Back to Journals » Journal of Pain Research » Volume 15
Fire Needling Acupuncture for Adult Patients with Acute Herpes Zoster: Protocol of a Systematic Review and Meta-Analysis
Authors Liu L , Chen Q, Yang J, Gang W , Zhao L , Lyu T, Jing X, Zhang CS, Li B
Received 12 April 2022
Accepted for publication 8 July 2022
Published 2 August 2022 Volume 2022:15 Pages 2161—2170
DOI https://doi.org/10.2147/JPR.S370484
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Lu Liu,1 Qiuyi Chen,1 Jiwei Yang,2 Weijuan Gang,2 Luopeng Zhao,1 Tianli Lyu,1 Xianghong Jing,2 Claire Shuiqing Zhang,3 Bin Li1
1Department of Acupuncture and Moxibustion, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Key Laboratory of Acupuncture Neuromodulation, Beijing, People’s Republic of China; 2Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 3School of Health and Biomedical Sciences, RMIT University, Melbourne, VIC, Australia
Correspondence: Claire Shuiqing Zhang, School of Health and Biomedical Sciences, RMIT University, Plenty Road, Bundoora, Melbourne, VIC, Australia, Tel +61 3 9925 7002, Fax +61 3 9925 7178, Email [email protected] Bin Li, Department of Acupuncture and Moxibustion, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Key Laboratory of Acupuncture Neuromodulation, 23, Meishuguanhou Street, Beijing, People’s Republic of China, Tel +86 18910781852, Fax +86 52176055, Email [email protected]
Background: Acute herpes zoster (HZ) is characterized as a vesicular rash with unilateral distribution produced by the reactivation of varicella zoster virus. It can induce various comorbidities that can adversely influence the quality of life of patients. The purpose of this systematic review is to assess the effect and safety of fire needling acupuncture on acute HZ.
Methods: Three English databases (PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials) and four Chinese Databases (China National Knowledge Infrastructure, Wanfang database, Chongqing VIP database, and China Biology Medicine database) will be searched from their inceptions to July 2022. Randomized controlled trials investigating fire needling acupuncture therapy for acute HZ will be included, regardless of publication status. Two reviewers will independently conduct the study screening, data extraction, and research quality assessments. The primary outcome measures are the Pain Visual Analogue Scale and the occurrence of postherpetic neuralgia. Secondary outcomes measures are the evaluation of skin lesions, time to resolution of pain, tolerance evaluation, total effective rate, adverse events and changes in inflammatory and immune suppression markers in peripheral plasma. All included studies will be assessed for methodological quality via the Cochrane Collaboration’s bias risk assessment tool. Meta-analyses will be undertaken using Review Manager V.5.3 software. The findings will be reported as the risk ratio of the binary data and the mean difference (MD) or standardized MD of the continuous data. Subgroup analyses and sensitivity analyses will be conducted where appropriate. The Grading of Recommendations Assessment, Development, and Evaluation will be used to assess evidence certainty.
Results: From the study, we will ascertain the effects and safety of fire needling acupuncture on acute HZ.
Conclusion: This study will validate the effects and safety of fire needling acupuncture in the management of acute HZ, generating new evidence to guide acupuncture interventions for acute HZ in the future.
Registration Number: PROSPERO CRD42020199047.
Keywords: fire needling acupuncture, herpes zoster, postherpetic neuralgia, randomized controlled trials, systematic review
Introduction
Acute herpes zoster (HZ) is a common dermatological condition. Symptoms of acute HZ typically start with pain sensation in the affected dermatome, which is followed by a vesicular eruption within 2–3 days after the onset. Acute HZ is often accompanied by continuous or episodic sensations such as pain, paresthesia (eg, burning and tingling), dysesthesia (altered or painful sensitivity to touch), allodynia (pain associated with nonpainful stimuli), or hyperesthesia (exaggerated or prolonged response to painful stimuli).1,2 Although acute HZ is self-limited, 13–47% of patients may have comorbidities such as postherpetic neuralgia (PHN), herpes zoster ophthalmicus (HZO), cutaneous and visceral dissemination, with PHN being the most common one.3,4 Severe cases of these comorbidities may necessitate hospitalization.5 In some patients, particularly the elderly, the pain persists after the rash cures and proceeds into PHN.
The incidence of HZ is counted as 3 to 5 per 1000 person-years globally and 3 to 10 per 1000 person-years in the Asia-Pacific region, with an annual increase of 2.5–5.0%.6 After the age of 50, the specific cellular immune function of varicella-herpes zoster gradually decreases. Hence, the incidence, hospitalization rate, and mortality rate of HZ increase progressively along with the increase in age.7 The burden of HZ is significant worldwide, with millions affected and the incidence rising.8 As shown by the latest Global Burden of Disease study 2017,9 HZ is ranked the 5th leading cause of years lived with disability (YLDs) of infectious diseases (0.30 million YLDs, 95% UI 0.18 to 0.46). Globally, between 1990 and 2007, the number of all-age YLDs attributed to HZ increased by 39.5% (95% UI 36.3 to 42.8). From 2007 to 2017, there were further increases in the number of all-age YLDs attributable to HZ (22.1%, 95% UI 19.5–24.8). The pain symptoms caused by HZ significantly affect people’s daily activities and sleep, cause a negative psychological state, impact their quality of life, and lead to medical burden on patients, their families and society.10
Acute HZ is triggered by the varicella-zoster virus (VZV) reactivating. Primary infection of VZV causes varicella. Once varicella resolves, the virus remains latent in the dorsal root ganglia. With advancing age or immunosuppression, cell-mediated immunity to VZV declines and the virus reactivates to cause acute HZ, occurring anywhere in the body.11 The risk factors for acute HZ are elderly cell immune deficiency, genetic susceptibility, mechanical trauma, systemic diseases (such as glucose and urine disease, kidney disease, fever and hypertension), recent mental stress, fatigue and other common causes. The risk of HZ in women is higher than that in men.12 In the general population, the estimated lifetime risk of HZ is roughly 30%, with the risk increasing dramatically after the age of 50.13
The treatments of acute HZ aim to reduce the intensity and duration of acute zoster-associated pain (ZAP) and cutaneous symptoms and improve patients’ quality of life. According to the European consensus-based (S2k) Guideline on the Management of HZ, these treatments consist of antiviral medication, pain management, and local therapy. Usually, they are used for seven days, commencing 72 hours after the rash appears. Where HZ presents signs of the visceral or central nervous system, these treatments can be provided for up to 21 days.14 PHN is often refractory to treatment. It is unclear whether the current acute HZ treatments could prevent the occurrence or shorten the duration of PHN.15,16 Treatments with established efficacy for PHN consist of gabapentin, pregabalin, topical lidocaine patch, tricyclic antidepressants, and opioid analgesics.17 However, for many patients, whether taken alone or in combination, these medications are either partially or completely ineffective. The pain caused by PHN can persist for months or even years. The long-term pain often leads to depression, fatigue, insomnia, altered activities of daily living and decreased socialization.18 The efficacy of acupuncture in the treatment of pain diseases has been confirmed by evidence-based medicine, especially for HZ.19 Relevant meta-analysis proved that fire acupuncture therapy can be used as an effective method to treat pain diseases.20
Fire needling acupuncture is one subtype of acupuncture therapy, which is widely used for the treatment of acute HZ in Chinese medicine hospitals in China.21 Fire needling acupuncture involves inserting a red-hot needle through a person’s skin at specific points on the body,22 hence it combines conventional acupuncture and cauterization therapy. Clinical studies have been conducted to investigate the effects of fire needling acupuncture for treating HZ.23–26 The findings from these studies suggested certain benefits of fire needling acupuncture in reducing pain intensity, improving rash healing, and preventing the occurrence of PHN and other complications of HZ.
Recent clinical research showed that fire-needling acupuncture was effective for HZ, in terms of shortening the duration and intensity of pain and reducing the occurrence of PHN.27–32 Although fire needling acupuncture has shown effects for relieving HZ, its mechanisms remain unclear. Previous studies have found that fire needling acupuncture might promote microcirculation in the lesion area by regulating cutaneous nerves, which potentially absorb inflammatory mediators and exert neuroprotective effects.33 Furthermore, the high temperature of the red-hot needle may generate an effect that directly eliminates the microorganisms and achieves anti-inflammatory effects.34 Specifically, the mechanism of fire needling acupuncture alleviating mechanical and thermal pain intensity is supported by animal studies in which fire needling acupuncture activated pain-related PKA/TRPV1 pathway.35 Furthermore, fire needling acupuncture has been found to show significant effects on inflammatory and immune regulation in patients’ peripheral plasma, such as inhibited CD8+ T-cell counts, increased CD4+ T-cell counts and CD4/CD8 T-cell ratio.36
Two systematic reviews on acupuncture for PHN have been published in 201837 and 2019,38 suggesting that acupuncture may reduce pain intensity in patients with PHN. However, neither review focused on fire needling acupuncture and acute HZ. The clinical evidence of fire needling acupuncture for acute HZ needs to be systematically evaluated. Therefore, we plan this systematic review to synthesize currently available clinical evidence of fire needling acupuncture for acute HZ.
Materials and Methods
Study Registration
The systematic review protocol has been registered at the PROSPERO database (CRD42020199047). The Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) is used to format our publication (Supplementary Table 1).39 The review will be carried out in compliance with the PRISMA-P statement.40,41
Inclusion Criteria for Study Selection
Types of Studies
This review will encompass clinical randomized controlled trials (RCTs) regardless of their publication status.
Types of Participants
This study will follow the diagnostic criteria of herpes zoster defined in the “Consensus of Chinese experts on herpes zoster” published in 2018 by the dermatologist group of the Chinese Medical Association.42 This review will encompass studies that enrolled adults (≥18 years) with acute HZ diagnosed according to the clinical diagnostic criteria.42,43 No limitation will be placed on participants’ gender, race, ethnicity, education and economic status.
Types of Interventions
We will include studies comparing fire needling acupuncture with pharmacotherapy or comparing the combination of fire needling acupuncture and pharmacotherapy with pharmacotherapy alone. No limitation will be placed on the fire needling acupuncture details such as acupoint selection, duration of treatment sessions, and manipulation techniques. Pharmacotherapy for acute HZ includes antiviral medication (acyclovir, valacyclovir, famciclovir, brivudine, vidarabine, etc.) and pain management (acetaminophen, dipyrone, ibuprofen, diclofenac, naproxen, cyclooxygenase-2 inhibitors, tramadol, hydromorphone, oxycodone, tilidine, naloxone, gabapentin, pregabalin, lidocaine, amitriptyline, etc.).14 The dosage and treatment duration of pharmacotherapy will not be limited. Studies involve other Chinese medicine therapies as the comparator will be excluded.
Types of Outcome Measures
Primary Outcomes
The primary outcomes are the pain intensity assessed by Visual Analogue Scale (VAS) and the occurrence of PHN at 1, 3, and 6 months after HZ.44
Secondary Outcomes
Secondary outcomes are the evaluation of skin lesions (time to resolution of rash, time to cessation of new lesion formation, time to crust formation), time to resolution of pain, tolerance evaluation, and total effective rate.45 There are four levels of effectiveness: (1) Clinically healed: The skin lesions have crusted, and the pain sensation has entirely vanished; (2) Remarkably effective: More than 70% of the skin lesions have disappeared, and the pain sensation has been significantly reduced; (3) Effective: The skin lesions have disappeared by around 30%, and the pain sensation has been reduced; (4) Ineffective: The skin lesions have disappeared less than 30%, no relief of pain.45 The total effective rate is calculated as the proportion of people who obtained clinical effectiveness of the first three levels. Adverse events and changes in inflammatory and immune suppression markers (interleukin (IL)-2, IL-6, IL-10, CD4+ T-cell counts, CD8+ T-cell counts and CD4/CD8 T-cell ratio) in plasma will also be evaluated as secondary outcome measures.
Search Methods for Identification of Studies
Electronic Searches
From their inception to July 2022, electronic searches will be conducted in the following databases: PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure (CNKI), Wanfang database, Chongqing VIP database (CQVIP), and China Biology Medicine database (CBM). The following search terms will be used: HZ, zona, zoster, shingles. zoster-associated pain, herpetic pain, herpetic neuralgia, PHN, HZ ophthalmicus, HZ oticus, zoster sine herpete, fire needling acupuncture, fire needling, fire needle, fire acupuncture, red hot needle, burn needle, caloripuncture, ignipuncture, pyronyxis and pyropuncture. The Cochrane Handbook for Systematic Reviews of Interventions has been used to construct a search strategy.46 Table 1 presents the search strategy for the PubMed database. The following clinical trial registries will be searched for ongoing trials with unpublished data: International Clinical Trial Registration Platform (ICTRP) (https://www.who.int/clinical-trials-registry-platform), National Institute of Health clinical registry ClinicalTrials.gov (https://clinicaltrials.gov/), Chinese Clinical Trial Registry (ChiCTR) (http://www.chictr.org.cn/), ISRCTN registry (https://www.isrctn.com/) and Australian New Zealand Clinical Trials Registry (ANZCTR) (https://www.anzctr.org.au/). The trial investigators will be approached for the most recent clinical data in ongoing RCTs by E-mail.
 |
Table 1 Search Strategy for PubMed |
Searching Other Resources
Other trials will be found by searching and browsing the list of all verified websites, which includes related associations, organizations, institutions, and preprint servers. The following websites will be searched for preprint studies: medRxiv (https://www.medrxiv.org/), Research Square (https://www.researchsquare.com/) and Arxiv (https://arxiv.org/). The citation searching will be conducted to find the references of published studies and identify additional trials. Grey literature, including theses or dissertations, conference proceedings, and government reports, will also be searched.
Data Collection and Analysis
Selection of Studies
All citations obtained from the search will be entered into an Endnote library. After screening the titles and abstracts, all relevant trials’ full-text articles will be retrieved for subsequent eligibility screening. Two review authors (LL and QC) will independently conduct the screening; any dispute will be resolved through discussion with a third author (CSZ). Where necessary, the trial investigators will be contacted for more information. A PRISMA-P flow chart depicts the primary selection process (Figure 1).
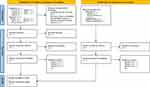 |
Figure 1 PRISMA flow diagram of studies identified. Abbreviations: CNKI, China National Knowledge Infrastructure; CQVIP, Chongqing Chinese Scientific Journal Database; CBM, China Biology Medicine Database; ICTRP, International Clinical Trial Registration Platform; ChiCTR, Chinese Clinical Trial Registry; ANZCTR, Australian New Zealand Clinical Trials Registry. Notes: Adapted from Moher D, Shamseer L, Clarke M et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1.40 Creative Commons CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/legalcode). |
Data Extraction and Management
Two researchers will extract data from the included studies in a separate and independent manner and fill a data extraction form. The following information will be extracted: study title, first author, journal, publication year, inclusion and exclusion criteria, sample size, study design, randomization methods, allocation concealment, blinding methods, treatment and control interventions (duration, frequency, acupoints, acupoint number, medication administration, dosage), outcomes, study population size (randomized and completed), dropouts, age, gender, disease duration, participant withdrawals, and adverse events. We will email the authors of RCTs for clarification if the reported data is ambiguous. Any dispute will be settled by discussion between the two authors, and any subsequent dispute will be resolved through arbitration by a third author (CSZ).
Risk of Bias Assessment in Included Studies
The risk of bias was evaluated using the Cochrane Risk of Bias 2.0 tool.47 Two review authors (JY and WG) will assess the risk of bias for all included studies using the Cochrane Collaboration’s tool for assessing risk of bias. The following items will be assessed for potential risk of bias: the sequence generation, allocation sequence concealment, blinding of participants, investigators and care providers, blinding of outcome assessment, incomplete outcome reporting, selective reporting of results, and conflict of interest.43 This review uses high, low, or unclear risk of bias as the key judgements to these assessments. If there are disagreements in the results, the final decision will be taken after consulting with a third author (CSZ). The study’s information on risk of bias assessment will be presented in tabular form, and the findings and implications will be systematically evaluated. We will email the authors of RCTs for clarification if any data is unclear.
Measures of Treatment Effects
Using the Review Manager statistical software (RevMan) V.5.3, data on treatment effects will be synthesized and statistically analyzed. Continuous data will be analyzed by using a mean difference (MD) or standard MD with 95% confidence interval (CI). A risk ratio (RR) with a 95% CI will be used to analyze dichotomous data.
Unit of Analysis Issues
It is not feasible to perform cluster-randomized or cross-over studies to assess the effects of fire needling acupuncture for acute HZ. To prevent unit-of-analysis errors, we will define several different outcomes and perform separate analyses. In studies comparing more than two intervention groups, we will include the pertinent pair of intervention groups in the analysis.
Dealing with Missing Data
Reviewers will contact the trial authors to clarify any missing or inadequate data. An intent-to-treat analysis (containing data from all participants) will be undertaken if feasible.
Heterogeneity Assessment
Heterogeneity will be evaluated using a visual inspection of the forest plot, a Mantel-Haenszel 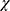 2 test and Higgins’ I square (I2) statistic of homogeneity by RevMan software. A high I2 value indicates statistically significant heterogeneity. When a high heterogeneity (I2 > 50%) is detected, a meta-analysis with the random-effects model will be utilized to determine the overall treatment effect. If the I2 is <50%, the data will be pooled using a fixed-effects model. The I2 value can be categorized into four classifications based on the Cochrane Handbook for systematic reviews:43 a value of 0%–40% denotes little or no heterogeneity; a value of 30%–60% denotes moderate heterogeneity; a value of 50%–90% denotes substantial heterogeneity; and a value of 75%–100% denotes considerable heterogeneity. Furthermore, a subgroup analysis or meta regression will be used to investigate the causes of heterogeneity in the trial results.
2 test and Higgins’ I square (I2) statistic of homogeneity by RevMan software. A high I2 value indicates statistically significant heterogeneity. When a high heterogeneity (I2 > 50%) is detected, a meta-analysis with the random-effects model will be utilized to determine the overall treatment effect. If the I2 is <50%, the data will be pooled using a fixed-effects model. The I2 value can be categorized into four classifications based on the Cochrane Handbook for systematic reviews:43 a value of 0%–40% denotes little or no heterogeneity; a value of 30%–60% denotes moderate heterogeneity; a value of 50%–90% denotes substantial heterogeneity; and a value of 75%–100% denotes considerable heterogeneity. Furthermore, a subgroup analysis or meta regression will be used to investigate the causes of heterogeneity in the trial results.
Publication Bias Assessment
To examine publication bias and the impact of small-scale research, a funnel plot will be utilized. When more than 10 trials are included, Begg’s and Egger’s tests will be conducted to evaluate the asymmetry of the funnel plot using Stata software V.14.0. A value of p < 0.05 will imply a significant publication bias.46,48
Synthesis of Data
For data synthesis, RevMan will be employed. If the meta-analysis is not practicable owing to significant clinical and methodological heterogeneity, the results will be summarized in a descriptive qualitative summary.
Subgroup Analysis
If applicable, subgroup analysis will be undertaken to identify plausible reasons of heterogeneity in the previous analysis. The analysis will be carried out based on variations in the characteristics of the treatment frequency, controls and disease severity.
Sensitivity Analysis
Sensitivity analysis will be performed based on the risk of bias by redoing the estimation after excluding studies with a high or unclear risk of bias in specific domains. When inconsistent results are confirmed, the results will be compared and reviewed, and conclusions will be drawn with caution.
Grading the Certainty of Evidence
The Grading of Recommendation, Assessment, Development, and Evaluation (GRADE)49 will be utilized independently by two authors (LL and QC) to evaluate the certainty of the evidence. Specifically, the certainty of evidence will be downgraded for several reasons: study limitations, results inconsistency, indirectness of evidence, imprecision and reporting bias. The evaluation findings will also be cross-checked, and any further disagreement will be arbitrated by a third author (CSZ).
Patient and Public Involvement
Patients will not be engaged in the development of the research question, study design, data collection, outcome measures, preparation of this study protocol or analysis of the systematic review and meta-analysis. The findings will not be disseminated to research participants.
Ethics and Dissemination
Ethical approval will not be required since the included studies in our review do not comprise private data from individuals or violate patients’ privacy. The main information will be extracted from published literature.
The results of this study will be published in a peer-reviewed journal or disseminated in a relevant conference report to provide a reference for the effects and safety of acupuncture treatment for acute herpes zoster.
Discussion
Previously published clinical studies have demonstrated that fire needling acupuncture is beneficial in the treatment of acute HZ and has no significant side effects.23,24 One systematic review published in Chinese language concluded that fire needling acupuncture was superior to pharmacological treatments for HZ in terms of pain relief and prevention of PHN occurrence.50 However, this review did not limit the participants to be acute HZ only. The intervention evaluated by this study was not limited to fire needling acupuncture, authors also included studies that applied the combination of fire needling acupuncture with electro-acupuncture, cupping, or blood-letting therapies. The control methods consist of varied pharmacological treatments. The authors did not conduct subgroup analyses based on the stage of herpes zoster, different intervention and control treatments.50 Therefore, the results of this systematic review could not translate to clinical practice directly. A recently published systematic review protocol is proposed to evaluate the treatment effects of fire needling acupuncture for acute HZ.51 In this study, the authors will also include the combination of fire needling acupuncture with other acupuncture therapies such as electroacupuncture, cupping, moxibustion, bloodletting, etc. The outcome measures will be limited to pain intensity and incidence of PHN. Compared with these two systematic reviews, our study will focus on fire needling acupuncture only, the research questions of our study are more targeted, and therefore, we will provide more precise evidence to this area. The outcome measures evaluated by our study will include the evaluation of skin lesions, time to resolution of pain, tolerance evaluation, and changes in inflammatory and immune suppression markers in peripheral plasma. In addition, we will also conduct analyses on publication bias and GRADE. The results of our study will provide more robust and targeted evidence to this field to assist clinical decision-making, and therefore benefit patients with acute HZ from evidence-based clinical practice.
Abbreviations
HZ, Herpes zoster; MD, mean difference; PHN, postherpetic neuralgia; HZO, herpes zoster ophthalmicus; YLDs, years lived with disability; VZV, varicella-zoster virus; ZAP, zoster-associated pain; PRISMA-P, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs, randomized controlled trials; VAS, Visual Analogue Scale; CI, confidence interval; RR, risk ratio; GRADE, Grading of Recommendation, Assessment, Development, and Evaluation.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Supplementary Material
Supplementary Table 1: PRISMA-P checklist.
Funding
This study was supported by China National Natural Science Foundation (82074179), China Association for Science and Technology Young Talent Lifting Project (2019-2021ZGZJXH-QNRC001), Beijing Municipal Education Commission Science and Technology Plan General Project (KM202110025005), Capital health development scientific research project Excellent Young Talents (Capital development 2020-4-2236), National Key Research and Development Plan (2019YFC1709703), and National Administration of Traditional Chinese medicine: 2019 Project of building evidence-based practice capacity for TCM (2019XZZX-ZJ002).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: differences in demographic data and sensory symptoms. Pain. 2009;146(1–2):34–40.
2. Schmader K. Herpes Zoster. Ann Intern Med. 2018;169(3):Itc19–itc31.
3. Forbes HJ, Bhaskaran K, Thomas SL, et al. Quantification of risk factors for postherpetic neuralgia in herpes zoster patients: a cohort study. Neurology. 2016;87(1):94–102.
4. Borkar DS, Tham VM, Esterberg E, et al. Incidence of herpes zoster ophthalmicus: results from the Pacific Ocular Inflammation Study. Ophthalmology. 2013;120(3):451–456.
5. Johnson RW, Bouhassira D, Kassianos G, et al. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37.
6. Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81(10):928–930.
7. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833.
8. Marra F, Parhar K, Huang B, et al. Risk factors for herpes zoster infection: a meta-analysis. Open Infect Dis. 2020;7(1):548.
9. James SL, Abate D. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858.
10. Li Y, An Z, Yin D, et al. Disease burden due to herpes zoster among population aged ≥50 years old in China: a community based retrospective survey. PLoS One. 2016;11(4):e0152660.
11. Gershon AA, Gershon MD, Breuer J, et al. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J clin virol. 2010;48 Suppl 1(Suppl 1):S2–7.
12. Sauerbrei A. Diagnosis, antiviral therapy, and prophylaxis of varicella-zoster virus infections. Eur j Clin Microbiol Infect Dis. 2016;35(5):723–734.
13. Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clinic Proceedings. 2007;82(11):1341–1349.
14. Werner RN, Nikkels AF, Marinović B, et al. European consensus-based (S2k) Guideline on the Management of Herpes Zoster – guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), Part 2: treatment. J Eur Acad Dermatol Venereol. 2017;31(1):20–29.
15. Chen N, Li Q, Yang J, et al. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Sys Re. 2014;1(2):Cd006866.
16. Alper BS, Lewis PR. Does treatment of acute herpes zoster prevent or shorten postherpetic neuralgia? J Fam Pract. 2000;49(3):255–264.
17. Gross GE, Eisert L, Doerr HW, et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J German Soc Dermatol. 2020;18(1):55–78.
18. Du J, Sun G, Ma H, et al. Prevalence and risk factors of anxiety and depression in patients with postherpetic neuralgia: a retrospective study. Dermatology. 2020;1–5.
19. Liu WH, Chen C, Wang F, et al. trend and current situation of acupuncture-moxibustion indications. World J Acupuncture Moxibustion. 2020;30(04):245–250.
20. Deng KF, Li LHZ, Pan TZ, et al. Meta-analysis and trial sequential analysis on blood uric acid and joint function in gouty arthritis treated with fire needling therapy in comparison with western medication. World J Acupuncture Moxibustion. 2022;32(1):49–60.
21. Wang JX, Zhao WX, Zeng JC, et al. Systematic review and sequential analysis on treatment of herpes zoster pain mainly by fire needle therapy. Acupuncture Res. 2019;44(9):677–685.
22. Zhang Y, Peng W, Clarke J, et al. Acupuncture for uterine fibroids. Cochrane Database Sys Re. 2010;1:Cd007221.
23. Zhang Y, Li SH, Yang L, et al. Shallow fire-needle acupuncture stimulation plus cupping relieves neuralgia and down-regulates serum substance P level in patients with acute herpes zoster. Acupuncture Res. 2018;43(8):492–494.
24. Huang GF, Zhang HX, Xu ZS, et al. Comparison of therapeutic effects of different types of acupuncture interventions on herpes zoster in acute stage. Acupuncture Res. 2012;37(5):403–408.
25. Tian H, Wei L. Filiform fire needling plus cotton moxibustion for 126 cases of herpes zoster. Chin Acupuncture Moxibustion. 2015;35(10):1031–1032.
26. Gao XY, Xie J, Meng LY. Two proved cases of treating herpes zoster pain by needling wei. World J Acupunct Moxibust. 2019;29(1):28–30.
27. He KL, Lu TT, Ma RJ. Treatment of 32 cases of acute herpes zoster with Jiaji fire needle and Ashi point acupuncture. Zhejiang J Tradit Chin Med. 2019;54(11):842.
28. Jia YN, Gu JH. Treatment of 42 cases of acute herpes zoster with fire needle and cupping. Tradit Chin Med Clin Res. 2019;11(33):95–97.
29. Gui XH, Ma CY, Gao Y, et al. Clinical observation on the treatment of herpes zoster with fire needle combined with electroacupuncture and its effect on serum IL-4 and TNF-a. Liaoning J Tradit Chin Med. 2019;46(11):2399–2404.
30. Nie QL. Clinical study on the treatment of middle-aged and elderly herpes zoster with fibro-fire needle combined with surrounding acupuncture. Shenzhen J Integr Tradit Chin West Med. 2020;30(06):57–58.
31. Zhang Y, Liang ZH, Liu XH, et al. Efficacy observation of fire needle Zan needling method in the treatment of 30 cases of acute herpes zoster. Yunnan J Tradit Chin Med. 2016;39(01):50–53.
32. Jiao DH, Peng JY, Cao J. 30 cases of herpes zoster treated by fire needle plus cupping. Chin J Dermatol Venereol. 1994;1(2):119.
33. Xu J, Cheng S, Jiao Z, et al. Fire needle acupuncture regulates Wnt/ERK multiple pathways to promote neural stem cells to differentiate into neurons in rats with spinal cord injury. CNS Neurol Disord Drug Targets. 2019;18(3):245–255.
34. Lai HC, Lin YW, Hsieh CL. Acupuncture-analgesia-mediated alleviation of central sensitization. Evidence Complementary Alternative Med. 2019;2019:6173412.
35. Wang XD, Bao YC, Wei YZ, et al. Effects of Lingnan fire needling on PKA/TRPV1 pathway in dorsal root ganglia of rats with postherpetic neuralgia. Chin J Dermatol Venereol. 2021;35(06):684–690.
36. Deng SQ, Li Q, Jian XY, et al. Clinical effect of Lingnan fire acupuncture on herpes zoster and its effect on immunity. J Mathematical Med. 2019;32(05):698–700.
37. Wang Y, Li W, Peng W, et al. Acupuncture for postherpetic neuralgia: systematic review and meta-analysis. Medicine. 2018;97(34):e11986.
38. Pei W, Zeng J, Lu L, et al. Is acupuncture an effective postherpetic neuralgia treatment? A systematic review and meta-analysis. J Pain Res. 2019;12:2155–2165.
39. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
40. Moher D, Shamseer L, Clarke M, et al.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
41. Shamseer L, Moher D, Clarke M, et al.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;2(350):g7647.
42. Dermatologist Branch of Chinese Medical Association. Herpes zoster expert consensus in China. Chin J Dermatol. 2018;51(6):403–408.
43. Kramer S, Baeumler P, Geber C, et al. Somatosensory profiles in acute herpes zoster and predictors of postherpetic neuralgia. Pain. 2019;160(4):882–894.
44. Coyle ME, Liang H, Wang K, et al. Acupuncture plus moxibustion for herpes zoster: a systematic review and meta-analysis of randomized controlled trials. Dermatol Ther. 2017;30(4):87.
45. National Administration of Traditional Chinese Medicine. Standards for TCM industry of the People’s Republic of China. Criteria of diagnosis and therapeutic effect of diseases and syndromes in TCM (ZY/T001.1-94). Nanjing Univ Press. 1994;1:617–618.
46. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane. 2021.
47. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;28(366):l4898.
48. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
49. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926.
50. Wang JX, Zhao WX, Zeng JC, et al. Systematic review and sequential analysis on treatment of herpes zoster pain mainly by fire needle therapy. Zhen Ci Yan Jiu. 2019;44(9):677–685.
51. Wang J, Wang X, Xia H, et al. An update of fire needle acupuncture for acute herpes zoster and prevention of postherpetic neuralgia in adults: a protocol for systematic review and meta-analysis. Medicine. 2021;100(1):e24180.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
