Back to Journals » Infection and Drug Resistance » Volume 16
Comparison of a One- versus Two-Week Treatment with Famciclovir Upon Reductions in Pain and Occurrence of Postherpetic Neuralgia in Herpes Zoster: A Randomized Open-Label Trial
Authors Peng F , He H, Xia T, Lv S
Received 8 August 2022
Accepted for publication 16 January 2023
Published 2 February 2023 Volume 2023:16 Pages 721—726
DOI https://doi.org/10.2147/IDR.S385442
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Fen Peng,1,* Haiyang He,2,* Tianbao Xia,2 Shichao Lv2
1Department of Dermatology, Peking University Third Hospital, Beijing, People’s Republic of China; 2Department of Dermatology, Special Medical Center of Strategic Support Force of PLA, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tianbao Xia; Shichao Lv, Department of Dermatology, Special Medical Center of Strategic Support Force of PLA, No. 9 Anxiang Beili Road, Chaoyang District, Beijing, 100101, People’s Republic of China, Email [email protected]; [email protected]
Background: Herpes zoster (HZ) is an acute herpetic skin disease resulting from the varicella-zoster virus. Typically, this condition is treated with a one-week administration of antiviral drugs, including famciclovir, which can effectively control the symptoms during the acute phase and prevent the occurrence of postherpetic neuralgia (PHN).
Objective: To investigate whether a longer, two-week, regimen would enhance the capacity for famciclovir to reduce pain and prevent the occurrence of postherpetic neuralgia.
Methods: HZ patients were randomly divided into two groups who were treated with famciclovir for either a one- or two-week period. Following their respective famciclovir treatments, patients were assessed for potential differences in pain intensity as evaluated at 1, 2, 4, 8 and 12 weeks post-treatment. In addition, the occurrence of postherpetic neuralgia at three months after treatment was compared between the two groups.
Results: Of the 86 patients initially enrolled, 80 completed the study with N=40 randomly assigned to each of the two groups. Pain scores decreased significantly at 1, 2, 4, 8 and 12 weeks after famciclovir treatments. There were no significant differences in pain scores, and the incidence of postherpetic neuralgia occurrence between the two groups. There were no statistically significant differences in reducing pain intensity or frequency of postherpetic neuralgia between the one-week and two-week treatment protocols.
Conclusion: It suggests that longer administration of famciclovir has no further benefit in the treatment of herpes zoster in our study.
Keywords: herpes zoster, postherpetic neuralgia, famciclovir
Introduction
Herpes zoster results from a reactivation of the varicella zoster virus (VZV), the same virus responsible for chickenpox. It presents as painful blistering and occurs when VZV cell-mediated immunity wanes with age or under conditions of immunocompromise.1,2 Herpes zoster is estimated to have a risk level of approximately 30% in the general population, with an increase to greater than 50% in those >85 years of age.3,4 The clustered blisters distribute along unilateral peripheral nerves of the torso and are associated with significant neuralgia and occasional local lymphadenopathy. Not only does the incidence increase with age, but the severity of herpes zoster pain also increases with age and PHN often occurs even after the rash is resolved.5 PHN, a persistent and severe chronic pain after the recovery from herpes zoster, represents one of the most common complications of herpes zoster.6 PHN can persist for 30 days or longer after the disappearance of skin lesions. Approximately 30% to 50% of the patients experience PHN lasting for more than 1 year, and several studies report that PHN can even last up to 10 years.7 The use of antiviral drugs during the herpes zoster attack can effectively control symptoms in the acute phase and reduce the occurrence of PHN.
Previous study demonstrated that a two-week administration of acyclovir appeared to reduce the severity of the pain experienced in acute herpes zoster compared with one-week acyclovir treatment.8 Acyclovir is a cyclic nucleoside analogue in chemical structure, mainly phosphorylated in cells infected with varicella zoster to produce triphosphate compounds, thereby terminating the extension of DNA strands and achieving antiviral activity. Acyclovir and its prodrug valacyclovir (L-valyl ester of acyclovir) are the gold standard for prophylaxis and therapy of HSV and VZV associated diseases. Famciclovir is a prodrug of penciclovir, which is converted in vivo to penciclovir and phosphorylated into triphosphate compounds, which inhibit viral DNA replication. The bioavailability of oral famciclovir is higher than that of acyclovir.9 Moreover, famciclovir is considered to have advantages in the prevention of PHN.8,10 In clinic, we believe that the use of famciclovir for two weeks may reduce the occurrence of PHN compared with one-week treatment. However, no study has proved whether prolonging the treatment time of famciclovir will reduce the occurrence of PHN in the treatment of herpes zoster.
In this study, we compared the safety, efficacy and incidence of PHN occurrence of famciclovir one-week and two-week treatment for herpes zoster.
Materials and Methods
Study Populations
All the patients with herpes zoster in this study were recruited from Special Medical Center of Strategic Support Force of PLA over the period between February and December 2013. The inclusion criteria were as follows: 1) either gender between age 18–75 years; 2) clinically diagnosed as herpes zoster, with skin lesions being present within 3 days of onset; 3) herpes zoster outside of head and face. Key exclusion criteria included patients 1) with a malignant neoplasm; 2) systemic diseases affecting immune functions, such as hyperthyroidism, hypothyroidism or diabetes; 3) having used glucocorticoids and immunosuppressants in the past 2 weeks; 4) pregnant and lactating females; 5) with renal dysfunction; 6) with allergies to famciclovir; 7) having used oral or external antiviral drugs within 1 week; and 8) HIV-positive. A total of 80 patients were enrolled. The Medical Ethics Committee of the 306 Hospital of the PLA, China approved the study. The study was conducted in accordance with the Declaration of Helsinki and all the study participants provided written informed consent.
Interventions
The patients randomly received either a one- or two-week period of an oral 250 mg famciclovir 3 times per day. We keep the two treatments in sealed opaque envelopes. The envelopes were sorted randomly by the nurse. Patients choose envelopes with corresponding numbers according to the order of enrollment and adopt the treatment methods in the envelopes. In addition, both groups were given oral vitamin B1 and intramuscular injection of mecobalamin for two weeks. Calamine lotion was applied for external use. No physical treatments were used in either group. Patients can take ibuprofen orally when they unable to bear pain. We asked patients to score when they feel the most intense pain during whole day and 10 hours after taking ibuprofen.
Study Assessments
Efficacy
The primary outcome of this study was “pain score”, and secondary outcome was the occurrence of PHN. Pain intensity was assessed in patients as reported by the patient. A numeric pain rating scale was used to evaluate their pain intensity. The values ranged from 0 to 10, with 0 indicating a complete absence of pain and 10 as maximal and unbearable pain. The investigators explained the method of pain assessment to the patients to ensure that they fully understood the use of the scale when assessing their pain intensity and assigning a pain score for themselves.
Pain intensity scores were recorded at 1, 2, 4, 8 and 12 weeks after famciclovir treatment. The occurrence of PHN was also evaluated at three months after the treatment in both groups. Assessments at weeks one and two after treatment were conducted in the clinic, while those at 4, 8 and 12 weeks were performed via phone interviews.
Safety
Adverse reactions (common adverse reactions included headache, nausea, vomiting and diarrhea)
In the event of an emergency in the patient, the investigator involved in the project initiated appropriate treatments as based on the symptoms observed and informed the clinician as to the results of this emergency and its treatment. The investigator then recorded the details, processing results and signature on the case report form. If an adverse reaction occurred, the investigator provided the appropriate treatment and the reported reaction was recorded.
Statistical Analysis
Qualitative variables were expressed in N (%), quantitative variables in mean (standard deviation SD) or median percentiles as appropriate. Pearson chi-squared tests or Fisher’s exact tests were used for comparison of categorical variables. Variables not normally distributed were analysed using t-test and Mann–Whitney U-test. The mutually adjusted association was defined as significant if P<0.05. Statistical analysis was carried out using SPSS 18.0 (IBM SPSS, Armonk, NY, USA).
Results
Demographic Characteristics
Table 1 provides a description of all the relevant data of the study subjects separately for the one- and two-week treatment. All the data derived from the two groups were completely analysed. A total of 40 persons in the one-week group and 40 in the two-week group were analysed. All the patients were aged between 18 and 75 years. Approximately half of the patients were males in both two groups. The height and weight of patients from two groups were almost the same. About 35% patients from one-week group and 37.5% patients from two-week group had bachelor's degrees or above. About 32.5% patients from one-week group and 40% patients from two-week group were smokers. A higher number of patients drink alcohol in the one-week group (32.5%) than in the two-week group (27.5%). There are no statistically significant differences in demographics between the two groups (Table 1).
 |
Table 1 Characteristics of Study Population |
Clinical Manifestation
The manifestations including Impact on life, locations and symptoms in one-week and two-week groups are shown separately in Table 2. We asked patients to choose whether HZ has an impact on their daily life including work, sleep, sports and if they worry about contagion. There were no significant differences in clinical manifestations in the two groups.
 |
Table 2 Herpes Zoster (HZ) Clinical Manifestations |
Efficacy
Of the 80 patients, 12 patients took ibuprofen in case of pain. Ibuprofen metabolism takes about 10 hours. We asked patients to score 10 hours after take ibuprofen and when they feel the most intense pain during whole day.
Within both treatment groups, pain scores significantly decreased at 1, 2, 4, 8 and 12 weeks after famciclovir treatments of the study (p<0.05) (Figure 1, *p<0.05). There were no statistically significant differences between the two groups on pain scores as determined at 1, 2, 4, 8 and 12 weeks after famciclovir treatments (Table 3).
 |
Table 3 Comparisons of One (Group 1)- versus Two (Group 2)-Week of Famciclovir on Pain Scores |
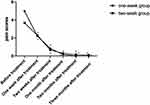 |
Figure 1 Pain scores significantly decreased at 1, 2, 4, 8 and 12 weeks after famciclovir treatments of the study (*p <0.05). |
At the 3-month after zoster rash resolved follow-up, 3/40 patients (7.5%) in the one-week group and 2/40 (5.0%) in the two-week group had PHN. There were no differences in the incidence of PHN between the two groups that were not statistically significant (Table 4).
 |
Table 4 Comparisons of One (Group 1)- versus Two (Group 2)-Week of Famciclovir the Occurrence of PHN |
Safety Results
There were no emergencies experienced by any of the patients in this study. Within both groups, 3 patients reported mild headaches while 1 patient reported a mild headache in the one-week group. Two patients (one in each group) experienced mild diarrhea. No treatments were administered for any of these patients.
Discussion
Our study compared the safety and efficacy of famciclovir as administered for either a one- or two-week period in the treatment of herpes zoster and prevention of PHN. Famciclovir was found to be safe when administered for either the one- or two-week periods, with no emergencies or serious adverse reactions observed. Only three cases of mild headache and two cases of diarrhea were reported in these patients, with no statistically significant differences in these effects between the two groups. We found that both protocols of famciclovir treatment were equally effectively in reducing pain as determined at 1, 2, 4, 8 and 12 weeks after the treatment. As we all know, the dose of 500 mg famciclovir three times a day is used in the USA, EU, Japan, etc. We used the dose of 250 mg three times a day based on the Chinese guidelines.
A common complication of HZ is PHN, which occurs when pain persists ≥90 days after the zoster rash has resolved. PHN can disrupt all aspects of daily life, including sleep, mood, work, and daily activities, and it can last for several months and occasionally for several years or more.11 When comparing the incidence of PHN at three months after treatment, there were no statistically significant differences between the one-week (7.5%) and two-week (5.0%) treatment groups. As the two weeks of treatment with famciclovir offers no superior beneficial effects on reducing the pain and occurrence of PHN, our results suggest that the one-week treatment regimen be utilized for herpes zoster. Such a protocol would reduce costs and potential side effects of this medication. PHN is often refractory to guideline-recommended treatments. In clinic, pulsed radiofrequency, short-term spinal cord stimulation and acupuncture therapy are commonly used method for the treatment of zoster-related pain.12,13 Moreover, the recombinant zoster vaccine (RZV) vaccine prevents shingles and, therefore, may reduce the occurrence of PHN.11
Prior to famciclovir, acyclovir was one of the most commonly used antiviral drugs for herpes zoster. It was reported that a longer term period of acyclovir administration after the occurrence of rash in herpes zoster patients (21 days), could shorten the healing time for the pain as compared to the 7-day treatment procedure and that pain reduction was greater during the acute phase of this disease in patients treated for 21 days with acyclovir.14 However, no differences were observed with regard to the time for the first or complete cessation of pain and the overall benefits of using acyclovir for 21 days were only slightly better than that for the standard 7-day treatment period. Nor were differences observed in the frequency of PHN at the 6-month follow-up period between the 7 versus 21 periods of acyclovir administration.14 Several clinical studies demonstrated that valacyclovir has a safety profile comparable to that of acyclovir in HZ patients. Valacyclovir became a better option in the treatment of VZV infections since it requires a less frequent dosing regimen than acyclovir, contributing to increased patient adherence to therapy.15 Famciclovir has been shown to have better efficacy in vitro antiviral activity due to better stability of penciclovir triphosphate form in HSV- and VZV-infected cells and stronger antiviral efficacy in neurological infection in mice than acyclovir. Therefore, famciclovir is expected to show better efficacy in neurological complications. To the best of our knowledge, this is the first study about evaluation of the difference between one-week and two-week use of famciclovir.
Accordingly, these results regarding the duration of treatment with acyclovir are similar to our present findings with famciclovir. In this way, although patients continue to experience severe pain during the recovery process and there remains a potential for PHN in response to a 7-day treatment regimen, there appears to be little, if any advantage, for a more prolonged administration of these treatments with acyclovir or famciclovir.
A limitation of this study was the small sample of patients, although according to statistical methods, the sample size is sufficient. Therefore, our future studies will include more patients to avoid this potential bias.
In conclusion, the findings of this study provide clinical evidence for use of a one-week period of famciclovir administration in the routine treatment of herpes zoster. The addition of a second week of famciclovir therapy is not associated with any better clinical outcomes. Moreover, this one-week treatment protocol has the advantage of reducing the cost of treatment and potential side effects of this drug.
Acknowledgment
The study was funded by the Scientific Training Section of Special Medical Center of Strategic Support Force of PLA. We thank all medical staff of dermatological department for their support of this study.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Sauerbrei A. Diagnosis, antiviral therapy, and prophylaxis of varicella-zoster virus infections. Eur J Clin Microbiol Infect Dis. 2016;35:723–734. doi:10.1007/s10096-016-2605-0
2. O’Connor KM, Paauw DS. Herpes zoster. Med Clin North Am. 2013;97:503–522. doi:10.1016/j.mcna.2013.02.002
3. Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–1349. doi:10.4065/82.11.1341
4. Johnson R, McElhaney J, Pedalino B, Levin M. Prevention of herpes zoster and its painful and debilitating complications. Int J Infect Dis. 2007;11:S43–S48. doi:10.1016/S1201-9712(07)60021-6
5. Fashner J, Bell AL. Herpes zoster and postherpetic neuralgia: prevention and management. Virus. 2011;83:1432–1437.
6. Wu CL, Marsh A, Dworkin RH. The role of sympathetic nerve blocks in herpes zoster and postherpetic neuralgia. Pain. 2000;87:121–129. doi:10.1016/S0304-3959(00)00230-X
7. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. doi:10.1136/bmjopen-2014-004833
8. Rasi A, Heshmatzade Behzadi A, Rabet M. The efficacy of time-based short-course Acyclovir therapy in treatment of postherpetic pain. J Infect Dev Ctries. 2010;4:754–760. doi:10.3855/jidc.849
9. Perry CM, Wagstaff AJ. Famciclovir: a review of its pharmacological properties and therapeutic efficacy in herpes virus infections. Drugs. 1995;50(2):396. doi:10.2165/00003495-199550020-00011
10. Stoopler ET, Balasubramanlam R. Topical and systemic therapies for oral and perioral herpes simplex virus infections. J Calif Dent Assoc. 2013;41:259–262.
11. Poirrier JE, DeMartino JK, Nagar S, et al. Burden of opioid use for pain management among adult herpes zoster patients in the US and the potential impact of vaccination. Hum Vaccin Immunother. 2022;2022:1–11.
12. Xue S, Yang WJ, Cao ZX, Sun T. Comparing the efficacy and safety of short-term spinal cord stimulation and pulsed radiofrequency for zoster-related pain: a systematic review and meta-analysis. Medicine. 2022;101:e29073.
13. Bian Z, Yu J, Tu M. Acupuncture therapies for postherpetic neuralgia: a protocol for a systematic review and Bayesian network meta-analysis. BMJ Open. 2022;12:e056632. doi:10.1136/bmjopen-2021-056632
14. Wood MJ, Johnson RW, McKendrick MW, et al. A randomized trial of Acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896–900. doi:10.1056/NEJM199403313301304
15. Andrei G, Snoeck R. Advances and perspectives in the management of varicella-zoster virus infections. Molecules. 2021;26:1132. doi:10.3390/molecules26041132
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
