on average 8.5 years before arthritis symptoms.33
Dermatologists should conduct a thorough anamnesis and physical examination to distinguish PsA from degenerative joint disease (illustrated in Table 3). Imaging and laboratory tests to evaluate for signs of inflammation (erythrocyte sedimentation rate, C-reactive protein) can be helpful in distinguishing these 2 conditions. However, such testing is neither sensitive nor specific for PsA. When the diagnosis PsA is suspected, referral to a rheumatologist is warranted.31
 |
Table 3 Comparison of Inflammatory and Degenerative Pain Pattern |
Children
PsA can occur at any age, but the onset in children is more common between 2 to 3 years and 9 to 12 years of age. In contrast to adults, 80% of children with PsA develop arthritis 2 to 3 years prior to cutaneous manifestations.48 In younger children, especially girls, PsA presents with an oligoarticular disease or dactylitis, while in older children, especially boys, it presents with enthesitis and axial joint involvement.33,49 Those patients typically present with inflammatory joint pain, joint swelling and morning stiffness. Osier et al suggests that dermatologists question these symptoms in children and conduct a thorough clinical examination with attention to signs of dactylitis, enthesitis, joint swelling, and decreased axial mobility.33 We recommend educating patients and parents about this comorbidity.
Non-Alcoholic Fatty Liver Disease
Adults
Non-Alcoholic Fatty Liver Disease (NAFLD) is defined by the presence of steatosis in 5% of hepatocytes or more. It is strongly associated with metabolic risk factors such as obesity or type 2 diabetes without a history of excessive alcohol consumption (≥30 g per day for men and ≥20 g per day for women) or chronic liver disease. In time, NAFLD can progress to non-alcoholic steatohepatitis (NASH). Untreated, NASH can evolve to irreversible scarring of the liver tissue (cirrhosis).50 The prevalence of NAFLD is estimated at a 25% in the general population and was seen 1.5 to 3 times more in psoriasis patients.51,52
While the exact underlying mechanisms are yet to be fully elucidated, both psoriasis and NAFLD are strongly associated with low-grade, chronic inflammation, insulin resistance and increased levels of oxidative stress.53–55 When NAFLD is suspected, it is important to assess risk factors (Table 4) and to check for advanced fibrosis or cirrhosis. Elastography is a frequently used non-invasive measurement of liver fibrosis. Other non-invasive scores to determine if NAFLD is present are the NAFLD score (http://nafldscore.com) and the FIB-4 index. The FIB-4 index uses a formula (https://www.hepatitisc.uw.edu/page/clinical-calculators/fib-4) based on a combination of age, platelets, AST and ALT.52
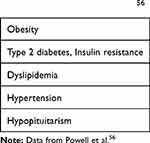 |
Table 4 Risk Factors NAFLD56 |
When NAFLD is suspected, referral to a hepatologist for confirmation of the diagnosis is recommended. Patients without advanced fibrosis should be monitored with biochemistry and repeat elastography every 3 to 5 years.56 Since ALT can remain normal for a long time in NAFLD, a degree of caution remains necessary.
Concerns about the toxicity of systemic medications (eg methotrexate or cyclosporine) have been raised: we recommend additional screening for NAFLD in psoriasis patients with risk factors.54,57
Children
NAFLD is one of the most common chronic liver diseases in children and adolescents: Anderson et al reported a prevalence of 7.6% in the overall pediatric population, and 34.2% in obese children.58,59 In a retrospective cohort study by Tollefson et al the rate of NAFLD in children with psoriasis was significantly higher compared to a healthy control group (HR, 1.76; 95% CI, 1.16–2.65).60
Osier et al advises ALT measurement as a screening tool for NAFLD in all overweight children with additional risk factors for insulin resistance (Table 2), starting at the age of 9–11 years. When initial assessment is normal, ALT measurement should be repeated every 2–3 years. Earlier reassessment may be needed if new risk factors develop. The upper limit of ALT is 22 U/L in girls and 25 U/L in boys.33
Inflammatory Bowel Disease
Patients with psoriasis have a higher risk of inflammatory bowel disease (IBD). Identified risk factors are onset of psoriasis at young age, low BMI, lower intestinal symptoms and smoking.61 Patient education on this comorbidity is warranted and anamnesis should target IBD.22 For pediatric patients, a referral to a pediatrician with expertise in the field is advised.37
Discussion
Psoriasis is a chronic inflammatory skin disease that manifests in genetically predisposed patients.55 The current pathophysiologic model identifies the IL23-Th17 axis as the predominant immunologic cell-line involved. The high clinical response observed when targeting these immunological mediators seems an in vivo confirmation of this pathophysiological model.14
More than a decade ago the concept of the psoriatic march was introduced, referring to the clinical observation that psoriatic disease is often accompanied by metabolic diseases like diabetes mellitus, hypertension, dyslipidemia, obesity, insulin resistance and NAFLD.11 Ultimately, these comorbidities lead to an increased risk of atherosclerosis and cardiovascular mortality in psoriasis patients.16 Notably, psoriasis severity was identified as an independent cardiovascular risk factor.
In recent years, the pathophysiological model of atherosclerosis and NAFLD has been elucidated and, interestingly, the IL23-Th17 axis seems to be involved in these processes. It seems feasible that patients with a genetic predisposition to upregulate the IL23-Th17 axis as part of a skin disease, might also display this upregulation in other organs (eg liver, artery wall, …).17 Indeed, these insights emphasize the importance of the dermatologist as gatekeeper who consequently screens for these comorbidities and timely refers when necessary. The GP who knows the patients’ history as well as patients’ family history is well placed to consequently follow-up and treat the comorbidities. This review aims to provide a literature overview on published recommendations, as well as a practical guide on how and when to screen for comorbidities in patients in daily practice (Tables 5–8). Furthermore, we recommend patient education and prevention, starting at the first symptoms of psoriatic disease.
 |
Table 5 Comorbidity Screening Tool for Adult Psoriasis Patients |
 |
Table 6 Comorbidity Screening Tool for Children and Adolescents with Psoriasis |
 |
Table 7 Treatment Recommendations for Comorbidities in Adult Psoriasis Patients |
 |
Table 8 Treatment Recommendations for Comorbidities in Children and Adolescents with Psoriasis |
For follow-up on hypertension and diabetes mellitus, the guidelines are unambiguous in terms of cutoff values. However, suggested screening intervals differ between the guidelines for both adults and children. We recommend to measure blood pressure during every patient visit across all ages since it is non-invasive and might convey an educational message to the patient. We recommend annual screening for diabetes through fasting blood sugar and/or HbA1c. In patients with uncontrolled psoriasis, more frequent monitoring (2x/year) should be considered.
Guidelines for the screening of dyslipidemia are somewhat conflicting when it comes to the fasting state of the patient. Here, we follow the guidelines and statements from Denmark, the United Kingdom, Europe, Canada, Brazil, and the United States which endorse non-fasting lipid profiles (total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol, and triglycerides) for routine screening.56,57
We emphasize the importance of educating the patient on the increased risk of PsA and IBD. Appropriate education of patients about the signs and symptoms of PsA and the potential consequences of delaying diagnosis and management is imperative to prevent permanent joint destruction. We recommend a targeted anamnesis every visit. When PsA is suspected, immediate referral to a collaborating rheumatologist is in place. Screening tools that may be of help are the Psoriasis Epidemiology Screening Tool, the Toronto Psoriatic Arthritis Screen, the Psoriatic Arthritis Screening and Evaluation, and the Early Arthritis for Psoriatic Patients questionnaire.58 When IBD is suspected, we endorse determining fecal calprotectin as first-line screening.
Currently, NAFLD is a diagnosis of exclusion, rather than a diagnosis based on positive inclusive criteria that are present in the patient. In 2020, the term metabolic dysfunction-associated fatty liver disease (MAFLD) was proposed by an expert panel.62 Even though the latter term is not the currently accepted nomenclature by the American Association for the Study of Liver Diseases or the European Association for the Study of Liver Diseases, we believe this term very accurately identifies the liver disease in the psoriatic population.49 The dermatologist should be aware that weight reduction and physical activity are the cornerstones for prevention and treatment.
A limitation of this review is the non-systematic approach in literature search and inclusion of articles. Furthermore, some literature on the effect of anti-inflammatory therapy on comorbidities is available, however we deemed it outside the scope of this manuscript.
Conclusion
The dermatologist is in charge of the psoriasis patient and its comorbidities. A holistic approach of this multimorbid disease is warranted. The dermatologist should function as gatekeeper and screen for comorbidities, in order to make timely referrals when a comorbidity is suspected or diagnosed.
Abbreviations
AAP, American Academy of Pediatrics; ACE-I, angiotensin-converting enzyme inhibitor; aHT, arterial hypertension; ALT, alanine-amino-transferase; ARB, angiotensin receptor blockers; AST, aspartate-amino-transferase; BMI, body mass index; BP, blood pressure; CCB, calcium channel blockers; CVD, cardiovascular disease; DM, diabetes mellitus; GE, gastroenterologist; GP, general practitioner; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; IBD, inflammatory bowel disease; IFG, Impaired fasting glycemia; LDL, low-density lipoprotein; MetS, metabolic syndrome; MAFLD, metabolic-associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PsA, psoriatic arthritis.
Funding
No funding to report.
Disclosure
The authors report no conflict of interest.
References
1. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi:10.1038/jid.2012.339
2. Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231(1):35–40. doi:10.1159/000381913
3. Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. doi:10.1210/er.2008-0024
4. Singh S, Young P, Armstrong AW. Relationship between psoriasis and metabolic syndrome: a systematic review. G Ital Dermatol Venereol. 2016;151(6):663–677.
5. Chan WMM, Yew YW, Theng TSC, Liew CF, Oon HH. Prevalence of metabolic syndrome in patients with psoriasis: a cross-sectional study in Singapore. Singapore Med J. 2020;61(4):194–199. doi:10.11622/smedj.2019152
6. Sarafidis PA, Nilsson PM. The metabolic syndrome: a glance at its history. J Hypertens. 2006;24(4):621–626. doi:10.1097/01.hjh.0000217840.26971.b6
7. Pinter A, Schwarz P, Gerdes S, et al. Biologic treatment in combination with lifestyle intervention in moderate to severe plaque psoriasis and concomitant metabolic syndrome: rationale and methodology of the METABOLyx Randomized Controlled Clinical Trial. Nutrients. 2021;13(9):3015. doi:10.3390/nu13093015
8. Ma C, Harskamp CT, Armstrong EJ, Armstrong AW. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168(3):486–495. doi:10.1111/bjd.12101
9. Wohltmann W. JAAD Game Changers: psoriasis and the risk of diabetes: a prospective population-based cohort study. J Am Acad Dermatol. 2019;81(6):1459. doi:10.1016/j.jaad.2019.06.1296
10. Suárez-Fariñas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132(11):2552–2564. doi:10.1038/jid.2012.184
11. Boehncke WH, Boehncke S, Tobin AM, Kirby B. The “psoriatic march”: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20(4):303–307. doi:10.1111/j.1600-0625.2011.01261.x
12. Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: a comprehensive review. Adv Ther. 2020;37(5):2017–2033. doi:10.1007/s12325-020-01346-6
13. Lai YC, Yew YW. Psoriasis as an independent risk factor for cardiovascular disease: an epidemiologic analysis using a national database. J Cutan Med Surg. 2016;20(4):327–333. doi:10.1177/1203475415602842
14. Gooderham MJ, Papp KA, Lynde CW. Shifting the focus - the primary role of IL-23 in psoriasis and other inflammatory disorders. J Eur Acad Dermatol Venereol. 2018;32(7):1111–1119. doi:10.1111/jdv.14868
15. Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. doi:10.3390/ijms20061475
16. Kakarala CL, Hassan M, Belavadi R, et al. Beyond the skin plaques: psoriasis and its cardiovascular comorbidities. Cureus. 2021;13(11):e19679. doi:10.7759/cureus.19679
17. Lockshin B, Balagula Y, Merola JF. Interleukin 17, inflammation, and cardiovascular risk in patients with psoriasis. J Am Acad Dermatol. 2018;79(2):345–352. doi:10.1016/j.jaad.2018.02.040
18. Bajaj S, Mandal S, Singh KG, Prajapati R. Metabolic diseases and associated complications in patients with psoriasis. J Assoc Physicians India. 2020;68(10):44–46.
19. Hillary T, Lambert J. The use of metrics in daily practice and the perception of psoriasis-associated comorbidities: discrepancies between research and real-world. Psoriasis. 2021;11:169–175. doi:10.2147/ptt.S341215
20. Egan BM, Kjeldsen SE, Grassi G, Esler M, Mancia G. The global burden of hypertension exceeds 1.4 billion people: should a systolic blood pressure target below 130 become the universal standard? J Hypertens. 2019;37(6):1148–1153. doi:10.1097/hjh.0000000000002021
21. Bhagavathula AS, Shah SM, Suliman A, Oulhaj A, Aburawi EH. Hypertension control and guideline-recommended target blood pressure goal achievement at an early stage of hypertension in the UAE. J Clin Med. 2021;11(1):47. doi:10.3390/jcm11010047
22. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113. doi:10.1016/j.jaad.2018.11.058
23. Wu S, Han J, Li WQ, Qureshi AA. Hypertension, antihypertensive medication use, and risk of psoriasis. JAMA Dermatol. 2014;150(9):957–963. doi:10.1001/jamadermatol.2013.9957
24. Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148(11):1244–1250. doi:10.1001/archdermatol.2012.2502
25. Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52(2):262–267. doi:10.1016/j.jaad.2004.06.017
26. Mosca M, Hong J, Hadeler E, et al. Psoriasis and cardiometabolic comorbidities: an evaluation of the impact of systemic treatments in randomized clinical trials. Dermatol Ther (Heidelb). 2021;11(5):1497–1520. doi:10.1007/s13555-021-00590-0
27. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212. doi:10.1111/jdv.13854
28. de Jesus Janet M. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5(Suppl5):S213–S256. doi:10.1542/peds.2009-2107C
29. Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159(4):895–902. doi:10.1111/j.1365-2133.2008.08707.x
30. Arnett DK, Blumenthal RS, Albert MA. Correction to: 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2020;141(16):e773. doi:10.1161/cir.0000000000000770
31. Chat VS, Uppal SK, Kearns DG, Han G, Wu JJ. Translating the 2019 AAD-NPF guidelines of care for psoriasis with attention to comorbidities. Cutis. 2021;108(2s):7–11. doi:10.12788/cutis.0309
32. Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198–208. doi:10.1542/peds.2008-1349
33. Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153(7):698–704. doi:10.1001/jamadermatol.2017.0499
34. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149(2):166–176. doi:10.1001/jamadermatol.2013.1078
35. Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161(2):484–486. doi:10.1111/j.1365-2133.2009.09276.x
36. Thomas J, Parimalam K. Treating pediatric plaque psoriasis: challenges and solutions. Pediatric HealthMed Ther. 2016;7:25–38. doi:10.2147/phmt.S75834
37. Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82(1):161–201. doi:10.1016/j.jaad.2019.08.049
38. Skilton MR, Moulin P, Sérusclat A, Nony P, Bonnet F. A comparison of the NCEP-ATPIII, IDF and AHA/NHLBI metabolic syndrome definitions with relation to early carotid atherosclerosis in subjects with hypercholesterolemia or at risk of CVD: evidence for sex-specific differences. Atherosclerosis. 2007;190(2):416–422. doi:10.1016/j.atherosclerosis.2006.02.019
39. Gisondi P, Cazzaniga S, Chimenti S, et al. Metabolic abnormalities associated with initiation of systemic treatment for psoriasis: evidence from the Italian Psocare Registry. J Eur Acad Dermatol Venereol. 2013;27(1):e30–e41. doi:10.1111/j.1468-3083.2012.04450.x
40. Malhotra A, Shafiq N, Rajagopalan S, Dogra S, Malhotra S. Thiazolidinediones for plaque psoriasis: a systematic review and meta-analysis. Evid Based Med. 2012;17(6):171–176. doi:10.1136/ebmed-2011-100388
41. DeBoer MD. Assessing and managing the metabolic syndrome in children and adolescents. Nutrients. 2019;11(8):1788. doi:10.3390/nu11081788
42. Bronckers IM, Paller AS, van Geel MJ, van de Kerkhof PC, Seyger MM. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatr Drugs. 2015;17(5):373–384. doi:10.1007/s40272-015-0137-1
43. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32 Suppl 1(Suppl1):S62–S67. doi:10.2337/dc09-S062
44. Augustin M, Reich K, Glaeske G, Schaefer I, Radtke M. Co-morbidity and age-related prevalence of psoriasis: analysis of health insurance data in Germany. Acta Derm Venereol. 2010;90(2):147–151. doi:10.2340/00015555-0770
45. Kwa L, Kwa MC, Silverberg JI. Cardiovascular comorbidities of pediatric psoriasis among hospitalized children in the United States. J Am Acad Dermatol. 2017;77(6):1023–1029. doi:10.1016/j.jaad.2017.08.034
46. Vandenbriele C, Sun Y, Criel M, et al. Dextran sulfate triggers platelet aggregation via direct activation of PEAR1. Platelets. 2016;27(4):365–372. doi:10.3109/09537104.2015.1111321
47. Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–265.e19. doi:10.1016/j.jaad.2018.06.027
48. Stoll ML, Punaro M. Psoriatic juvenile idiopathic arthritis: a tale of two subgroups. Curr Opin Rheumatol. 2011;23(5):437–443. doi:10.1097/BOR.0b013e328348b278
49. Lewkowicz D, Gottlieb AB. Pediatric psoriasis and psoriatic arthritis. Dermatol Ther. 2004;17(5):364–375. doi:10.1111/j.1396-0296.2004.04039.x
50. Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175–1183. doi:10.1001/jama.2020.2298
51. Younossi ZM, Henry L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021;3(4):100305. doi:10.1016/j.jhepr.2021.100305
52. Magdaleno-Tapial J, Valenzuela-Oñate C, Ortiz-Salvador JM, et al. Prevalence of non-alcoholic fatty liver and liver fibrosis in patients with moderate-severe psoriasis: a cross-sectional cohort study. Australas J Dermatol. 2020;61(2):105–109. doi:10.1111/ajd.13175
53. Ganzetti G, Campanati A, Molinelli E, Offidani A. Psoriasis, non-alcoholic fatty liver disease, and cardiovascular disease: three different diseases on a unique background. World J Cardiol. 2016;8(2):120–131. doi:10.4330/wjc.v8.i2.120
54. Balak DMW, Piaserico S, Kasujee I. Non-Alcoholic Fatty Liver Disease (NAFLD) in patients with psoriasis: a review of the hepatic effects of systemic therapies. Psoriasis. 2021;11:151–168. doi:10.2147/ptt.S342911
55. Ganzetti G, Campanati A, Offidani A. Non-alcoholic fatty liver disease and psoriasis: so far, so near. World J Hepatol. 2015;7(3):315–326. doi:10.4254/wjh.v7.i3.315
56. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–2224. doi:10.1016/s0140-6736(20)32511-3
57. Bath RK, Brar NK, Forouhar FA, Wu GY. A review of methotrexate-associated hepatotoxicity. J Dig Dis. 2014;15(10):517–524. doi:10.1111/1751-2980.12184
58. Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One. 2015;10(10):e0140908. doi:10.1371/journal.pone.0140908
59. Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi:10.1016/s2468-1253(19)30039-1
60. Tollefson MM, Van Houten HK, Asante D, Yao X, Maradit Kremers H. Association of psoriasis with comorbidity development in children with psoriasis. JAMA Dermatol. 2018;154(3):286–292. doi:10.1001/jamadermatol.2017.5417
61. King D, Chandan JS, Thomas T, et al. The risk of later diagnosis of inflammatory bowel disease in patients with dermatological disorders associated with inflammatory bowel disease. Inflamm Bowel Dis. 2021;27(11):1731–1739. doi:10.1093/ibd/izaa344
62. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
63. Amin M, Lee EB, Tsai TF, Wu JJ. Psoriasis and co-morbidity. Acta Derm Venereol. 2020;100(3):adv00033. doi:10.2340/00015555-3387
64. Nordestgaard BG. A test in context: lipid profile, fasting versus nonfasting. J Am Coll Cardiol. 2017;70(13):1637–1646. doi:10.1016/j.jacc.2017.08.006
65. Wan MT, Shin DB, Hubbard RA, Noe MH, Mehta NN, Gelfand JM. Psoriasis and the risk of diabetes: a prospective population-based cohort study. J Am Acad Dermatol. 2018;78(2):315–322.e1. doi:10.1016/j.jaad.2017.10.050
66. Roubille C, Richer V, Starnino T, et al. Evidence-based recommendations for the management of comorbidities in rheumatoid arthritis, psoriasis, and psoriatic arthritis: expert opinion of the Canadian dermatology-rheumatology comorbidity initiative. J Rheumatol. 2015;42(10):1767–1780. doi:10.3899/jrheum.141112
67. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). European. Heart Journal. 2018;39(33):3021–3104. doi:10.1093/eurheartj/ehy339
68. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi:10.1016/S0140-6736(05)67402-8
69. Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the cardiovascular risk profile of obese patients with psoriasis. Acta Derm Venereol. 2014;94(6):691–694. doi:10.2340/00015555-1824
70. Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–441. doi:10.1007/s40265-014-0191-y
71. Loomis AK, Kabadi S, Preiss D, et al. Body mass index and risk of nonalcoholic fatty liver disease: two electronic health record prospective studies. J Clin Endocrinol Metab. 2016;101(3):945–952. doi:10.1210/jc.2015-3444
72. Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64(2):319–334. doi:10.1097/mpg.0000000000001482
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
