Back to Journals » ImmunoTargets and Therapy » Volume 12
Managing Cardiovascular Risk in Systemic Lupus Erythematosus: Considerations for the Clinician
Authors Semalulu T, Tago A, Zhao K , Tselios K
Received 10 July 2023
Accepted for publication 8 November 2023
Published 8 December 2023 Volume 2023:12 Pages 175—186
DOI https://doi.org/10.2147/ITT.S377076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Michael Shurin
Teresa Semalulu,1 Achieng Tago,2 Kevin Zhao,3 Konstantinos Tselios1
1Division of Rheumatology, Department of Medicine, McMaster University, Hamilton, Ontario, Canada; 2Division of Cardiology, Department of Medicine, McMaster University, Hamilton, Ontario, Canada; 3Michael G. DeGroote School of Medicine, McMaster University, Hamilton, Ontario, Canada
Correspondence: Konstantinos Tselios, Division of Rheumatology, Department of Medicine, McMaster University, 1280 Main St W, HSC 3H1B, Hamilton, Ontario, L8S 4K1, Canada, Tel +1 905 521 2100 Ext. 76714, Fax +1 905 521 4971, Email [email protected]
Abstract: A significant improvement in the survival of patients with systemic lupus erythematosus (SLE) over recent decades is largely attributed to the impact of disease-modifying therapies on end-organ damage. Thus, cardiovascular disease now represents the leading cause of mortality in SLE. Various disease-specific mechanisms are responsible for advanced atherosclerosis, as they lead to premature endothelial dysfunction, arterial stiffness, arterial wall thickening, and plaque formation. Consequently, in the assessment of cardiovascular risk in SLE, we must not only consider traditional risk factors (ie, age, gender, dyslipidemia) but also the additional role of non-traditional risk factors such as persistent disease activity and prolonged corticosteroid use. Cardiovascular risk assessment incorporates general cardiovascular screening, as existing risk prediction scores underestimate cardiovascular risk in this patient population. There is also an expanding role of imaging modalities in screening. Risk reduction strategies integrate unique considerations for the use of low-dose aspirin and more stringent hypertension targets. Hydroxychloroquine is the only disease-modifying therapy with known cardiovascular benefit in SLE, though this is a promising area of study.
Keywords: cardiovascular disease, atherosclerosis, endothelial dysfunction, coronary artery disease, risk stratification, prevention
Introduction
Systemic lupus erythematosus (SLE) is a complex multisystemic autoimmune disease that primarily impacts premenopausal women.1 This heterogeneous disease has the potential to cause severe renal, central nervous system, and cardiac dysfunction. The bimodal distribution of mortality in SLE was recognized in 1976, with late-stage mortality primarily attributed to infections and atherosclerotic heart disease.2 Increased survival rates in SLE over recent decades can be attributed to the prevention of end-organ damage through early diagnosis and prompt aggressive immunosuppressive management at disease onset.3 This has rendered cardiovascular disease (CVD) the leading cause of mortality in SLE.3
Advanced atherosclerosis in SLE occurs through various mechanisms related to autoimmunity and chronic inflammation, vascular injury, and disease-related factors.4,5 Thus, patients not only have premature atherosclerotic disease, but they are three times more likely to die from atherosclerotic disease compared to the general population.6,7 Traditional risk factors for CVD do not completely account for this increased risk, with disease activity and corticosteroids likely accelerating the development of atherosclerosis.8
Due to the multisystemic nature of SLE, these patients often require management from numerous generalists and specialists. Thus, it is essential for all clinicians to understand the nature of atherosclerosis in SLE. The objectives of this review are to describe the mechanisms of premature atherosclerosis in SLE and an approach to cardiovascular risk assessment and risk reduction in this high-risk population.
Cardiovascular Outcomes in SLE
SLE provides a similar risk for the development of cardiovascular disease as type 1 diabetes, thus it is considered an independent predictor of cardiovascular events (CVE).9 The prevalence of cardiovascular disease in SLE has been estimated between 6% and 16% of patients, with one large population study identifying atherosclerotic CVD in 25.6% of hospitalized SLE patients.10–12 One study showed that people with SLE had almost 4 times higher odds of developing CVD within the first two years of diagnosis, compared to the general population.13 Compared to age-matched controls, premenopausal SLE patients are more likely to be hospitalized for acute myocardial infarction and congestive heart failure.14 These patients also have more prolonged hospitalizations compared to age-matched diabetic patients.15 Accelerated atherosclerosis also contributes to peripheral vascular disease, cerebrovascular disease, and their associated morbidity and mortality.
The impact of accelerated atherosclerosis on mortality in SLE was first recognized in 1976, when atherosclerosis was identified as a leading cause of late-stage mortality.2 Survival has improved in SLE over recent decades, with 5-year and 10-year survival rates exceeding 90%, though much of this is related to advancements in therapies.3,16 Although we have also seen significant improvements in SLE mortality related to atherosclerotic disease, CVD remains a leading cause of death in SLE.17,18
Mechanisms of Advanced Atherosclerosis
Accelerated atherosclerosis in SLE has been attributed to both the higher prevalence of traditional risk factors such as hypertension and dyslipidemia as well as disease-related risk factors and the deleterious effects of pharmaceutical agents that are used for disease management.7 Endothelial dysfunction is believed to be the initial event in the pathophysiologic cascade as demonstrated by the enhanced expression of adhesion molecules such as ICAM-1 (intercellular adhesion molecule-1), VCAM (vascular adhesion molecule-1), VEGF (vascular endothelial growth factor), pentraxin-3, thrombomodulin, IP-10 (interferon-γ-induced protein 10) and MCP-1 (monocyte chemoattractant protein-1).19 In parallel to the endothelial activation, there is increased endothelial apoptosis, mediated through Fas/FasL (CD95/CD95 ligand) and TNF/TNFRII (tumor necrosis factor/tumor necrosis factor receptor II) interactions. All these cytokines have been shown to be increased in patients with SLE, particularly during the active phases of the disease.20 The increased rate of apoptosis of endothelial cells is not compensated by repair from the endothelial progenitors as IFN-a (interferon-a) induces dysregulation of these cells.21 The increased rate of endothelial apoptosis leads to insufficient apoptotic debris clearance from monocytes/macrophages and amplifies the autoimmune inflammation.
The proinflammatory cytokine milieu in SLE in tandem with the increased oxidative stress (as demonstrated by increased levels of reactive oxygen species, ROS) augments the oxidation of the low-density lipoprotein (LDL). Under normal circumstances, high-density lipoprotein (HDL) inhibits this pathway effectively. However, in SLE, a certain proportion of the HDL molecules are pro-inflammatory and do not regulate LDL oxidation. Oxidized LDL (oxLDL) further activates the endothelial cells to overexpress the aforementioned adhesion molecules. Consequently, circulating monocytes/macrophages are recruited in the subendothelial space and eventually phagocytose the oxLDL molecules and other lipids; this leads to the formation of “foam” cells that are considered to represent the earlier stages of the atherosclerotic plaque.22
Atherosclerotic plaques have also been shown to contain abundant numbers of neutrophils and plasmacytoid dendritic cells (DCs). Under inflammatory conditions (such as in SLE), neutrophils release neutrophil extracellular traps (NETs) that induce endothelial damage and activate the macrophages towards the production of IL-1β (interleukin-1β), TNFα, and MCP-1. On the other hand, plasmacytoid DCs and low-density granulocytes secrete IFN-a, which induces platelet activation among other actions. Certain T cell subpopulations are also involved in the atherogenic process, mainly Th17 and Th1, while T regulatory cells (Tregs) are found in reduced numbers in the periphery and in the vessel wall. Both Th1 and Th17 cells contribute to the perpetuation of inflammation by secreting pro-inflammatory cytokines such as Il-17. This has been associated with increased vulnerability of the atherosclerotic plaques that may result in platelet aggregation and thrombosis. These mechanisms are poorly regulated by Tregs, which are quantitatively and qualitatively impaired in SLE. Tregs may suppress the principal effectors of arterial wall inflammation, namely Th1 and Th17 cells, and down-regulate IFN-a and TNF-α. This action is mediated by IL-10 and TGF-β, by cell-to-cell contact mechanisms (including CTLA-4, cytotoxic T cell antigen 4), and by modulation of DC function. Tregs are also able to steer macrophage differentiation toward the M2 anti-inflammatory phenotype by down-regulating CD36 and scavenger receptor A (SRA). This mechanism reduces the uptake of oxLDL, thus inhibiting foam cell formation.23
OxLDL is highly immunogenic and stimulates the crosstalk between T and B cells for the production of antibodies against it (anti-oxLDL antibodies). The formed immune complexes will accelerate the rate of “foam” cell generation and enhance IFN-a secretion from the DCs. Similar actions have been demonstrated by immune complexes containing b2GPI, which is a potent anticoagulant protein and neutralized by these antibodies. Moreover, the immune complexes containing oxLDL and/or anti-b2GPI and other phospholipid epitopes bind to the C1q receptor of the endothelial cells and induce the expression of VCAM-1, resulting in an auto-amplification loop.24
In parallel, various cytokines have been shown to affect other risk factors. TNF-α induces a dyslipidemic profile with increased triglycerides, decreased HDL, inhibition of lipoprotein lipase, and induction of very low-density lipoprotein (VLDL) synthesis. Serum levels of TNF-α are strongly correlated with disease activity and drive the activation of endothelial cells, smooth muscle cells, and macrophages, thus augmenting the atherogenic process. TNF-α is also implicated in endothelial cell apoptosis (through p55 receptor) and vulnerability of the atherosclerotic plaque. Low levels of the transforming growth factor β (TGF-b) in SLE are associated with the breakdown of immune tolerance and have demonstrated a strong correlation with premature atherosclerosis.25 Other cytokines that were shown to increase CV risk in lupus patients are tumor necrosis factor-like weak inducer of apoptosis (TWEAK), IL-6, VEGF, and type I interferons. Soluble CD40 ligand (sCD40L) is also overexpressed in SLE and associated with increased activation of the endothelial cells through CD40 binding on their surface. It induces the coagulation cascade through the increase in tissue factor (TF) expression and is also related to plaque vulnerability.20 The complexity of the pathophysiologic basis of atherosclerosis in SLE as well as its clinical impact warrants further investigation aiming to identify biomarkers for its early identification and prevention.
Traditional and Non-Traditional Risk Factors for Atherosclerosis
Traditional non-modifiable and modifiable risk factors for atherosclerosis contribute to the development on CVD in SLE (Figure 1); however, SLE patients have elevated risk for CVEs even after controlling for traditional risk factors, therefore non-traditional risk factors (ie, chronic inflammation) must be taken into consideration in the assessment and management of CVD in SLE.6
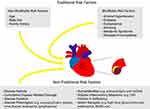 |
Figure 1 Risk factors for the development of atherosclerotic disease in systemic lupus erythematosus. |
Traditional Risk Factors
Non-modifiable risk factors include age, male sex, and family history of premature coronary artery disease. SLE patients over the age of 48 and/or with postmenopausal status have a fivefold increased risk of cardiovascular events (CVEs).26 These patients are also at increased risk of subclinical disease. Male sex also predicts subclinical atherosclerosis and CVEs. A family history of early coronary artery disease in a first-degree relative (male age less than 55 years or female age less than 65 years) is associated with increased risk of CVEs.7
Modifiable risk factors include smoking, hypertension, dyslipidemia, metabolic syndrome, obesity, and hyperglycemia. SLE patients who smoke have more subclinical disease and are at almost 4 times greater risk of CVEs relative to those who do not smoke.7 Multiple factors contribute to the development of hypertension in SLE, including renal disease and the use of non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids.7,27,28 General population targets for blood pressure (BP) may be inadequate for SLE patients, as studies have found a higher risk of CVEs in SLE patients with systolic BPs between 130 and 139 mmHg and diastolic pressures of 80–89 mmHg, compared to those with levels below 130/80 mmHg.29
Autoantibody production against various lipoproteins and cytokine-related hepatic synthesis of very-low density lipoproteins (VLDLs) are the primary mechanisms by which SLE leads to dyslipidemia.7,30 The most common pattern of dyslipidemia in SLE consists of elevated triglycerides (TGs) and very low-density lipoproteins (VLDL), and decreased high-density lipoproteins (HDL).13,31 Furthermore, modified pro-inflammatory high-density lipoproteins (HDLs) accelerate atherosclerosis in SLE by inducing vascular inflammation.32
Metabolic syndrome is associated with subclinical cardiovascular disease and cardiovascular mortality in SLE and is three times more common in SLE patients compared to the general population.7,28,33,34 SLE patients are known to have impaired glucose homeostasis.35 Hyperglycemia appears to modulate the risk of cardiovascular events and disease.36,37 Pre-diabetes is associated with arterial stiffness.38 Diabetes is associated with subclinical CVD and CVEs.39–41
Non-Traditional Risk Factors
Non-traditional risk factors are not only attributed to disease-related processes but also therapies, namely prolonged corticosteroid use.7 Disease-related processes that contribute to increased cardiovascular risk in SLE include ongoing disease-related activity, more disease-related damage, and longer duration of disease. Select organ manifestations, such as renal disease and neuropsychiatric diseases, have been associated with increased subclinical and clinical CVEs. Laboratory markers associated with subclinical and clinical cardiovascular disease include lupus anticoagulant, anticardiolipin antibodies (aCL), anti-β2 glycoprotein 1 (b2GPI) antibodies, and anti-dsDNA antibodies, high-sensitivity C-reactive protein (hsCRP), C3 complement fragment, and uric acid. Associations with additional inflammatory mediators have been described (eg, vascular cell adhesion molecule (VCAM), E-selectin, type 1 interferons, adipocytokines, leptin), though their clinical utility is unclear.7 High-dose glucocorticoids are associated with greater than two times increased risk of CVEs, and exacerbate many traditional risk factors such as hypertension, dyslipidemia, and hyperglycemia. Azathioprine has also been associated with increased CVEs.7
Management of Cardiovascular Disease in SLE
Multiple considerations are required for the appropriate management of cardiovascular disease in SLE. Clinicians must recognize the limitations of risk assessment tools, adjust their screening compared to the general population, consider the role of imaging modalities, and the use of SLE-specific anti-atherosclerotic therapies.
Risk Assessment
Screening
Cardiovascular risk in SLE not only begins at an earlier age compared to the general population, but these patients also experience accelerated atherosclerosis due to chronic inflammation and prolonged glucocorticoid use.8,42 Existing cardiovascular risk scores have not been validated in this patient population, which makes accurate risk assessment challenging. The Framingham risk score (FRS) underestimates cardiovascular risk in SLE, with a modified score shown to more accurately predict risk by multiplying each item by 2.6 The Predictors of Risk for Elevated Flares, Damage Progression, and Increased Cardiovascular Disease in Patients with SLE (PREDICTS) score was developed with these limitations in mind, as it incorporates certain biomarkers (proinflammatory high-density lipoprotein [piHDL], leptin, soluble TNF-like weak inducer of apoptosis [sTWEAK] and homocysteine levels) in the risk calculation.43 Although the PREDICTS score has been shown to predict subclinical atherosclerosis and major adverse CVEs (MACEs), its use is likely limited by clinical availability of the incorporated biomarkers.43,44
The European Alliance of Associations for Rheumatology (EULAR) previously recommended using the Systemic Coronary Risk Evaluation (SCORE) for CV risk assessment in SLE patients.45 Although this score has been shown to predict subclinical disease, it has also been shown to underestimate cardiovascular risk in SLE patients.7,8,46 Other CV risk prediction tools were assessed in the setting of SLE; however, the more recent EULAR recommendations recognize the limitations and lack of validation of existing cardiovascular risk prediction tools in SLE and, thus, do not endorse any particular risk calculator.47,48 Rather, they recommend comprehensive assessments and initiation of preventative strategies to reduce the impact of the traditional and non-traditional atherosclerotic risk factors.47
More recent studies have identified promising risk prediction tools for future consideration, including the QRESEARCH risk estimator version 3 (QRISK3) calculator, which was shown to have a greater discriminating capacity for the presence of carotid plaque compared to SCORE.49 Additionally, an updated SCORE prediction model, SCORE2, was developed using more recent CVD rates and by accounting for fatal and non-fatal CVD events, and demonstrated a higher ability to predict the presence of subclinical atherosclerosis in a cross-sectional study, compared to SCORE.50
Imaging Modalities
Flow-Mediated Dilation
Various modalities can be used to assess cardiovascular risk in SLE. Flow-mediated dilation (FMD) of the brachial artery can provide clinicians with information regarding endothelial dysfunction and can therefore be used as a surrogate in the assessment of early atherosclerosis. It can also monitor the risk of progression to coronary heart disease in SLE.51,52 A meta-analysis of 22 studies found that the FMD in SLE was significantly lower when compared to the general population. Similar to findings from other high cardiovascular risk populations; however, there have been no studies assessing the ability of FMD to predict clinically significant CVD in SLE.22,52
Pulse Wave Velocity
Pulse wave velocity (PWV) measures the stiffness of the carotid and femoral arteries and increased PWV can be a marker of early atherosclerotic disease.53,54 In a meta-analysis of 14 studies, patients with SLE had a significantly greater PWV when compared to the general population.53
Intima Media Thickness and Carotid Plaque
Intima media thickness (IMT) of the carotid artery as assessed by ultrasound is inversely related to FMD, with an increase in IMT acting as a predictor for vascular events including CHD.51 Studies have identified an IMT of >0.83 mm as abnormal in the general population, with an IMT >1.0 mm corresponding to plaque formation; conversely, in SLE an IMT >0.9 mm is considered to be abnormal with plaque formation corresponding to an IMT >1.3 mm.55 IMT progression is associated with a number of traditional and disease-related risk factors as noted in Table 1.
 |
Table 1 Overview of Cardiac Imaging Modalities in SLE and Associated Risk Factors |
In SLE, the presence of carotid plaque, as assessed by ultrasound, has a detection rate of 7% to 50% and is associated with a four-fold increased risk for cardiovascular events, furthermore, progression of carotid plaques occurs more frequently in SLE when compared to the general population.56–58 When compared to IMT of the carotid artery, the total plaque area has a stronger association with clinically significant CAD.58 As well, the combined presence of carotid plaques and femoral plaques has a higher predictive value when compared to isolated carotid plaques.58
Coronary Artery Calcification
Coronary artery calcification (CAC) is predictive of atherosclerotic disease in the general population, and in SLE, it is associated with various traditional and non-traditional risk factors as seen in Table 1. However, even with optimal management of traditional risk factors, the degree of CAC, as described by the Agatston score, has been shown to be higher in SLE when compared to the general population.59 Since they are often more metabolically active, non-calcified coronary plaques carry a higher risk for acute coronary events when compared to calcified plaques; it has previously been shown that up to 54% of SLE patients had non-calcified coronary plaques.60
Coronary Angiogram
Coronary angiogram is the “gold standard” modality for diagnosing CVD in the general population.61 In a retrospective cohort, SLE was associated with angiographic CVD.62 This study also found that although the traditional risk factors were associated with CVD in patients with SLE, certain factors, specifically hypercholesterolemia and diabetes mellitus, were seen less in the SLE group compered to non-SLE sex-matched controls. As well, patients with SLE were noted to be 20 years younger than controls with similar severity of CVD.62 Of note, studies have shown that up to two-thirds of SLE patients with perfusion defects did not have disease on coronary angiogram.7
Myocardial Perfusion Evaluation with Single-Photon Emission Computed Tomography (SPECT)
Perfusion abnormalities found in SPECT are a strong predictor of mortality, with general population studies showing the risk of myocardial infarction and cardiac death is almost four times greater among those with myocardial perfusion defects compared to those without.63,64 In patients with SLE, a high rate of myocardial perfusion defects in SPECT analysis has been seen, despite a lack of clinical symptoms suggestive of CVD.63,64 Up to two-thirds of SLE patients may have perfusion defects without associated disease on coronary angiogram, indicating that SPECT analysis may detect CVD earlier.7
Cardiac Magnetic Resonance Imaging (CMR)
One study demonstrated that greater than 40% of SLE patients had abnormalities noted by cardiac MR and the majority of abnormal findings included late gadolinium enhancement (LGE) in non-ischemic patterns, pericardial effusions, and at least 20% had adenosine-stress perfusion deficits indicating underlying CHD.65 Another study showed that in patients with connective tissue diseases with normal echocardiograms, 25.2% of them were found to have myocardial fibrosis; also, in patients where the LGE within the left ventricle was greater than 5%, the risk of future events occurring was increased.66
Positron Emission Tomography (PET)
Combined use of PET with other non-invasive modalities mentioned above is an emergent method of assessing CVD in patients with chronic inflammatory diseases, including SLE.67 PET assesses both systemic and vascular inflammation, and as prior studies have found a direct association with increased inflammation with both plaque burden and coronary obstruction, PET can act as a surrogate marker indicating CVD.67,68
See Table 1 for an overview of cardiac imaging modalities in SLE and associated risk factors. The only modalities that have proven to be predictive for coronary vascular events in SLE are IMT of the carotid and plaque assessment by ultrasound.58
Risk Reduction
Anti-Atherosclerotic Therapies
The initiation of anti-atherosclerotic therapies in SLE is directed by general population guidelines, with a few important considerations highlighted in the EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal disease, including systemic lupus erythematosus and antiphospholipid syndrome.47 There is no additional benefit of the use of low-dose aspirin for primary prevention, thus low-dose aspirin use should generally be reserved for secondary prevention in SLE. However, low dose ASA should be recommended in SLE patients with high-risk antiphospholipid (aPL) risk profiles regardless of the occurrence of previous thromboembolic events, based on 2a level of evidence (LoE) on the Oxford Centre for Evidence-Based Medicine system. ASA should also be considered in patients with low aPL risk profiles. Blood pressure targets also differ from the general population targets of less than 140/90. Clinicians should target a BP of less than 130/80 to prevent increased CVEs.69,70 Angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) should be used for hypertension management in lupus nephritis.47,70 Additional unique considerations for reducing cardiovascular risk in SLE include maintaining low disease activity and the use of the lowest possible dose of corticosteroids. Statins use is guided by general population recommendations, as there is insufficient evidence to show benefit with use of statins for primary prevention in SLE.45,47
Immunomodulatory Therapies
To date, hydroxychloroquine (HCQ) is the only immunomodulatory therapy recommended for cardioprotective benefit in SLE.47 HCQ is an antimalarial agent with multiple immunomodulatory and cardioprotective effects.71 It modifies the intracellular pH to block T-cell proliferation, inhibits Toll-like receptor (TLR) activation, and reduces the production of select cytokines (ie, TNF-a, IL-17, IL-6, IFNα and IFNγ). HCQ also modifies antibody and self-antigen presentation and reduces oxidative stress. Furthermore, HCQ effectively reduces platelet aggregation, lipid levels, and insulin resistance, all mechanisms that counteract the inflammatory effects on traditional risk factors. A multivariate analysis showed benefit in reducing plaque burden (adjusted OR 0.49, 95% CI 0.21–1.12).72 Use of HCQ has also been associated with lower aortic stiffness in premenopausal women and a significant reduction of thromboembolic events (OR 0.32, 95% CI 0.14–0.74).73
In vivo studies with mycophenolate mofetil (MMF) have shown promising results in mouse models with lupus.74 MMF may reduce cardiovascular mortality in renal transplant patients with diabetes and has been shown to reduce the development of carotid artery plaques in non-SLE patients, by decreasing T cell activation and increasing regulatory T cells.75 However, there was no improvement in subclinical cardiovascular disease in a small prospective cohort study of SLE patients.76
Apart from HCQ and MMF, canakinumab was the first commercially available drug to demonstrate efficacy in reducing cardiovascular risk in patients with a recent acute coronary syndrome and residual inflammation.77
Canakinumab is a monoclonal antibody against Il-1β and disrupts the IL-6 signaling pathway. Canakinumab-treated patients showed a significant decrease of the C-reactive protein without any effect on the levels of LDL and HDL, implying that targeting of inflammation alone may be adequate to decrease the atherosclerotic burden in selected patients. Colchicine affects the activation of the NLRP3 inflammasome and disrupts lymphocyte activation. Several studies have shown modest benefit in chronic atherosclerotic coronary disease as well as acute coronary syndromes.78 On the contrary, methotrexate, a potent suppressor of certain pro-inflammatory cytokines (IL-6, IL-12, TNFa), did not reduce the rate of cardiovascular events in CAD patients with residual inflammation.79
Harnessing other inflammatory mediators such as IFNa may prove beneficial in reducing the atherosclerotic burden in SLE. The recent development of anifrolumab, an IFN receptor antagonist, may achieve this goal not only by regulating the IFN-induced autoimmune phenomena but also by reducing disease activity and the need for glucocorticoids.
Strengths and Limitations
The major strength of this review is that we have highlighted essential considerations for the management of CVD in SLE, allowing all clinicians managing patients with SLE to participate in this important aspect of this patient populations care. Given that practice patterns among rheumatologists and cardiologists may vary, a systematic review or meta-analysis might provide further insight for those requiring more in-depth knowledge about this aspect of care.
Future Directions
Optimizing screening strategies will enable early intervention to reduce the risk of cardiovascular disease among SLE patients. This may include the development of CV risk prediction tools validated in this population and identifying the role of biomarkers and imaging modalities in pre-clinical disease screening.4 Also, the development of anti-atherosclerotic therapies in SLE, including recognizing the role of novel immunomodulatory therapies in mediating atherosclerotic risk in these patients.80 As ongoing advancements continue to reduce the impact of CVD in SLE, we must address poorer outcomes seen in certain subsets of the population, related to socioeconomic, racial, and ethnic disparities.15,81 These should be explored further to reduce disparate CV risk.
Conclusion
We have highlighted the significant impact of cardiovascular disease on morbidity and mortality in SLE. Disease-related processes directly contribute to the development of premature atherosclerosis among the patient population. This is further compounded by traditional cardiovascular risk factors and non-traditional risk factors, such as disease activity and prolonged glucocorticoid use. Cardiovascular risk calculators are not generalizable to this predominantly young and female patient population, and they have consistently been shown to underestimate risk. Carotid IMT assessed via ultrasound independently predicts CVEs. The utility of other forms of vascular imaging to assess atherosclerotic burden in SLE is unclear.
EULAR recommends comprehensive monitoring and management of cardiovascular risk factors based on general population guidelines. Unique considerations include the use of low dose ASA based on aPL risk profile and tighter BP control (targeting a BP of less than 130/80). Also, the use of hydroxychloroquine in all SLE patients without contraindications, as this therapy has additional cardioprotective effects (ie, lipid lowering, insulin tolerance). Previous studies have failed to identify additional immunomodulatory therapies with anti-atherosclerotic effect. Advancements in therapy are promising, as they relate to direct anti-atherosclerotic effects (ie, canakinumab) or perhaps by improving disease activity and reducing the need for prolonged glucocorticoid use.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2(1):16039. doi:10.1038/nrdp.2016.39
2. Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60(2):221–225. doi:10.1016/0002-9343(76)90431-9
3. Borchers AT, Keen CL, Shoenfeld Y, Gershwin ME. Surviving the butterfly and the wolf: mortality trends in systemic lupus erythematosus. Autoimmun Rev. 2004;3(6):423–453. doi:10.1016/j.autrev.2004.04.002
4. Piga M, Arnaud L. The Main Challenges in Systemic Lupus Erythematosus: where Do We Stand? J Clin Med. 2021;10(2). doi:10.3390/jcm10020243
5. Skaggs BJ, Hahn BH, Mcmahon M. Accelerated atherosclerosis in patients with SLE—mechanisms and management. Nat Rev Rheumatol. 2012;8(4):214–223. doi:10.1038/nrrheum.2012.14
6. Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum Oct. 2001;44(10):2331–2337. doi:10.1002/1529-0131(200110)44:10
7. Tselios K, Sheane BJ, Gladman DD, Urowitz MB. Optimal Monitoring For Coronary Heart Disease Risk in Patients with Systemic Lupus Erythematosus: a Systematic Review. J Rheumatol. 2016;43(1):54–65. doi:10.3899/jrheum.150460
8. de Leeuw K, Smit AJ, de Groot E, van Roon AM, Kallenberg CG, Bijl M. Longitudinal study on premature atherosclerosis in patients with systemic lupus erythematosus. Atherosclerosis. 2009;206(2):546–550. doi:10.1016/j.atherosclerosis.2009.03.018
9. Koenig KF, Ribi C, Radosavac M, Zulewski H, Trendelenburg M. Prevalence of vascular disease in systemic lupus erythematosus compared with type-1 diabetes mellitus: a cross-sectional study of two cohorts. Lupus. 2015;24(1):58–65. doi:10.1177/0961203314550223
10. Katz G, Smilowitz NR, Blazer A, Clancy R, Buyon JP, Berger JS. Systemic Lupus Erythematosus and Increased Prevalence of Atherosclerotic Cardiovascular Disease in Hospitalized Patients. Mayo Clin Proc. 2019;94(8):1436–1443. doi:10.1016/j.mayocp.2019.01.044
11. McMahon M, Hahn BH, Skaggs BJ. Systemic lupus erythematosus and cardiovascular disease: prediction and potential for therapeutic intervention. Expert Rev Clin Immunol. 2011;7(2):227–241. doi:10.1586/eci.10.98
12. Zeller CB, Appenzeller S. Cardiovascular disease in systemic lupus erythematosus: the role of traditional and lupus related risk factors. Curr Cardiol Rev. 2008;4(2):116–122. doi:10.2174/157340308784245775
13. Bartels CM, Buhr KA, Goldberg JW, et al. Mortality and Cardiovascular Burden of Systemic Lupus Erythematosus in a US Population-based Cohort. J Rheumatol. 2014;41(4):680–687. doi:10.3899/jrheum.130874
14. Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42(2):338–346. doi:10.1002/1529-0131(199902)42:2<338::aid-anr17>3.0.co;2-u
15. Shah MA, Shah AM, Krishnan E. Poor Outcomes After Acute Myocardial Infarction in Systemic Lupus Erythematosus. J Rheumatol. 2009;36(3):570–575. doi:10.3899/jrheum.080373
16. Bakshi J, Segura BT, Wincup C, Rahman A. Unmet Needs in the Pathogenesis and Treatment of Systemic Lupus Erythematosus. Clin Rev Allergy Immunol. 2018;55(3):352–367. doi:10.1007/s12016-017-8640-5
17. Tselios K, Gladman DD, Sheane BJ, Su J, Urowitz M. All-cause, cause-specific and age-specific standardised mortality ratios of patients with systemic lupus erythematosus in Ontario, Canada over 43 years (1971-2013). Ann Rheum Dis. 2019;78(6):802–806. doi:10.1136/annrheumdis-2018-214802
18. Ståhl-Hallengren C, Jönsen A, Nived O, Sturfelt G. Incidence studies of systemic lupus erythematosus in Southern Sweden: increasing age, decreasing frequency of renal manifestations and good prognosis. J Rheumatol. 2000;27(3):685–691.
19. Bergkamp SC, Wahadat MJ, Salah A, et al. Dysregulated endothelial cell markers in systemic lupus erythematosus: a systematic review and meta-analysis. J Inflamm. 2023;20(1):18. doi:10.1186/s12950-023-00342-1
20. Guzmán-Martínez G, Marañón C, Network CR. Immune mechanisms associated with cardiovascular disease in systemic lupus erythematosus: a path to potential biomarkers. Front Immunol. 2022;13:974826. doi:10.3389/fimmu.2022.974826
21. Ding X, Xiang W, He X. IFN-I Mediates Dysfunction of Endothelial Progenitor Cells in Atherosclerosis of Systemic Lupus Erythematosus. Front Immunol. 2020;11:581385. doi:10.3389/fimmu.2020.581385
22. Liu Y, Yu X, Zhang W, Zhang X, Wang M, Ji F. Mechanistic insight into premature atherosclerosis and cardiovascular complications in systemic lupus erythematosus. J Autoimmun. 2022;132:102863. doi:10.1016/j.jaut.2022.102863
23. Tselios K, Sarantopoulos A, Gkougkourelas I, Boura P. T regulatory cells: a promising new target in atherosclerosis. Crit Rev Immunol. 2014;34(5):389–397. doi:10.1615/critrevimmunol.2014010802
24. Liu Y, Kaplan MJ. Cardiovascular disease in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2018;30(5):441–448. doi:10.1097/BOR.0000000000000528
25. Jackson M, Ahmad Y, Bruce IN, Coupes B, Brenchley PE. Activation of transforming growth factor-beta1 and early atherosclerosis in systemic lupus erythematosus. Arthritis Res Ther. 2006;8(3):R81. doi:10.1186/ar1951
26. Schoenfeld SR, Kasturi S, Costenbader KH. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum. 2013;43(1):77–95. doi:10.1016/j.semarthrit.2012.12.002
27. Pope JE, Anderson JJ, Felson DT. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med. 1993;153(4):477–484.
28. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413–1432. doi:10.1016/j.clinthera.2011.09.009
29. Tselios K, Gladman DD, Su J, Urowitz M. Impact of the new American College of Cardiology/American Heart Association definition of hypertension on atherosclerotic vascular events in systemic lupus erythematosus. Ann Rheum Dis. 2020;79(5):612–617. doi:10.1136/annrheumdis-2019-216764
30. Tselios K, Koumaras C, Gladman DD, Urowitz MB. Dyslipidemia in systemic lupus erythematosus: just another comorbidity? Semin Arthritis Rheum. Apr. 2016;45(5):604–610. doi:10.1016/j.semarthrit.2015.10.010
31. Borba EF, Bonfá E. Dyslipoproteinemias in systemic lupus erythematosus: influence of disease, activity, and anticardiolipin antibodies. Lupus. 1997;6(6):533–539. doi:10.1177/096120339700600610
32. Mcmahon M, Grossman J, Skaggs B, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009;60(8):2428–2437. doi:10.1002/art.24677
33. Kip KE, Marroquin OC, Kelley DE, et al. Clinical Importance of Obesity Versus the Metabolic Syndrome in Cardiovascular Risk in Women. Circulation. 2004;109(6):706–713. doi:10.1161/01.cir.0000115514.44135.a8
34. Chung CP, Avalos I, Oeser A, et al. High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: association with disease characteristics and cardiovascular risk factors. Ann Rheum Dis. 2006;66(2):208–214. doi:10.1136/ard.2006.054973
35. Kuo CY, Tsai TY, Huang YC. Insulin resistance and serum levels of adipokines in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus. 2020;29(9):1078–1084. doi:10.1177/0961203320935185
36. Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L, Investigators EHS. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J. 2008;29(2):177–184. doi:10.1093/eurheartj/ehm519
37. Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;152(1):27–38. doi:10.1016/j.ahj.2005.09.015
38. Selzer F, Sutton-Tyrrell K, Fitzgerald SG, et al. Comparison of risk factors for vascular disease in the carotid artery and aorta in women with systemic lupus erythematosus. Arthritis Rheum. 2004;50(1):151–159. doi:10.1002/art.11418
39. Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol. 2012;176(8):708–719. doi:10.1093/aje/kws130
40. Rho YH, Chung CP, Oeser A, et al. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J Rheumatol. 2008;35(9):1789–1794.
41. Sella EM, Sato EI, Leite WA, Oliveira Filho JA, Barbieri A. Myocardial perfusion scintigraphy and coronary disease risk factors in systemic lupus erythematosus. Ann Rheum Dis. 2003;62(11):1066–1070. doi:10.1136/ard.62.11.1066
42. Quintana P-EG, Serrano R, Pons-Estel BA, Bruce IN. Accelerated atherosclerosis and cardiovascular disease in systemic lupus erythematosus. Revista Colombiana de Reumatología. 2021;1(28):21–30.
43. Mcmahon M, Skaggs BJ, Grossman JM, et al. A Panel of Biomarkers Is Associated With Increased Risk of the Presence and Progression of Atherosclerosis in Women With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2014;66(1):130–139. doi:10.1002/art.38204
44. Skaggs BJ, Grossman J, Sahakian L, et al. A Panel of Biomarkers Associates With Increased Risk for Cardiovascular Events in Women With Systemic Lupus Erythematosus. ACR Open Rheumatol. 2021;3(4):209–220. doi:10.1002/acr2.11223
45. Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–745. doi:10.1136/annrheumdis-2019-215089
46. Gustafsson JT, Simard JF, Gunnarsson I, et al. Risk factors for cardiovascular mortality in patients with systemic lupus erythematosus, a prospective cohort study. Arthritis Res Ther. 2012;14(2):R46. doi:10.1186/ar3759
47. Drosos GC, Vedder D, Houben E, et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis. 2022:annrheumdis–202. doi:10.1136/annrheumdis-2021-221733
48. Sivakumaran J, Harvey P, Omar A, et al. Assessment of cardiovascular risk tools as predictors of cardiovascular disease events in systemic lupus erythematosus. Lupus Sci Med. 2021;8(1):548. doi:10.1136/lupus-2020-000448
49. Quevedo-Abeledo JC, Caceres L, Palazuelos C, Llorca J, González-Gay M, Ferraz-Amaro I. QRISK3 relation to carotid plaque is higher than that of score in patients with systemic lupus erythematosus. Rheumatology. 2022;61(4):1408–1416. doi:10.1093/rheumatology/keab531
50. Quevedo-Abeledo JC, González-Gay M, Ferraz-Amaro I. SCORE2 versus SCORE in patients with systemic lupus erythematosus. Ther Adv Musculoskelet Dis. 2022;14:1759720X221092373. doi:10.1177/1759720X221092373
51. Kiss E, Soltesz P, Der H, et al. Reduced flow-mediated vasodilation as a marker for cardiovascular complications in lupus patients. J Autoimmun. 2006;27(4):211–217. doi:10.1016/j.jaut.2006.09.008
52. Wang DG, Tang XW, Fan Y, et al. Decreased flow-mediated dilatation in patients with systemic lupus erythematosus: a meta-analysis. Inflammation. 2014;37(6):2067–2075. doi:10.1007/s10753-014-9940-z
53. Wang P, Mao YM, Zhao CN, et al. Increased Pulse Wave Velocity in Systemic Lupus Erythematosus: a Meta-Analysis. Angiology. 2018;69(3):228–235. doi:10.1177/0003319717715964
54. Tso TK, Huang WN, Huang HY, Chang CK. Association of brachial-ankle pulse wave velocity with cardiovascular risk factors in systemic lupus erythematosus. Lupus. 2005;14(11):878–883. doi:10.1191/0961203305lu2234oa
55. Lertratanakul A, Sun J, Wu PW, et al. Risk factors for changes in carotid intima media thickness and plaque over 5 years in women with systemic lupus erythematosus. Lupus Sci Med. 2021;8(1):548. doi:10.1136/lupus-2021-000548
56. Thompson T, Sutton-Tyrrell K, Wildman RP, et al. Progression of carotid intima-media thickness and plaque in women with systemic lupus erythematosus. Arthritis Rheum. 2008;58(3):835–842. doi:10.1002/art.23196
57. Kao AH, Lertratanakul A, Elliott JR, et al. Relation of carotid intima-media thickness and plaque with incident cardiovascular events in women with systemic lupus erythematosus. Am J Cardiol. 2013;112(7):1025–1032. doi:10.1016/j.amjcard.2013.05.040
58. Nussinovitch U. The Heart in Rheumatic, Autoimmune, and Inflammatory Diseases: Pathophysiology, Clinical Aspects, and Therapeutic Approaches. Academic Press; 2017:xxvi, 739.
59. Kiani AN, Magder LS, Post WS, et al. Coronary calcification in SLE: comparison with the Multi-Ethnic Study of Atherosclerosis. Rheumatology. 2015;54(11):1976–1981. doi:10.1093/rheumatology/kev198
60. Khan A, Arbab-Zadeh A, Kiani AN, Magder LS, Petri M. Progression of noncalcified and calcified coronary plaque by CT angiography in SLE. Rheumatol Int. 2017;37(1):59–65. doi:10.1007/s00296-016-3615-z
61. Nikpour M, Urowitz M, Ibañez D, Gladman D. Relationship between cardiac symptoms, myocardial perfusion defects and coronary angiography findings in systemic lupus erythematosus. Lupus. 2011;20(3):299–304. doi:10.1177/0961203310381512
62. Kaul MS, Rao SV, Shaw LK, Honeycutt E, Ardoin SP. Association of systemic lupus erythematosus with angiographically defined coronary artery disease: a retrospective cohort study. Arthritis Care Res. 2013;65(2):266–273. doi:10.1002/acr.21782
63. Plazak W, Pasowicz M, Kostkiewicz M, et al. Influence of chronic inflammation and autoimmunity on coronary calcifications and myocardial perfusion defects in systemic lupus erythematosus patients. Inflamm Res. 2011;60(10):973–980. doi:10.1007/s00011-011-0358-x
64. Doukky R, Hayes K, Frogge N, et al. Impact of appropriate use on the prognostic value of single-photon emission computed tomography myocardial perfusion imaging. Circulation. 2013;128(15):1634–1643. doi:10.1161/CIRCULATIONAHA.113.002744
65. Burkard T, Trendelenburg M, Daikeler T, et al. The heart in systemic lupus erythematosus – a comprehensive approach by cardiovascular magnetic resonance tomography. PLoS One. 2018;13(10):e0202105. doi:10.1371/journal.pone.0202105
66. Mavrogeni S, Sfikakis PP, Gialafos E, et al. Cardiac tissue characterization and the diagnostic value of cardiovascular magnetic resonance in systemic connective tissue diseases. Arthritis Care Res. 2014;66(1):104–112. doi:10.1002/acr.22181
67. Parel PM, Berg AR, Hong CG, et al. Updates in the Impact of Chronic Systemic Inflammation on Vascular Inflammation by Positron Emission Tomography (PET). Curr Cardiol Rep. 2022;24(4):317–326. doi:10.1007/s11886-022-01651-2
68. Joshi AA, Lerman JB, Dey AK, et al. Association Between Aortic Vascular Inflammation and Coronary Artery Plaque Characteristics in Psoriasis. JAMA Cardiol. 2018;3(10):949–956. doi:10.1001/jamacardio.2018.2769
69. Tselios K, Koumaras C, Urowitz MB, Gladman DD. Do current arterial hypertension treatment guidelines apply to systemic lupus erythematosus patients? A critical appraisal. Semin Arthritis Rheum. 2014;43(4):521–525. doi:10.1016/j.semarthrit.2013.07.007
70. Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79(6):713–723. doi:10.1136/annrheumdis-2020-216924
71. Floris A, Piga M, Mangoni AA, Bortoluzzi A, Erre GL, Cauli A. Protective Effects of Hydroxychloroquine against Accelerated Atherosclerosis in Systemic Lupus Erythematosus. Mediators Inflamm. 2018;2018:1–11. doi:10.1155/2018/3424136
72. Romero-Díaz J, Vargas-Vóracková F, Kimura-Hayama E, et al. Systemic lupus erythematosus risk factors for coronary artery calcifications. Rheumatology. 2012;51(1):110–119. doi:10.1093/rheumatology/ker307
73. Jung H, Bobba R, Su J, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):863–868. doi:10.1002/art.27289
74. Van Leuven SI, Mendez-Fernandez YV, Wilhelm AJ, et al. Mycophenolate mofetil but not atorvastatin attenuates atherosclerosis in lupus-prone LDLr−/− mice. Ann Rheum Dis. 2012;71(3):408–414. doi:10.1136/annrheumdis-2011-200071
75. van Leuven SI, van Wijk DF, Volger OL, et al. Mycophenolate mofetil attenuates plaque inflammation in patients with symptomatic carotid artery stenosis. Atherosclerosis. 2010;211(1):231–236. doi:10.1016/j.atherosclerosis.2010.01.043
76. Kiani AN, Magder LS, Petri M. Mycophenolate mofetil (MMF) does not slow the progression of subclinical atherosclerosis in SLE over 2 years. Rheumatol Int. 2012;32(9):2701–2705. doi:10.1007/s00296-011-2048-y
77. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. doi:10.1056/NEJMoa1707914
78. Rocha VZ, Rached FH, Miname MH. Insights into the Role of Inflammation in the Management of Atherosclerosis. J Inflamm Res. 2023;16:2223–2239. doi:10.2147/JIR.S276982
79. Ridker PM, Everett BM, Pradhan A, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380(8):752–762. doi:10.1056/NEJMoa1809798
80. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi:10.1056/NEJM200003233421202
81. Barbhaiya M, Feldman CH, Guan H, et al. Race/Ethnicity and Cardiovascular Events Among Patients With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017;69(9):1823–1831. doi:10.1002/art.40174
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
