Back to Journals » Nature and Science of Sleep » Volume 14
Has the COVID-19 Pandemic Traumatized Us Collectively? The Impact of the COVID-19 Pandemic on Mental Health and Sleep Factors via Traumatization: A Multinational Survey
Authors Holzinger B , Nierwetberg F, Chung F, Bolstad CJ , Bjorvatn B, Chan NY, Dauvilliers Y, Espie CA, Han F , Inoue Y, Leger D , Macêdo T , Matsui K, Merikanto I , Morin CM, Mota-Rolim SA, Partinen M, Plazzi G , Penzel T , Sieminski M , Wing YK , Scarpelli S , Nadorff MR, De Gennaro L
Received 2 May 2022
Accepted for publication 15 August 2022
Published 26 August 2022 Volume 2022:14 Pages 1469—1483
DOI https://doi.org/10.2147/NSS.S368147
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Brigitte Holzinger,1,2 Franziska Nierwetberg,1 Frances Chung,3 Courtney J Bolstad,4 Bjørn Bjorvatn,5 Ngan Yin Chan,6 Yves Dauvilliers,7 Colin A Espie,8 Fang Han,9 Yuichi Inoue,10 Damien Leger,11 Tainá Macêdo,12 Kentaro Matsui,13,14 Ilona Merikanto,15,16 Charles M Morin,17 Sérgio A Mota-Rolim,18 Markku Partinen,19 Giuseppe Plazzi,20,21 Thomas Penzel,22 Mariusz Sieminski,23 Yun Kwok Wing,6 Serena Scarpelli,24 Michael R Nadorff,4,25 Luigi De Gennaro24,26
1Institute for Consciousness and Dream Research, Vienna, Austria; 2Medical University Vienna, Postgraduate Master ULG Sleep Coaching, Vienna, Austria; 3Department of Anesthesiology and Pain Medicine, University Health Network, University of Toronto, Toronto, ON, Canada; 4Mississippi State University, Mississippi State, MS, USA; 5Department of Global Public Health and Primary Care, University of Bergen and Norwegian Competence Center for Sleep Disorders, Haukeland University Hospital, Bergen, Norway; 6Li Chiu Kong Family Sleep Assessment Unit, Department of Psychiatry, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong, Special Administrative Region, People’s Republic of China; 7Sleep-Wake Disorders Unit, Department of Neurology, Gui-de-Chauliac Hospital, CHU Montpellier, INM, Univ Montpellier, INSERM, Montpellier, France; 8Sir Jules Thorn Sleep & Circadian Neuroscience Institute (SCNi), University of Oxford, Oxford, UK; 9Department of Pulmonary and Critical Care Medicine, Peking University People’s Hospital, Beijing, People’s Republic of China; 10Tokyo Medical University, Tokyo, Japan; 11Université de Paris, APHP, Hôtel-Dieu, Centre du Sommeil et de la Vigilance, Paris, France; 12Department of Psychology, Federal University of Rio Grande do Norte, Natal, Brazil; 13Department of Laboratory Medicine, National Center Hospital, National Center of Neurology and Psychiatry, Kodaira, Japan; 14Department of Psychiatry, Tokyo Women’s Medical University, Tokyo, Japan; 15SleepWell Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland; 16Department of Public Health and Welfare, Finnish Institute for Health and Welfare, Helsinki, Finland; 17École de Psychologie, Centre d’étude des troubles du sommeil, Centre de recherche CERVO/Brain Research Center, Université Laval, Québec, QC, Canada; 18Brain Institute, Physiology and Behavior Department, and Onofre Lopes University Hospital - Federal University of Rio Grande do Norte, Natal, Brazil; 19Helsinki Sleep Clinic, Terveystalo Healthcare, and Department of Neurosciences, Clinicum, University of Helsinki, Helsinki, Finland; 20IRCCS, Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy; 21Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy; 22Sleep Medicine Center, Charité Universitätsmedizin Berlin, Berlin, Germany; 23Department of Emergency Medicine, Medical University of Gdansk, Gdansk, Poland; 24Department of Psychology, Sapienza University of Rome, Rome, Italy; 25Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX, USA; 26IRCCS Fondazione Santa Lucia, Rome, Italy
Correspondence: Brigitte Holzinger, Tel +43 699 101 99 042, Email [email protected]
Purpose: The COVID-19 pandemic affects mental health and sleep, resulting in frequent nightmares. Therefore, identifying factors associated with nightmare frequency is important, as it can indicate mental health issues. The study aimed to investigate increases in nightmare frequency comparing the pre-pandemic and pandemic period, and identify its risk factors. Further, the mediating role of post-traumatic stress disorder symptoms between the pandemic and nightmares is explored.
Patients and Methods: For this cross-sectional survey data were obtained via self-rating online survey (ICOSS: details in Partinen et al, 2021), which was open to anyone older than 18 years. The final volunteer sample consisted of 15,292 participants, divided according to their nightmare frequency (high: ≥ 1– 2 nights/week; low: < 1– 2 nights/week). A total of 9100 participants were excluded if answers on variables of interest were missing or receiving rewards for participation. Chi-square tests identified changes of nightmare frequency. Predictors of high nightmare frequency were assessed using logistic regression and presented as Odds Ratios. Post-hoc mediation models were used to investigate the role of post-traumatic stress symptoms (PTSS).
Results: The mean age was 41.63 (SD=16.55) with 64.05% females. High nightmare frequency increased significantly from 13.24% to 22.35% during the pandemic. Factors associated with it included self-reported PTSS (OR=2.11), other mental disorders and various sleep disorders or problems. Financial burden due to the pandemic, confinement, having had COVID-19, and work situation during the pandemic were associated with nightmare frequency, those relations were partly mediated through PTSS.
Conclusion: Our results display the pandemic influence on nightmare frequency, which in turn connects to multiple mental health and sleep factors. These relations were partly mediated through PTSS. The COVID-19 pandemic appears to have caused traumatization of a substantial proportion of society. Health care workers should consider nightmares in their screening routines, as it might indicate PTSS and/or other mental and sleep disorders.
Keywords: COVID-19, nightmares, mental health, sleep, post-traumatic stress disorder, collective trauma
Introduction
The COVID-19 pandemic presents a variety of challenges which burdened almost all aspects of life. This may translate into the potential for increased nightmare frequency (NMF)2 and a variety of sleep and mental disorders, for which high NMF is a symptom.3 Notably, nightmares are not only a symptom of wider syndromes but also a separate disorder that should be treated accordingly.4 Thus, we propose that NMF can be used as an indicator of sleep disorders, psychological problems, and overall well-being.
Dream function is to integrate new experiences in the autobiographical memory.5–7 They are reactive to experiences that are highly emotional and personally relevant.8,9 Similarly, dreams often involve the most significant stressors of one’s life, and help with emotion regulation.10 Nightmares can also display an attempt to cope with stress, especially when other stress buffering factors, like social support are missing.11 During this crisis both stress levels12 and NMF13 have been found increased. Generally, an increase in nightmares and bad dreams has been observed in periods of high stress.14,15 During the pandemic, the stressful factors are uncertainty, social isolation, and confinement. Social distancing and isolation are very conflictive to our human nature, that is to live in herd- or swarm-like alignments.16–19 Additionally, the uncertainty of the current situation evokes feelings of insecurity and powerlessness, as it is unknown when life will get back to normal and the individual influence on the course of the pandemic is limited.20,21
Nightmares are symptoms of, or related to a variety of sleep and mental health-related problems such as insomnia,22 post-traumatic stress disorder (PTSD)3,23 and depression.22 Even more concerning is that insomnia symptoms and nightmares are independently significantly related to suicide risk.24 Importantly, levels of anxiety and depression improved in PTSD patients receiving lucid dreaming therapy.23 Nightmares severely impact not only our night but also our day life and can cause mental health problems in the affected person. Lemyre et al (2019)25 found that, apart from depression and PTSD, nightmares show a positive correlation with bipolar disorders, anxiety disorders, obsessive-compulsive disorders, attention-deficit and hyperactivity disorder, schizophrenia spectrum disorders, substance use disorders, eating disorders, personality disorders, and the general level of psychopathology.
Nightmares may have a diagnostic value, especially during this pandemic. Indeed, several studies reported increasing sleep problems and disorders, as well as mental disorders. Specifically, studies reported high levels of distress during the pandemic,12 as well as an increase of depressive symptoms,26,27 anxiety-related symptoms28 and PTSD.26 Moreover, panic attacks, irrational fears, post-traumatic stress, fatigue, reduced sleep quality, and sleep disturbances have been reported more frequently during the pandemic.1,29
The pandemic is also likely to affect our circadian rhythm. This is because we are forced to work from home, which resulted in waking up later.30 Additionally, confinements led to restricted daylight exposure affecting our circadian rhythm.29 But there is still no clear answer to the question whether the pandemic, which clearly changed our sleeping patterns, resulted in better31 or worsened sleep quality,32 or if sleep quality changed in different directions for different populations. Merikanto et al found that especially the evening-type population suffered from increasing sleep problems.33 But COVID-19 is not just influencing our sleep, as sleep could also have an impact on the severity of an infection. There are reports that improving sleeping habits is vital to our immune system.34–36 Sleep deprivation is proven to impair the immune systems’ ability to combat infections and inflammations, because cytokine levels, anti-bodies and cells that are fighting infections are decreasing when sleep is missing.35 Therefore, investigating nightmares, that affect sleep quality, is of high interest.
We hypothesized that NMF increased during the pandemic and that it can be predicted by mental health, sleep, and pandemic related measures. Our main objective is to examine which risk factors contribute to the increase of nightmares during the pandemic.
Materials and Methods
This paper is part of the International COVID-19 Sleep Study (ICOSS), and the research protocol and standardized survey questionnaire are described in detail elsewhere.1 The project is a cross-sectional online survey including 14 countries: Austria, Brazil, Canada, Hong Kong (China), Province Jilin (China), Finland, France, Italy, Japan, Norway, Sweden, Poland, the UK and the USA. Data was obtained from May to August 2020. Participants received a link or QR-code to access the questionnaire. All subjects were at least 18 years old and agrees voluntarily to participate anonymously, which was considered as implied consent, as approved by the local Institutional Review Board in each country. Informed consent was obtained as every subject voluntarily agreed to participate in the survey Investigators from each country obtained ethical approval from the local ethics committee. For the survey in Austria, the ethics commission of the Medical University of Vienna approved the survey and stated, that no further approval is needed as participation is voluntarily and anonymously and no clinical population is targeted. General data protection regulations were applied and the study complies with the Declaration of Helsinki.
The questionnaire collected data on sociodemographic variables (eg, age, gender, marital status, education, work) and COVID-19 related information (ie, infection, treatment, confinement). To assess psychological variables, standardized and validated measures were included. Depressive symptoms were measured using the Patient Health Questionnaire – 2 (PHQ-2, Kroenke et al, 200937) and the Generalized Anxiety Disorder – 2 (GAD-2, Kroenke et al, 200937) was used to assess symptoms of anxiety. For both scales, sum scores are calculated and the cut-off score is ≥3. The Abbreviated PTSD Checklist (PCL-2, Lang et al, 2012) assessed post-traumatic stress symptoms (PTSS) and consists of two items referring to repeated disturbing thoughts of stressful events in the past and feeling very upset when thinking about stressful events of the past, answered on a five-point scale (1 to 5). If the sum score is ≥4 it indicates PTSS. A single item assessed stress39 and the WHO-540 measured overall psychological well-being. Quality of life (QoL) and health (QoH) data were measured through linear visual analogue scales.
Questionnaires on sleep problems included items of the Basic Nordic Sleep Questionnaire,41 the STOP Questionnaire (STOP, Chung et al, 200842), the Insomnia Severity Index (ISI; Morin et al, 201143) and a single question on REM sleep behavior disorder (RBD).44 If a person answers yes on two or more items on the STOP questionnaire, an elevated risk for obstructive sleep apnoea (OSA) is indicated. Cutoff scores for the ISI were ≥8 for no insomnia, ≥10 for threshold insomnia and ≥15 for severe insomnia.
Participants reported their NMF and dream recall frequency (DRF) before and during the pandemic on a five-point scale (ie 1=“Never or less frequently than once/month”, 2=“Less frequently than once/week”, 3=“1–2 days/week”, 4=“3–5 days/week”, 5=“Daily or almost daily”). NMF and DRF were categorized as low (NMF: <1–2 nights/week; DRF: <3–5 nights/week) and high (NMF: ≥1–2 nights/week; DRF: ≥3–5 nights/week).13,45
Statistical Analysis
Statistical procedures were carried out using Stata/SE 16.1.46 Data from the USA were excluded, because collection method differed as participants received a monetary reward. Data from Poland was excluded due to too many missing values. Additionally, any participants with missing values on any of the variables were excluded. This led to the exclusion of 9100 respondents.
Chi-square tests were calculated to detect differences in NMF between the pre-pandemic and pandemic period, and to investigate differences between individuals high and low in NMF on pandemic related and sociodemographic characteristics. The participants characteristics were described using frequencies (percentages).
Logistic regressions were conducted to examine associations between high NMF during the pandemic (dependent variable) and PTSS, insomnia, sleep quality, stress, anxiety, RBD, OSA, depressive symptoms, sleep talking, sleep maintenance problems, early morning awakenings, sleep onset problems, use of sleep medicine/hypnotics, daytime sleepiness, fatigue, financial burden due to COVID-19, confinements, having had COVID-19, work situation during the pandemic that impacts how much everyday life changed, living alone, QoL, QoH and wellbeing (independent variables). Adjustments were made stepwise for DRF to see whether NMF would just be a by-product, for age and gender, as these are known to impact NMF47 and for pre-pandemic NMF, because NMF is considered relatively time-stable.48 P-values less than 0.05 were considered significant.
Variable Inflation Factors (VIF) of the independent variables were calculated to check for multicollinearity before running the regression. All VIFs were <5 indicating no critical collinearity.
Results
The final sample consisted of 15,292 participants (the initial sample size was 24,392, 9100 participants have been excluded), with 64.05% females. The mean age was 41.63 (SD = 16.55) years. 63.23% reported no confinements, whereas 16.97% were confined more than eight weeks. Further, 2.21% reported having had COVID-19.
NMF had indeed increased during the pandemic. Before the pandemic, 86.76% reported low and 13.24% high NMF. During the pandemic, these percentages changed significantly (p < 0.001) to 77.65% and 22.35%, respectively. During the pandemic, NMF decreased for 4.80% of participants, did not change for 75.37%, and increased for 19.83%.
The results comparing pandemic related variables and sociodemographic characteristics of participants by low and high NMF are shown in Table 1. Participants who were female, younger, unemployed, students, had COVID-19 were confined for a longer period and experienced higher financial burden due to the pandemic showed higher NMF.
 |
Table 1 Sociodemographic and Pandemic Related Characteristics of Participants (Low NMF vs High NMF) |
Predictors of High NMF
Predictors for high NMF during the pandemic are shown in Table 2 as odds ratios (OR) with the 95% confidence intervals. Unadjusted and adjusted results are displayed in Table 2, results for every adjustment step can be found in eTable 1 in the supplements. The fully adjusted model explained 45.29% of NMF variance. All models were significant at p<0.001.
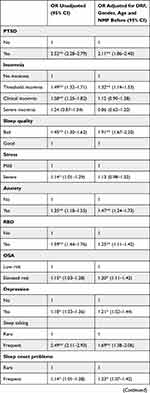 | 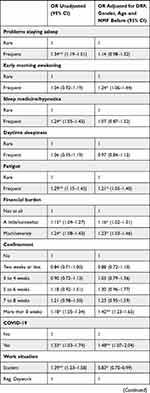 | 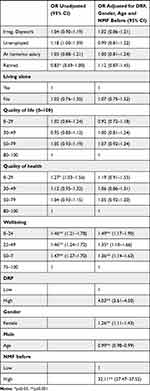 |
Table 2 Unadjusted and Adjusted Results of Predictors for High NMF During the Pandemic |
In the adjusted model, PTSS, threshold insomnia, poor sleep quality, anxiety, RBD, depression, and OSA significantly predicted high NMF. Further, sleep talking, sleep onset problems, early morning awakenings, fatigue, poor wellbeing, high DRF, being a woman, younger age and high pre-pandemic NMF were significantly associated with pandemic NMF. Concerning pandemic-related variables, suffering financially, being confined for more than 8 weeks, being a student and having had COVID-19 were significantly associated with NMF.
Post-Hoc Mediation Analysis
When looking at the predictors for high NMF, PTSS was highly significant and the OR was especially high (OR=2.11, p<0.001). This finding is unsurprising as nightmares are a common symptom of PTSD. But additionally, other significant predictors seem coherent with the diagnostic criteria of PTSD.3 Further, 46.69% of participants reported PTSS. Therefore, it seems possible, that effects of pandemic related variables on NMF might be mediated through PTSS.
To explore this possibility a mediation analysis was conducted. Pandemic related variables significantly predicting NMF were used as independent variables, to see whether the effect these pandemic factors had on NMF were a manifestation of post-traumatic stress. These pandemic factors were financial burden, confinement, having had COVID-19, and work situation. The mediator was PTSS and the dependent variable was NMF in all models. We conducted mediation analysis for each independent variable, thus resulting in four models, that are displayed in Figure 1.
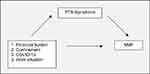 |
Figure 1 Mediation models 1, 2, 3, 4. Note: Mediation models of the effect of pandemic-related variables on NMF through PTSD. |
The analysis of Model 1 yielded a significant total effect of financial burden on NMF, B=0.36, p<0.001. Further, financial burden predicted PTSS, B=0.067, p<0.001, and PTSS predicted NMF, B=0.27, p<0.001. The relation between financial burden and NMF was still significant after including the mediator in the model, B=0.018, p<0.001. Therefore, PTSS only partially mediated the effect of financial burden on NMF, indirect effect=0.018, 95% CI [0.016; 0.020].
The results of Model 2 indicated a significant total effect of confinement on NMF, B=0.033, p<0.001. Confinement predicted PTSS, B=0.41, p<0.001 and PTSS predicted NMF, B=0.26, p<0.001. The significant effect of confinement on NMF persisted after including the mediator, B=0.022, p<0.001. Thus, the effect of confinement on NMF was partially mediated by PTSS, indirect effect=0.01, 95% CI [0.009; 0.012].
Model 3 showed a significant total effect of COVID-19 on NMF, B=0.14, p<0.001. Specifically, COVID-19 significantly predicted PTSS, B=0.18, p<0.001, and PTSS significantly predicted NMF, B=0.27, p<0.001. The direct effect of COVID-19 on NMF remained highly significant after including the mediator, B=0.09, p<0.001. It was found that the effect of COVID-19 on NMF was partially mediated by PTSS, indirect effect=0.05, 95% CI [0.035; 0.065].
Model 4 resulted in a significant total effect of work situation on NMF, B=−0.01, p<0.001. Work situation significantly predicted PTSS, B=−0.01, p<0.001, and PTSS significantly predicted NMF, B=0.27, p<0.001. The direct effect of work situation on NMF remained significant after including the mediator, B=−0.01, p=0.003. The indirect effect was significant, indicating a partial mediation, indirect effect=−0.003, 95% CI [−0.005; −0.002]. The results of all four mediation models are displayed in Figure 2.
 |
Figure 2 Results of mediation analysis. Note: c=total effect. c’=direct effect. *Indicates p<0.05. **Indicates p<0.01. |
Discussion
Did COVID-19 Traumatize Us Collectively?
The logistic regression yielded that insomnia symptoms, anxiety, depression, OSA and RBD symptoms, sleep quality, sleep talking, sleep onset problems, early morning awakenings and fatigue significantly predicted high NMF and are associated with PTSS.
Hoffman and Kruczek49 developed a Bioecological Model of Mass Trauma. It involves nested systems ordered hierarchically and the so-called chronosystem referring to time and developmental processes. The hierarchical systems are the biophysical (dispositions, fear conditioning, temperament), the microsystem (family, peers, work), the exosystem (school system, mass media, community system, health care system) and the macrosystem (societal norms, economic factors, governmental systems, socio-economic factors). The pandemic influenced all of these systems, and therefore has the potential to lead to a mass trauma.
Therefore, we conducted mediation analyses for pandemic related variables, significantly predicting NMF, to see whether their effects are mediated through PTSS. These were financial burden, confinement, having had COVID-19 and work situation. Results supported our hypothesis that pandemic effects on NMF are partly mediated though PTSS, as all models found significant indirect effects. Even though the effects were small, they still support our assumption. Additionally, we found that almost half of the participants (46.69%) scored above the cutoff on the PCL-2.38 This screening tool was used as it is very brief, and therefore suitable for this survey asking about a broad variety of different variables. The cost of this briefness is that it results in a high proportion of false positives; nevertheless, it indicates PTSS, but the percentage of positives should be interpreted with great caution. Further, we conducted mediation analyses for only four pandemic related variables that represent only a small part of the complex nature of the pandemic. Nevertheless, our findings replicate results on former pandemic or epidemic situations, which left a remarkable proportion of society with symptoms of PTSD.50 Pagel (2021) suggested that if more than 20% of a society shows PTSS, this indicates societal stress, potentially resulting in insufficient treatment resources. PTSD, in such situations, was more likely to develop and maintain amongst medical staff,52 individuals that have been isolated and if the traumatic experience persists or is repeated.51 This fact might gain relevance as many countries have already declared several lockdowns and the pandemic persists longer than people anticipated. Therefore, we suggest healthcare workers to pay additional attention to NMF in combination with identified risk factors as they may point to developing or existing PTSD.
These observations led to the assumption, that the pandemic collectively traumatized us, which helps explain the elevated NMF. A recent review demonstrated that pandemic situations indeed have the power to traumatize a society.50 Collective traumas can lead to increased dream activity and incorporation of trauma themes.47 Research results show not only heightened NMF, but also pandemic related nightmare content.53 A qualitative study analyzing dream content confirmed COVID-19 related nightmare plots in 37% of the sample.54 The authors interpreted them as related to post-traumatic nightmares. Therefore, it makes sense that in the present study factors connected to PTSS, either symptoms or risk factors, also predict high NMF.
General Discussion
Comparing NMF in subpopulations of our sample via chi-square tests revealed differences between groups. Consistent with former research, that is broadly consistent about the association that women and younger people recall dreams more frequently than men and older people,47,55–57 female and younger participants had higher NMF. Further, students and unemployed had significantly higher NMF. Additionally, people under longer confinement who had COVID-19 or greater financial burden showed higher NMF. Concerning students, lower NMF might merely be due to age effects. In contrast, unemployment, longer confinement, COVID-19 infection, and financial burden could influence NMF because those factors increase anxiety.58 The regression revealed anxiety is predictive of high NMF. In line with this, it was found that waking day negative emotions were positively related to NMF.59 Partinen et al60 found that especially financial burden led to nightmares during the pandemic.
NMF indeed increased during the pandemic, which is consistent with previous studies with different populations.13,53,61 Scarpelli et al13 found that younger age, female gender, sleep problems, symptoms of anxiety and depression significantly predicted NMF. Those results were fully replicated.
Increased DRF has frequently been reported during the pandemic.13,53,61–63 Therefore, we included DRF stepwise in our regression, to see if NMF might only be a byproduct. Results indicated that this was not the case, as all significant associations with NMF in the unadjusted model remained significant after including DRF. Exceptions were being student and stress. Concerning students this might depict that younger individuals generally recall dreams more frequently than older individuals,64–66 as aging leads to less REM- and more NREM-sleep.67 The non-significance of stress after adjusting for DRF, might be because the effect of stress on DRF68 outdid the effect on NMF or because a one-item measure was used.
For insomnia, anxiety, depressive and OSA symptoms, the effects on NMF were even larger after controlling for DRF. The results on insomnia and depression are in line with former research.22,24,63,69 The prevalence of nightmares is significantly increased in patients with insomnia with awakenings, daytime anxiety and female gender strongly related to nightmares in insomniacs22 and also to NMF in the present study. Scarpelli et al2 found people that had COVID-19 differed from people that did not have COVID-19 in NMF but not in DRF. This also supports results of the fully adjusted regression that having been infected with COVID-19 predicts high NMF.
Anxiety is likely to play a major role in the effects that the pandemic has on NMF, and also fits our hypothesis of the mediating role of PTSS as it is one of PTSDs characteristics.3 Former results on anxiety and nightmares are contradictory, with some pointing towards a positive relationship70 and some indicating no relationship.71 Schredl72 found state or situational factors, like this pandemic, influencing NMF more strongly than trait factors. This could have an impact on the association between pandemic related variables (having been infected, financial burden and confinement) and NMF.
Adjusting for pre-pandemic NMF revealed very interesting results. Pre-pandemic NMF itself unsurprisingly has an enormous impact on pandemic NMF, as nightmares are generally considered relatively time stable.48 Nevertheless, most significant predictors remained significant after adjusting. After the adjustment only threshold but not clinical or severe insomnia was significantly associated with high pandemic NMF. This might be because people with severe insomnia symptoms had high NMF before the pandemic, whereas people on the threshold of insomnia got pushed over the edge by the pandemic, as studies report increasing insomnia rates.58 This also corresponds to the ORs of poor sleep quality, early morning awakenings and fatigue increasing by adjusting for pre-pandemic NMF and to frequently reported rise of sleep-related problems during this pandemic.1,13,60,62,63,73 QoH, that is associated with NMF,74,75 significantly positively predicted pandemic NMF before including pre-pandemic NMF in the model but not after. Poor overall wellbeing, that is also associated with high NMF,76 in contrast still significantly predicted NMF in the adjusted model. This might display, that wellbeing was more affected by the pandemic, whereas QoH remained more stable across time, and therefore rather impacts pre-pandemic NMF.
Another unexpected finding was that being retired significantly positively predicted NMF until pre-pandemic NMF was included in the regression model. This might be explained by findings indicating an increase in nightmares after the age of 70,77 so NMF might have already been higher for this population in the pre-pandemic period. Students in contrast showed significantly lower NMF after adjustments were made. This can be explained by well-proofed age effects.64–66 Additionally, the virus might be less frightening for students, as it is less dangerous for younger people.78 According to our findings, anxiety increases the chances for high NMF and people perceiving themselves at greater risk for COVID-19 have higher chances for high NMF.79 This might also explain that being confined more than 8 weeks, higher financial burden and having had COVID-19 led to higher NMF. These three factors might have contributed to pandemic anxiety and to how traumatic the pandemic was experienced. Taking a closer look at the results, the higher the financial burden is, the higher the OR for high NMF and the same trend can be seen with confinements.
Even though OSA cannot be diagnosed purely by symptoms, our results imply a predictive value of some symptoms for high NMF and add to an ongoing debate. How OSA influences NMF is still unclear. There are studies stating more nightmares with more severe OSA.80,81 Other studies find the opposite.82 One explanation could be that it depends on the OSA being REM-stage specific or not.83 OSA is relevant during the pandemic as people at high risk for OSA were more likely to contract COVID-19, to be hospitalized or receive intensive unit care treatment.73
To summarize, the adjusted model identified the following predictors for NMF: PTSD, insomnia, poor sleep quality, anxiety, symptoms of RBD, high risk for OSA, depression, sleep talking, sleep onset problems, early morning awakenings, fatigue, financial burden, being confined more than 8 weeks, having had COVID-19, being a student, low wellbeing, high DRF, female sex, and older age.
Limitations
The present study included self-reported measures, which are prone to bias. This is especially true for self-reported sleep measures, as questionnaires are considered the least accurate measure in sleep assessment.84 Second, with online assessments, no researcher can clarify misunderstandings or help and access was limited to persons with internet connections. Third, sex was unevenly distributed in our sample. Further, European countries with similar economic and societal background and well-developed countries in general are over-represented in our sample. Consequently, one should be careful generalizing our results, as they might hold true only for the studied sample. Finally, our assessments for anxiety, depression, and PTSS were brief on what could reduce variability affecting the strength of relations and the power to detect differences.
Conclusion
The pandemic might have caused collective traumatization. Further research is necessary to clarify this possibility. This would shed light on the collective stress experienced and allow for the development of innovative “treatment options” not only for individual but also for collective suffering.
We found that pandemic NMF was connected to a variety of mental and sleep disorders. It may be useful to implement questions about NMF in screening processes as it can help indicate psychopathology or the presence of trauma.
Author Contributions
Brigitte Holzinger: Data collection, conception, study design, writing, analysis and interpretation, substantial revision, critical review. Franziska Nierwetberg: Conception, study design, execution, analysis and interpretation, drafting, writing. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Prof. Dr. Colin A Espie reports personal fees from Big Health (Sleepio), grants from NIHR, outside the submitted work. Dr Kentaro Matsui reports personal fees from Eisai, personal fees from Meiji Seika Pharma, personal fees from Mochida, personal fees from MSD, personal fees from Otsuka Pharmaceutical, personal fees from Takeda Pharmaceutical, personal fees from Yoshitomi Pharmaceutical, outside the submitted work. Prof. Dr. Charles M Morin reports grants from Eisai, Idorsia, Lallemand, personal fees from Eisai, Idorsia, Pear Therapeutics, royalities from Mapi Research Trust, outside the submitted work. Prof. Dr. Markku Partinen reports clinical trials from Bioprojet, Jazz Pharmaceuticals and TEVA, personal fees from GSK and Takeda, personal fees and clinical trials from MSD, outside the submitted work. Prof. Dr. Giuseppe Plazzi reports personal fees from Jazz Pharmaceutical, personal fees from Takeda, personal fees from Idorsia, personal fees from Bioprojet, outside the submitted work. Prof. Dr. Thomas Penzel reports grants from Cidelec, grants from Novartis, grants from Löwenstein Medical, personal fees from Löwenstein Medical, personal fees from Jazz Pharma, personal fees from Neuwirth, consultation to institution from Bayer Healthcare, personal fees from Cerebra, personal fees from National Sleep Foundation, grants from European Union, outside the submitted work; and Shareholder of Advanced Sleep Research, The Siestagroup GmbH, Nukute. Prof. Dr. Yun Kwok Wing reports personal fees from Eisai Co, Ltd (HK), personal fees from Lundbeck HK Ltd, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Partinen M, Bjorvatn B, Holzinger B, et al. Sleep and circadian problems during the coronavirus disease 2019 (COVID‐19) pandemic: the International COVID‐19 Sleep Study (ICOSS). J Sleep Res. 2021;30:1. doi:10.1111/jsr.13206
2. Scarpelli S, Nadorff MR, Bjorvatn B, et al. Nightmares in people with COVID-19: Did Coronavirus infect our dreams? Nat Sci Sleep. 2022;14:93–108. doi:10.2147/NSS.S344299
3. World Health Organization. ICD-10 Internationale statistische Klassifikation der Krankheiten und verwandter Gesundheitsprobleme 10. Revision –BMGF-Version 2017; 2017.
4. Spoormaker VI, Schredl M, Van den Bout J. Nightmares: from anxiety symptom to sleep disorder. Sleep Med Rev. 2006;10(1):19–31. doi:10.1016/j.smrv.2005.06.001
5. Hartmann E. Outline for a theory on the nature and functions of dreaming. Dreaming. 1996;6(2):147–170. doi:10.1037/h0094452
6. Horton CL, Malinowski JE. Autobiographical memory and hyperassociativity in the dreaming brain: implications for memory consolidation in sleep. Front Psychol. 2015;6. doi:10.3389/fpsyg.2015.00874
7. Wamsley EJ, Stickgold R. Dreaming and offline memory processing. Curr Biol. 2010;20(23):R1010–R1013. doi:10.1016/j.cub.2010.10.045
8. Malinowski JE, Horton CL. Memory sources of dreams: the incorporation of autobiographical rather than episodic experiences. J Sleep Res. 2014;23(4):441–447. doi:10.1111/jsr.12134
9. van Rijn E, Eichenlaub JB, Lewis PA, et al. The dream-lag effect: selective processing of personally significant events during rapid eye movement sleep, but not during slow wave sleep. Neurobiol Learn Mem. 2015;122:98–109. doi:10.1016/j.nlm.2015.01.009
10. Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135(5):731–748. doi:10.1037/a0016570
11. Picchioni D, Goeltzenleucher B, Green DN, et al. Nightmares as a coping mechanism for stress. Dreaming. 2002;12(3):155–169. doi:10.1023/A:
12. Gómez-Salgado J, Andrés-Villas M, Domínguez-Salas S, Díaz-Milanés D, Ruiz-Frutos C. Related health factors of psychological distress during the COVID-19 pandemic in Spain. Int J Environ Res Public Health. 2020;17(11):3947. doi:10.3390/ijerph17113947
13. Scarpelli S, Alfonsi V, Mangiaruga A, et al. Pandemic nightmares: effects on dream activity of the COVID‐19 lockdown in Italy. J Sleep Res. 2021;30. doi:10.1111/jsr.13300
14. Carr M, Nielsen T. A novel differential susceptibility framework for the study of nightmares: evidence for trait sensory processing sensitivity. Clin Psychol Rev. 2017;58:86–96. doi:10.1016/j.cpr.2017.10.002
15. Nielsen T. The Stress acceleration hypothesis of nightmares. Front Neurol. 2017;8:201. doi:10.3389/fneur.2017.00201
16. Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation; 1995: 33.
17. Cacioppo JT, Cacioppo S. Social relationships and health: the toxic effects of perceived social isolation: social relationships and health. Soc Personal Psychol Compass. 2014;8(2):58–72. doi:10.1111/spc3.12087
18. Raafat RM, Chater N, Frith C. Herding in humans. Trends Cogn Sci. 2009;13(10):420–428. doi:10.1016/j.tics.2009.08.002
19. Turner RH, Killian LM. The emergence of collective behaviour. In: Collective Behaviour.
20. Mazza C, Ricci E, Biondi S, et al. A nationwide survey of psychological distress among Italian people during the COVID-19 pandemic: immediate psychological responses and associated factors. Int J Environ Res Public Health. 2020;17(9):3165. doi:10.3390/ijerph17093165
21. Usher K, Durkin J, Bhullar N. The COVID‐19 pandemic and mental health impacts. Int J Ment Health Nurs. 2020;29(3):315–318. doi:10.1111/inm.12726
22. Ohayon MM, Morselli PL, Guilleminault C. Prevalence of nightmares and their relationship to psychopathology and daytime functioning in insomnia subjects. Sleep. 1997;20(5):340–348. doi:10.1093/sleep/20.5.340
23. Holzinger B, Saletu B, Klösch G. Cognitions in sleep: lucid dreaming as an intervention for nightmares in patients with posttraumatic stress disorder. Front Psychol. 2020;11:1826. doi:10.3389/fpsyg.2020.01826
24. Nadorff M, Nazem S, Fiske A. Insomnia symptoms, nightmares, and suicide risk: duration of sleep disturbance matters. Suicide Life Threat Behav. 2013;43(2):139–149. doi:10.1111/sltb.12003
25. Lemyre A, Bastien C, Vallières A. Nightmares in mental disorders: a review. Dreaming. 2019;29(2):144–166. doi:10.1037/drm0000103
26. Liu CH, Zhang E, Wong GTF, Hyun S, Hahm H. Factors associated with depression, anxiety, and PTSD symptomatology during the COVID-19 pandemic: clinical implications for U.S. young adult mental health. Psychiatry Res. 2020;290:113172. doi:10.1016/j.psychres.2020.113172
27. Pierce M, Hope H, Ford T, et al. Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry. 2020;7(10):883–892. doi:10.1016/S2215-0366(20)30308-4
28. Taylor S, Landry CA, Paluszek MM, Fergus TA, McKay D, Asmundson GJG. COVID stress syndrome: concept, structure, and correlates. Depress Anxiety. 2020;37(8):706–714. doi:10.1002/da.23071
29. Morin CM, Carrier J, Bastien C, Godbout R. On behalf of the Canadian sleep and Circadian Network. sleep and circadian rhythm in response to the COVID-19 pandemic. Can J Public Health. 2020;111(5):654–657. doi:10.17269/s41997-020-00382-7
30. Blume C, Schmidt MH, Cajochen C. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol. 2020;30(14):R795–R797. doi:10.1016/j.cub.2020.06.021
31. Holzinger B, Mayer L, Nierwetberg F, Klösch G. COVID-19 lockdown – are Austrians finally able to compensate their sleep debt? Sleep Med X. 2021;3:100032. doi:10.1016/j.sleepx.2021.100032
32. Gupta R, Grover S, Basu A, et al. Changes in sleep pattern and sleep quality during COVID-19 lockdown. Indian J Psychiatry. 2020;62(4):370. doi:10.4103/psychiatry.IndianJPsychiatry_523_20
33. Merikanto I, Kortesoja L, Benedict C, et al. Evening-types show highest increase of sleep and mental health problems during the COVID-19 pandemic—multinational study on 19 267 adults. Sleep. 2022;45(2):zsab216. doi:10.1093/sleep/zsab216
34. Besedovsky L, Lange T, Born J. Sleep and immune function. Eur J Physiol. 2012;463(1):121–137. doi:10.1007/s00424-011-1044-0
35. Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4(6):457–467. doi:10.1038/nri1369
36. Yousfi N, Bragazzi NL, Briki W, Zmijewski P, Chamari K. The COVID-19 pandemic: how to maintain a healthy immune system during the lockdown – a multidisciplinary approach with special focus on athletes. Biol Sport. 2020;37(3):211–216. doi:10.5114/biolsport.2020.95125
37. Kroenke K, Spitzer RL, Williams JBW, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ–4. Psychosomatics. 2009;50(6):613–621. doi:10.1016/S0033-3182(09)70864-3
38. Lang AJ, Wilkins K, Roy-Byrne PP, et al. Abbreviated PTSD Checklist (PCL) as a guide to clinical response. Gen Hosp Psychiatry. 2012;34(4):332–338. doi:10.1016/j.genhosppsych.2012.02.003
39. Elo AL, Leppänen A, Jahkola A. Validity of a single-item measure of stress symptoms. Scand J Work Environ Health. 2003;29(6):444–451. doi:10.5271/sjweh.752
40. Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom. 2015;84(3):167–176. doi:10.1159/000376585
41. Partinen M, Gislason T. Basic Nordic sleep questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4:150–155. doi:10.1111/j.1365-2869.1995.tb00205.x
42. Chung F, Yegneswaran B, Liao P, et al. STOP Questionnaire. Anesthesiology. 2008;108(5):812–821. doi:10.1097/ALN.0b013e31816d83e4
43. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi:10.1093/sleep/34.5.601
44. Postuma RB, Arnulf I, Hogl B, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study: REM Sleep Behavior Disorder Screen. Mov Disord. 2012;27(7):913–916. doi:10.1002/mds.25037
45. Li SX, Zhang B, Li AM, Wing YK. Prevalence and correlates of frequent nightmares: a community-based 2-phase study. Sleep. 2010;33(6):774–780. doi:10.1093/sleep/33.6.774
46. StataCorp. Stata Statistical Software: Release 16. TX: StataCorp LLC; 2019.
47. Nielsen TA, Stenstrom P, Levin R. Nightmare frequency as a function of age, gender, and September 11, 2001: findings from an Internet questionnaire. Dreaming. 2006;16(3):145–158. doi:10.1037/1053-0797.16.3.145
48. Schredl M, Gilles M, Wolf I, et al. Nightmares and stress: a Longitudinal Study. J Clin Sleep Med. 2019;15(09):1209–1215. doi:10.5664/jcsm.7904
49. Hoffman MA, Kruczek T, Bioecological A. Model of mass trauma: individual, community, and societal effects. Couns Psychol. 2011;39(8):1087–1127. doi:10.1177/0011000010397932
50. Yuan K, Gong YM, Liu L, et al. Prevalence of posttraumatic stress disorder after infectious disease pandemics in the twenty-first century, including COVID-19: a meta-analysis and systematic review. Mol Psychiatry. 2021;26:4982–4998. doi:10.1038/s41380-021-01036-x
51. Pagel JF. Disasters and societal trauma: complex and societal PTSD. In: Post-Traumatic Stress Disorder. Springer International Publishing; 2021:21–29. doi:10.1007/978-3-030-55909-0_3
52. Wanigasooriya K, Palimar P, Naumann DN, et al. Mental health symptoms in a cohort of hospital healthcare workers following the first peak of the COVID-19 pandemic in the UK. BJPsych Open. 2021;7(1):e24. doi:10.1192/bjo.2020.150
53. Pesonen AK, Lipsanen J, Halonen R, et al. Pandemic dreams: network analysis of dream content during the COVID-19 lockdown. Front Psychol. 2020;11:573961. doi:10.3389/fpsyg.2020.573961
54. Solomonova E, Picard-Deland C, Rapoport I, et al. Stuck in a lockdown: dreams, bad dreams, nightmares, and their relationship to stress, depression and anxiety during the COVID-19 Pandemic. PsyArXiv. 2021. doi:10.31234/osf.io/hbm84
55. Gieselmann A, Ait Aoudia M, Carr M, et al. Aetiology and treatment of nightmare disorder: state of the art and future perspectives. J Sleep Res. 2019;28(4):e12820. doi:10.1111/jsr.12820
56. Salvio MA, Wood JM, Schwartz J, Eichling PS. Nightmare prevalence in the healthy elderly. Psychol Aging. 1992;7(2):324–325. doi:10.1037/0882-7974.7.2.324
57. Schredl M, Goeritz AS. Nightmare frequency and nightmare distress: socio-demographic and personality factors. Sleep Sci. 2019;12:3. doi:10.5935/1984-0063.20190080
58. Morin CM, Bjorvatn B, Chung F, et al. Insomnia, anxiety, and depression during the COVID-19 pandemic: an International Collaborative Study. Sleep Med. 2021;87:38–45. doi:10.1016/j.sleep.2021.07.035
59. Davis K, DeCicco T. An examination of waking day stress, personality and emotions in relation to the prediction of nightmare frequency and distress: a pilot study. Int J Dream Res. 2021;14:21–29. doi:10.11588/IJODR.2021.1.73995
60. Partinen M, Holzinger B, Morin CM, et al. Sleep and daytime problems during the COVID-19 pandemic and effects of coronavirus infection, confinement and financial suffering: a multinational survey using a harmonised questionnaire. BMJ Open. 2021;11(12):e050672. doi:10.1136/bmjopen-2021-050672
61. Guerrero-Gomez A, Nöthen-Garunja I, Schredl M, et al. Dreaming in adolescents during the COVID-19 health crisis: survey among a sample of European school students. Front Psychol. 2021;12:652627. doi:10.3389/fpsyg.2021.652627
62. Fränkl E, Scarpelli S, Nadorff MR, et al. How our dreams changed during the COVID-19 pandemic: effects and correlates of dream recall frequency - a multinational study on 19,355 adults. Nat Sci Sleep. 2021;13:1573–1591. doi:10.2147/NSS.S324142
63. Gorgoni M, Scarpelli S, Alfonsi V, et al. Pandemic dreams: quantitative and qualitative features of the oneiric activity during the lockdown due to COVID-19 in Italy. Sleep Med. 2021;81:20–32. doi:10.1016/j.sleep.2021.02.006
64. Chellappa SL, Münch M, Blatter K, Knoblauch V, Cajochen C. Does the Circadian modulation of dream recall modify with age? Sleep. 2009;32(9):1201–1209. doi:10.1093/sleep/32.9.1201
65. Nielsen T. Variations in dream recall frequency and dream theme diversity by age and sex. Front Neurol. 2012;3. doi:10.3389/fneur.2012.00106
66. Scarpelli S, D’Atri A, Bartolacci C, et al. Dream recall upon awakening from non-rapid eye movement sleep in older adults: electrophysiological pattern and qualitative features. Brain Sci. 2020;10(6):343. doi:10.3390/brainsci10060343
67. Chellappa SL, Münch M, Knoblauch V, Cajochen C. Age effects on spectral electroencephalogram activity prior to dream recall: age effects on dream recall and EEG activity. J Sleep Res. 2012;21(3):247–256. doi:10.1111/j.1365-2869.2011.00947.x
68. Schredl M. Dream recall: models and empirical data. In: New Sci Dreaming Vol 2 Content Recall Personal Correl. Greenwood Publishing Group; 2007:79–114.
69. Schredl M. Nightmare Frequency in Patients with Primary Insomnia. Universitätsbibliothek der Universität Heidelberg; 2009.
70. Levin R, Hurvich MS. Nightmares and annihilation anxiety. Psychoanal Psychol. 1995;12(2):247–258. doi:10.1037/h0079625
71. Wood JM, Bootzin RR. The prevalence of nightmares and their Independence from anxiety. J Abnorm Psychol. 1990;99(1):64–68. doi:10.1037/0021-843X.99.1.64
72. Schredl M. Effects of state and trait factors on nightmare frequency. Eur Arch Psychiatry Clin Neurosci. 2003;253(5):241–247. doi:10.1007/s00406-003-0438-1
73. Chung F, Waseem R, Pham C, et al. The association between high risk of sleep apnea, comorbidities, and risk of COVID-19: a population-based international harmonized study. Sleep Breath. 2021;25(2):849–860. doi:10.1007/s11325-021-02373-5
74. Sandman N, Valli K, Kronholm E, Revonsuo A, Laatikainen T, Paunio T. Nightmares: risk factors among the Finnish general adult population. Sleep. 2015;38(4):507–514. doi:10.5665/sleep.4560
75. Pruiksma KE, Slavish DC, Taylor DJ, et al. Nightmares and insomnia in the US national guard: mental and physical health correlates. Int J Behav Med. 2021;28(2):238–249. doi:10.1007/s12529-020-09889-2
76. Zadra A, Donderi DC. Nightmares and bad dreams: their prevalence and relationship to well-being. J Abnorm Psychol. 2000;109(2):273–281. doi:10.1037/0021-843X.109.2.273
77. Park D, Kim S, Shin C, Suh S. Prevalence of and factors associated with nightmares in the elderly in a population based cohort study. Sleep Med. 2021;78:15–23. doi:10.1016/j.sleep.2020.11.039
78. Bonanad C, García-Blas S, Tarazona-Santabalbina F, et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J Am Med Dir Assoc. 2020;21(7):915–918. doi:10.1016/j.jamda.2020.05.045
79. Musse FCC, de S Castro L, Sousa KMM, et al. Mental violence: the COVID-19 nightmare. Front Psychiatry. 2020;11:579289. doi:10.3389/fpsyt.2020.579289
80. Fisher S, Lewis KE, Bartle I, Ghosal R, Davies L, Blagrove M. Emotional content of dreams in obstructive sleep Apnea hypopnea syndrome patients and sleepy snorers attending a sleep-disordered breathing clinic. J Clin Sleep Med. 2011;07(01):69–74. doi:10.5664/jcsm.28043
81. Gross M, Lavie P. Dreams in sleep apnea patients. Dreaming. 1994;4(3):195–204. doi:10.1037/h0094412
82. Lundetræ RS, Saxvig IW, Pallesen S, Aurlien H, Lehmann S, Bjorvatn B. Prevalence of parasomnias in patients with obstructive sleep Apnea. A registry-based cross-sectional study. Front Psychol. 2018;9:1140. doi:10.3389/fpsyg.2018.01140
83. BaHammam AS, Almeneessier AS. Dreams and nightmares in patients with obstructive sleep Apnea: a review. Front Neurol. 2019;10:1127. doi:10.3389/fneur.2019.01127
84. Ibáñez V, Silva J, Cauli O. A survey on sleep assessment methods. PeerJ. 2018;6:e4849. doi:10.7717/peerj.4849
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Effects of Obstructive Sleep Apnea on SARS-CoV-2 Antibody Response After Vaccination Against COVID-19 in Older Adults
Tufik S, Andersen ML, Rosa DS, Tufik SB, Pires GN
Nature and Science of Sleep 2022, 14:1203-1211
Published Date: 28 June 2022
The Multifaceted Influence of COVID-19 on Indian Dentists: A Cross-Sectional Survey
Shinde O, Jhaveri A, Pawar AM, Karobari MI, Banga KS, Arora S, Bhardwaj A, Djuanda AG, Wahjuningrum DA
Psychology Research and Behavior Management 2022, 15:1955-1969
Published Date: 1 August 2022
Initial Psychometric Development of the Fear and Anxiety to COVID-19 Scale in Nursing Professionals: An Occupational Health Assessment Tool
Morgado-Toscano C, Allande-Cussó R, Fagundo-Rivera J, García-Iglesias JJ, Climent-Rodríguez JA, Navarro-Abal Y, Gómez-Salgado J
Risk Management and Healthcare Policy 2022, 15:1947-1957
Published Date: 14 October 2022
Pre- and Post-Pandemic (COVID-19) Mental Health of International Students: Data from a Longitudinal Study
Jamshaid S, Bahadar N, Jamshed K, Rashid M, Imran Afzal M, Tian L, Umar M, Feng X, Khan I, Zong M
Psychology Research and Behavior Management 2023, 16:431-446
Published Date: 15 February 2023
Health-Related Quality of Life for Jordanian-Recovered Individuals During Post-COVID-19 Era: A Cross-Sectional Study
Abuhammad S, Khabour OF, Alzoubi KH, Hamaideh S, Khassawneh BY, Mehrass AAO, Alsmadi BF, Ababneh AM
Patient Preference and Adherence 2023, 17:1303-1310
Published Date: 22 May 2023
