Back to Journals » Risk Management and Healthcare Policy » Volume 16
Evaluation of Serum NLRC4 as a Potential Prognostic Biochemical Marker in Humans with Severe Traumatic Brain Injury: A Prospective Cohort Study
Authors Tang B, Zhong Z, Wu J, Ma J, Li L, Zhong X, Lin D, Hu J, Yu P
Received 14 January 2023
Accepted for publication 15 March 2023
Published 23 March 2023 Volume 2023:16 Pages 439—454
DOI https://doi.org/10.2147/RMHP.S404877
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jongwha Chang
Bei Tang, Ze Zhong, Jinping Wu, Jianping Ma, Li Li, Xuzheng Zhong, Dongmei Lin, Jiayuan Hu, Pingan Yu
Department of Critical Care Medicine, The First People’s Hospital of Jiande City, Jiande, People’s Republic of China
Correspondence: Ze Zhong, Department of Critical Care Medicine, The First People’s Hospital of Jiande City, Jiande, People’s Republic of China, Tel/Fax +86 571 64096607, Email [email protected]
Objective: Involvement of NLR CARD domain containing 4 (NLRC4) in neuroinflammation has been demonstrated. The aim of this study was to ascertain the prognostic role of serum NLRC4 in severe traumatic brain injury (sTBI).
Methods: In this prospective cohort study including 140 sTBI patients and 140 controls, serum NLRC4 levels were quantified. Follow-up time was 180 days after trauma and poor prognosis was designated as extended Glasgow outcome scale (GOSE) scores of 1– 4. Severity correlations and prognosis associations were determined under multivariate models.
Results: Enhanced serum NLRC4 levels after sTBI, in comparison to controls (median, 0.8 ng/mL versus 0.1 ng/mL; P < 0.001), were independently correlated with Glasgow coma scale (GCS) scores (β, − 0.091; 95% confidence interval (CI), − 0.161— 0.021; P = 0.011), Rotterdam computed tomography (CT) scores (β, 0.136; 95% CI, 0.024– 0.248; P = 0.018), serum C-reactive protein levels (β, 0.016; 95% CI, 0.002– 0.030; P = 0.025) and 180-day GOSE scores (β, − 0.906; 95% CI, − 1.632— 0.180; P = 0.015); and were independently predictive of 180-day death (odds ratio, 4.307; 95% CI, 1.706– 10.879; P = 0.014)), overall survival (hazard ratio, 2.360; 95% CI, 1.118– 4.981; P = 0.040) and poor prognosis (odds ratio, 6.705; 95% CI, 2.889– 15.561; P = 0.016). Under receiver operating characteristic curve, combination of serum NLRC4 levels, GCS scores and Rotterdam CT scores had significantly higher death predictive ability than Rotterdam CT scores (P = 0.040), but not than GCS scores (P = 0.070); and exhibited substantially higher predictive capability for poor prognosis than Rotterdam CT scores (P < 0.001) and GCS scores alone (P = 0.023).
Conclusion: There is a dramatical elevation of serum NLRC4 levels after sTBI, which has strong correlation with severity and inflammation, and is significantly associated with long-term death and poor outcome, substantializing serum NLRC4 as an inflammatory, prognostic biomarker in sTBI.
Keywords: traumatic brain injury, nucleotide-binding domain leucine-rich repeat-containing protein family caspase activation and recruitment domain-containing protein 4, severity, prognosis, biomarkers, inflammation
Introduction
Severe traumatic brain injury (sTBI) is a lethal neurosurgical disease in humans.1 Following external force, the two key pathophysiological processes, namely primary brain injury and secondary brain injury, are closely relevant to neurological dysfunction and even death after sTBI.2 In response to primary brain insult, secondary injury occurs, which triggers a series of cascading signaling events that potentiate the formation of sterile neuroinflammation, aggravation of brain edema, disruption of blood–brain barrier and development of neuronal injury.3 Besides treatments, severity assessment and prognostic prediction are of rather importance in clinical managements of sTBI.4 Conventionally, clinical and radiological scales, such as the Glasgow coma scale (GCS) and Rotterdam computed tomography (CT) scale, have been frequently utilized in routine work of sTBI.5 Intriguingly, biochemical biomarkers have recently emerged as an indispensable tool in decision-making of sTBI.6,7
Neuroinflammatory response accompanies sTBI.8 Inflammasome, an intracellular multi-protein complex, harbors inflammatory potentials and is involved in secondary brain injury after trauma.8,9 The nucleotide-binding and oligomerization domain-like receptor (NLR) family responds to innate immunity by forming inflammasomes.10 Following activation, NLR CARD domain containing 4 (NLRC4) inflammasomes facilitate conversion of precursor caspase-1 to cleaved caspase-1, whereby precursor interleukin-1beta and interleukin-18 are converted into biologically active mature pro-inflammatory cytokines, thereby finally triggering inflammatory cascade.11 NLRC4 expressions by microglia and astrocyte mediated sterile inflammasome activation in central nervous system.12 NLRC4 has been widely expressed in animal injured brain tissues after hemorrhagic or ischemic stroke.13,14 Clearly, NLRC4 inflammasome is implicated in neuroinflammation, and therefore contributes to increased cerebral edema, blood–brain barrier disruption and neuronal injury. In addition, its inhibition could markedly relieve acute brain injury, subsequently improving neurological function of experimental rats.13,14 Overall, NLRC4 may represent a potential biomarker for reflecting acute brain injury. Herein, we intended to investigate relationship between serum NLRC4 levels and trauma severity plus clinical outcome after human sTBI.
Materials and Methods
Study Design, Ethical Considerations and Participant Enrollment
In this prospective cohort study implemented between July 2017 and December 2020 at the First People’s Hospital of Jiande City, we consecutively recruited two groups of subjects, including controls and head trauma patients. The current study was performed in agreement with the ethical guidelines of the Declaration of Helsinki. The approval for the protocol of this study was acquired from the Institutional Review Committee at the First People’s Hospital of Jiande City (NO. JDYY2017023). The informed written consent to participate in this study was given by patients’ proxies or controls themselves.
Inclusion criteria for head trauma patients were that (1) blunt trauma; (2) age equal to or greater than 18 years; (3) post-resuscitation GCS scores of 3–8; (4) hospital admission within 12 hours since trauma and (5) injury severity score <9 in non-cranial aspects. Exclusion criteria for head trauma patients were that (1) previous or coexisting neurological diseases, for instance, stroke, intracranial tumors and multiple sclerosis; and (2) other specific conditions or diseases, for example, recent infections, autoimmune diseases, malignancies and severe hepatic, renal, pulmonary or cardiac diseases.
Inclusion criteria for controls were that (1) age of 18 years or greater; and (2) Normal results in some routine blood tests, including blood glucose levels, blood leucocyte count, blood platelet count, blood hemoglobin levels and blood albumin levels. Exclusion criteria for controls were (1) previous chronic diseases, eg, hypertension, diabetes mellitus and coronary heart disease; and (2) other specific conditions, such as use of immunosuppressants or dexamethasone, recent infections and pregnancies.
Data Collection, Outcome Assessment and Immune Analysis
Some conventional information should be collected, including age, gender, body mass index, cigarette smoking, alcohol drinking and admission time since trauma. Other specific diseases or conditions should be inquired, such as hypertension, diabetes mellitus, hyperlipidemia, medications (use of antihypertensive drugs, hypoglycemic drugs or insulin, and statins) and traumatic causes (automobile/motorcycle, fall/jump or others). We recorded some baseline vital signs, mainly including systolic and diastolic arterial blood pressure. Mean arterial blood pressure was calculated using the following equation: mean arterial blood pressure = diastolic arterial blood pressure + ((systolic arterial blood pressure-diastolic arterial blood pressure)/3). Clinical severity was under assessment using GCS. Positive radiologic characteristics included abnormal cisterns, midline shift, epidural hematoma, subdural hematoma, subarachnoid hemorrhage, intraventricular hemorrhage, intracerebral hematoma, brain contusion and pneumocephalus. Rotterdam CT scores were calculated based on the preceding positive radiological parameters. Follow-up time for patients was 180 days. Clinical outcome was evaluated via 8-grade Extended Glasgow Outcome Scale (GOSE). GOSE scores of 1–4 was referred to as a poor prognosis.15
Blood samples of participants were acquired and then blood was centrifugated. Obtained serum samples were preserved at −80°C until further analyses. Every 3 months, a batch of samples were melted and, using a commercially available Enzyme-Linked Immunosorbent Assay kit (SinoGeneclon Biotech, Hangzhou, China), serum NLRC4 levels were in duplicate quantified by the same technician, who was blinded to clinical data. Two measurements were averaged for ultimate clinical investigation.
Statistical Analysis
The MedCalc statistical software version 17.4 (MedCalc Software, Mariakerke, Belgium) was applied for receiver-operating characteristic (ROC) curve analysis and the SPSS 20.0 statistical package (SPSS Inc., Chicago, Illinois, USA) was in use for other data analyses. There are three types of variables, namely, qualitative data, normally and non-normally distributed quantitative variables, which were presented as frequencies (proportions), means (standard deviations, SDs) and medians (percentiles 25th-75th), respectively. In order to complete the data comparison between two groups, the used statistical methods included the Pearson’s Chi-square test, Fisher’s exact test, independent t-test and Mann–Whitney test. The Kruskal–Wallis H-test was done for data comparison among multiple groups. Baseline characteristics were compared between survivors and non-survivors as well as between patients with poor prognosis and those with good prognosis, and then the variables with substantial difference were enforced into the binary logistic regression model, thereby determining the independent predictors of death and poor prognosis. The Spearman test was done for bivariate correlative assessment, and afterwards the significantly correlated variables were incorporated into the multivariate linear regression model to identify independent factors of serum NLRC4 levels and GOSE scores. Patients were dichotomized based on the median value of serum NLRC4 levels, and the Log rank test was performed for comparing 180-day overall survival time between two groups. The univariate Cox’s proportional hazard analysis was implemented to screen significant variables, and such variables were subsequently entered into the multivariable model, wherein independent predictors were ascertained. ROC curves were established to assess the predictive performance of some designated parameters for mortality and poor prognosis. In consideration of this, the Youden index method was fulfilled to discern their optimal cut-off values. Differences with P-values <0.05 were deemed as statistical significance.
Results
Participant Selection and Characteristics
In this clinical survey, we at first enrolled 177 patients with head trauma, who fitted the inclusion criteria in the above-mentioned section, and then 37 patients were removed from the final analysis because of previous or coexisting neurological diseases (15 cases), other specific conditions or diseases (17 cases), loss to follow-up (1 case), incomplete clinical materials (1 case), unavailable blood samplings (2 cases) and refusal to participation (1 case). In summary, a total of 140 adults with blunt and isolated sTBI obtained an ultimate evaluation. Also, there were an aggregate of 140 healthy individuals as controls. Controls, 78 being males and 62 being females, comprised of 46 alcohol drinkers and 48 cigarette smokers and were aged from 18 to 79 years (mean, 40.7 years; SD, 13.9 years), with body mass index ranging from 18.3 to 34.7 kg/m2 (mean, 24.1 kg/m2; SD, 3.9 kg/m2). Age, gender, body mass index, alcohol drinking, cigarette smoking and other parameters of patients are listed in Table 1. By statistical analysis, it was revealed that proportions of males, smokers and drinkers, as well as mean age and body mass index were not significantly different between controls and patients (all P > 0.05).
 |
Table 1 Baseline Characteristics of Head Trauma Patients and Factors in Relation to Serum NLRC4 Levels After Head Trauma |
Serum NLRC4 Levels After sTBI and Its Correlative Factors
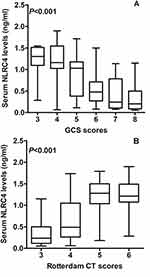
There was a significant elevation of serum NLRC4 levels in patients, in comparison to controls (P < 0.001; Figure 1). In Table 1, there were eight variables in close correlation with serum NLRC4 levels using spearman test, which were GCS scores, Rotterdam CT scores, unreactive pupils, abnormal cisterns, midline shift 5 mm, serum C-reactive protein levels, blood glucose levels and blood leucocyte count (all P < 0.05). Subsequently, the multivariate linear regression model was configured, wherein the aforementioned eight significantly correlated factors were forced. And, it was found that serum NLRC4 levels were independently correlated with GCS scores (β, −0.091; 95% CI, −0.161—0.021; P = 0.011), Rotterdam CT scores (β, 0.136; 95% CI, 0.024–0.248; P = 0.018) and serum C-reactive protein levels (β, 0.016; 95% CI, 0.002–0.030; P = 0.025). Also, its strong relations to GCS scores (P < 0.001; Figure 2A), Rotterdam CT scores (P < 0.001; Figure 2B) and serum C-reactive protein levels (P < 0.001; Figure 2C) were depicted in a diagram. When GCS scores and Rotterdam CT scores were considered as the two categorical variables, it was revealed that serum NLRC4 levels, which were significantly decreased in order of GCS scores from 3 to 8 (P < 0.001; Figure 3A), were substantially increased in order of Rotterdam CT scores from 3 to 6 (P < 0.001; Figure 3B).
 |
Figure 1 Serum NLRC4 levels after severe traumatic brain injury. Serum NLRC4 levels were substantially elevated after severe traumatic brain injury, in comparison to controls (P<0.001). |
Serum NLRC4 Levels and 180-Day Death After Head Trauma
There were 33 non-survivors (23.6%) within 180 days after head trauma. The dead had substantially higher percentages of alcohol drinking, unreactive pupils, epidural hematoma, subdural hematoma, abnormal cisterns and midline shift above 5 mm than the alive (all P < 0.05; Table 2); and, in comparison to survivors, GCS scores were significantly declined and Rotterdam CT scores, serum C-reactive protein levels, blood leucocyte count, blood glucose levels and serum NLRC4 levels were substantially increased (all P < 0.05; Table 2). The above significant variables were entered into the binary logistic regression model and then, it was confirmed that GCS scores, Rotterdam CT scores and serum NLRC levels were independently predictive of 180-day death with odds ratio values of 0.355 (95% CI, 0.192–0.659; P = 0.001), 2.789 (95% CI, 1.351–5.755; P = 0.006) and 4.307 (95% CI, 1.706–10.879; P = 0.014). Under ROC curve, serum NLRC4 levels substantially discriminated patients at risk of 180-day death (Figure 4A). Additionally, combination of serum NLRC4 levels, GCS scores and Rotterdam CT scores had insignificantly higher AUC than GCS scores (P = 0.070; Figure 4B), while displayed substantially higher AUC than Rotterdam CT scores (P = 0.040; Figure 4B).
 |
Table 2 Factors in Relation to 180-Day Mortality After Head Trauma |
Serum NLRC4 Levels and 180-Day Overall Survival After Head Trauma
This cohort of sTBI patients had the mean 180-day overall survival time at 145.8 days (95% CI, 135.0–156.6 days). Patients were divided into two subgroups in accordance with the median value of serum NLRC4 levels (ie, 0.83 ng/mL). In Figure 5, mean 180-day overall survival time was markedly shorter in patients with serum NLRC4 levels >0.83 ng/mL than in other remainders (P < 0.01). Additionally, variables, which were significantly associated with 180-day overall survival time using univariate Cox’s proportional hazard analysis, were alcohol drinking, GCS scores, Rotterdam CT scores, unreactive pupils, epidural hematoma, subdural hematoma, abnormal cisterns, midline shift above 5 mm, serum C-reactive protein levels, blood leucocyte count, blood glucose levels and serum NLRC4 levels (all P < 0.05; Table 3). Next, the aforementioned significant variables were incorporated into the multivariable model and afterwards, it was confirmed that GCS scores (hazard ratio, 0.592; 95% CI, 0.360–0.973; P = 0.013), Rotterdam CT scores (hazard ratio, 2.283; 95% CI, 1.186–4.393; P = 0.025) and serum NLRC4 levels (hazard ratio, 2.360; 95% CI, 1.118–4.981; P = 0.040) had independent association with 180-day overall survival after head trauma.
 |
Table 3 Factors in Relation to 180-Day Overall Survival After Head Trauma |
Serum NLRC4 Levels and 180-Day GOSE Scores After Head Trauma
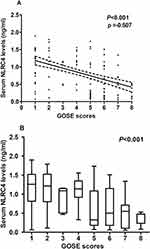
In Table 4, GOSE scores were in intimate correlation with cigarette smoking, GCS scores, Rotterdam CT scores, unreactive pupils, epidural hematoma, brain contusion, abnormal cisterns, midline shift more than 5 mm, serum C-reactive protein levels, blood glucose levels and serum NLRC levels (all P < 0.05; Table 4). The preceding factors were forced into the multivariate model and afterwards, it was verified that GCS scores (β, 0.504; 95% CI, 0.203–0.805; P = 0.001), Rotterdam CT scores (β, −0.907; 95% CI, −1.391—0.423; P = 0.005) and serum NLRC4 levels (β, −0.906; 95% CI, −1.632—0.180; P = 0.015) were independently correlative with 180-day GOSE scores after head trauma. Also, Figure 6A shows a tight relationship between GOSE scores and serum NLRC4 levels (P < 0.001). When GOSE was considered as the categorical variable, it was proved that serum NLRC4 levels were markedly reduced in order of GOSE scores from 1 to 8 (P < 0.001; Figure 6B).
 |
Table 4 Factors in Relation to Extended Glasgow Outcome Scale Scores at 180 Days After Head Trauma |
Serum NLRC4 Levels and 180-Day Poor Outcome After Head Trauma
An aggregate of 67 patients (47.9%) suffered from post-traumatic poor 180-day outcome. Just as depicted in Table 5, poor-outcome patients, in comparison to other remainders, tended to show markedly elevated proportions of unreactive pupils, epidural hematoma, brain contusion, abnormal cisterns and midline shift >5 mm, were prone to have significantly decreased GCS scores, and were likely to exhibited significantly increased Rotterdam CT scores, blood glucose levels, serum C-reactive protein levels and serum NLRC4 levels (all P < 0.05). Moreover, the binary logistic regression model was constructed, where the above-mentioned variables were enforced, and it was confirmed that GCS scores (odds ratio, 0.371; 95% CI, 0.209–0.689; P = 0.001), Rotterdam CT scores (odds ratio, 3.596; 95% CI, 1.646–7.857; P = 0.003) and serum NLRC4 levels (odds ratio, 6.705; 95% CI, 2.889–15.561; P = 0.016) were the three independent predictors for poor 180-day outcome after head trauma. Using ROC curve, serum NLRC4 levels pronouncedly distinguished patients with the development of 180-day poor outcome (Figure 7A). In addition, serum NLRC4 levels combined with GCS scores and Rotterdam CT scores exhibited substantially higher AUC than Rotterdam CT scores (P < 0.001; Figure 7B) and GCS scores alone (P = 0.023; Figure 7B).
 |
Table 5 Factors in Relation to 180-Day Poor Outcome After Head Trauma |
Discussion
To the best of our knowledge, there is a paucity of data available regarding NLRC4 levels in peripheral blood of subjects with acute brain injury. We found that (1) elevated serum NLRC4 levels after sTBI were independently correlated with serum C-reactive protein levels, GCS scores, Rotterdam CT scores and GOSE scores at 180 days after head trauma, were independently predictive of 180-day death and poor prognosis, as well as were independently associated with 180-day overall survival following sTBI; and (2) When combined with GCS scores and Rotterdam CT scores, serum NLRC4 levels showed efficient additive effect on prognostic prediction ability reflected by AUC. Hence, it is deduced that it may be of interest to measure serum NLRC4 levels for assessing trauma severity and predicting clinical outcome of patients with sTBI.
Neuroinflammation is a very well-known process, which, although complicated, is involved in pathophysiological mechanisms of secondary brain injury after TBI.8 Inflammasomes, which are recognized as large multimolecular complexes, play essential effects in activation of the proteolytic enzyme caspase-1.16 In other words, inflammasomes are implicated in pyroptosis via regulating the proteolytic maturation of interleukin-1β and interleukin-18.17 Besides pathogens (pathogen-associated molecular patterns), endogenous danger signals (danger-associated molecular patterns) derived from damaged or dying cells can lead to activation of inflammasomes, thereby triggering pathological inflammation in sterile inflammatory conditions or diseases, such as TBI, and finally inducing neurologic dysfunction.18 In summary, inflammasomes may harbor noxious potential in acute brain injury via participating in inflammatory processes.
NLRC4 is a very important component of inflammasome.19 NLRC4 was highly expressed in brain tissues of mice after intracerebral hemorrhage in a time-dependent manner, with peaking at post-hemorrhage 24 h.20 In addition, the highest expression of rat NLRC4 in ischemia area was revealed at 4h of transient cerebral ischemia/reperfusion.21 Moreover, NLRC4 was dominantly located in neurons after animal cerebral ischemia22 and in glial cells under lysophosphatidylcholine, a molecule associated with neurodegeneration and demyelination.12 Clearly, NLRC4 was highly expressed in brain tissues of TBI mice.23 The current study showed a significant elevation of serum NLRC4 levels after human sTBI, in comparison to controls. Taken together, it is inferred that NLRC4 in peripheral blood of sTBI patients may be at least partially derived from traumatized brain tissues.
NLRC4 inflammasome was activated in microglia and astrocytes subjected to lysophosphatidylcholine.12 Involvement of NLRC4 in neuroinflammation of rats after hemorrhagic stroke was confirmed because of its strong ability to increase accumulation of microglia and the release of interleukin-1beta and interleukin-18, induce neuronal death, disrupt blood–brain barrier, and aggravate brain edema.13 In contrast, knocking down NLRC4 using siRNA exerted neuroprotective effects in experimental intracerebral hemorrhage.20 In addition, similar results were found in ischemic stroke using siRNA knockdown of NLRC4.14 In the current study, serum NLRC4 levels were independently correlated with serum C-reactive protein levels. NLRC4 may act as an inflammatory protein to participate in the neuroinflammation. Moreover, targeting NLRC4 may be considered as another approach to lessen acute brain injury.
It is unclear regarding the relationship between serum NLRC4 levels and illness severity in addition to clinical outcome after sTBI. In this study, both GCS and Rotterdam CT classification were considered as the severity indicators. Interestingly, an independent correlation was found between serum NLRC4 levels and GCS scores, as well as between serum NLRC4 levels and Rotterdam CT scores. In addition, GOSE was regarded as the continuous and categorical variables. 180-day overall survival, death and poor prognosis were selected as the three prognostic parameters. Using multivariate analysis, serum NLRC4 levels had independent correlation with GOSE at 180 days after head trauma; and serum NLRC4 retained as an independent predictor of 180-day death, overall survival and poor prognosis. Of note, serum NLRC4 levels combined with GCS scores and Rotterdam CT scores exhibited substantially higher prognostic predictive capability than Rotterdam CT scores and GCS scores alone. In summary, such data were strongly supportive of the notion that serum NLRC4 may represent a promising prognostic biochemical biomarker of human sTBI.
There are several strengths and weaknesses in this study. The strengths are that (1) there was the follow-up time of 180 days after head trauma, thereby aiding in analysis of long-term prognosis after sTBI; and (2) all correlations or associations of serum NLRC4 levels with severity and prognosis following trauma were verified using multivariate analyses. The weaknesses are that (1) serum NLRC4 levels were only measured at a time point after head trauma and therefore, dynamic change of its levels is not discerned; and (2) using a medium sample size of patients, we investigated relationships between serum NLRC4 levels and trauma severity plus long-term prognosis of sTBI patients, and subsequently the conclusions need to be validated in a larger cohort study.
Conclusions
To the best of our knowledge, this is a first series for quantifying serum NLRC4 levels after human sTBI. Our main findings are that (1) serum NLRC4 levels are in independent relation to serum C-reactive protein levels, GCS scores, Rotterdam CT scores, 180-day GOSE scores, death, overall survival and poor prognosis; and (2) When combined with GCS scores and Rotterdam CT scores, serum NLRC4 levels show efficient additive effect on prognostic prediction ability reflected by AUC. In a word, serum NLRC4 may serve as an inflammatory predictive factor of clinical outcome in human sTBI.
Abbreviations
sTBI, severe traumatic brain injury; GCS, Glasgow coma scale; ROC, receiver operating characteristic; AUC, area under curve; OR, odds ratio; HR, hazard ratio; 95% CI, 95% confidence interval; GOSE, extended Glasgow Outcome Scale; NLR, nucleotide-binding and oligomerization domain-like receptor; NLRC4, NLR CARD domain containing 4.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics Statement
This study was carried out in compliance with the tenets of the Declaration of Helsinki and the protocol conformed to the ethical standards of the First People’s Hospital of Jiande City on human subjects. The study protocol was approved by the Human Investigation Committee at the First People’s Hospital of Jiande City (NO. JDYY2017023). We acquired written informed consent to participate in the current study from legal representatives of patients or controls themselves.
Acknowledgments
The authors thank all participants for providing us with their clinical information.
Funding
This study was financially supported by Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (NO. 021KY973).
Disclosure
The authors declare that there were no conflicts of interest.
References
1. Stocchetti N, Carbonara M, Citerio G, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16(6):452–464. doi:10.1016/S1474-4422(17)30118-7
2. Geeraerts T, Velly L, Abdennour L, et al. Management of severe traumatic brain injury (first 24hours). Anaesth Crit Care Pain Med. 2018;37(2):171–186. doi:10.1016/j.accpm.2017.12.001
3. Kowalski RG, Hammond FM, Weintraub AH, et al. Recovery of consciousness and functional outcome in moderate and severe traumatic brain injury. JAMA Neurol. 2021;78(5):548–557. doi:10.1001/jamaneurol.2021.0084
4. Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020;104(2):213–238. doi:10.1016/j.mcna.2019.11.001
5. Rakhit S, Nordness MF, Lombardo SR, Cook M, Smith L, Patel MB. Management and challenges of severe traumatic brain injury. Semin Respir Crit Care Med. 2021;42(1):127–144. doi:10.1055/s-0040-1716493
6. Wang KK, Yang Z, Zhu T, et al. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn. 2018;18(2):165–180. doi:10.1080/14737159.2018.1428089
7. Posti JP, Tenovuo O. Blood-based biomarkers and traumatic brain injury-A clinical perspective. Acta Neurol Scand. 2022;146(4):389–399. doi:10.1111/ane.13620
8. Sulhan S, Lyon KA, Shapiro LA, Huang JH. Neuroinflammation and blood-brain barrier disruption following traumatic brain injury: pathophysiology and potential therapeutic targets. J Neurosci Res. 2020;98(1):19–28. doi:10.1002/jnr.24331
9. Kursun O, Yemisci M, van den Maagdenberg AMJM, Karatas H. Migraine and neuroinflammation: the inflammasome perspective. J Headache Pain. 2021;22(1):55. doi:10.1186/s10194-021-01271-1
10. Mason DR, Beck PL, Muruve DA. Nucleotide-binding oligomerization domain-like receptors and inflammasomes in the pathogenesis of non-microbial inflammation and diseases. J Innate Immun. 2012;4(1):16–30. doi:10.1159/000334247
11. Duncan JA, Canna SW. The NLRC4 Inflammasome. Immunol Rev. 2018;281(1):115–123. doi:10.1111/imr.12607
12. Freeman L, Guo H, David CN, Brickey WJ, Jha S, Ting JP. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med. 2017;214(5):1351–1370. doi:10.1084/jem.20150237
13. Gan H, Zhang L, Chen H, et al. The pivotal role of the NLRC4 inflammasome in neuroinflammation after intracerebral hemorrhage in rats. Exp Mol Med. 2021;53(11):1807–1818. doi:10.1038/s12276-021-00702-y
14. Poh L, Kang SW, Baik SH, et al. Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav Immun. 2019;75:34–47. doi:10.1016/j.bbi.2018.09.001
15. Yan XJ, Li YB, Liu W, Wu HY, Yu GF. Elevated serum complement C1q levels after traumatic brain injury and its association with poor prognosis. Neuropsychiatr Dis Treat. 2022;18:47–55. doi:10.2147/NDT.S348682
16. Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–420. doi:10.1038/nri.2016.58
17. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. doi:10.1038/nm.3893
18. Lünemann JD, Malhotra S, Shinohara ML, Montalban X, Comabella M. Targeting Inflammasomes to treat neurological diseases. Ann Neurol. 2021;90(2):177–188. doi:10.1002/ana.26158
19. Sundaram B, Kanneganti TD. Advances in understanding activation and function of the NLRC4 inflammasome. Int J Mol Sci. 2021;22(3):1048. doi:10.3390/ijms22031048
20. Jin P, Qi D, Cui Y, et al. Aprepitant attenuates NLRC4-dependent neuronal pyroptosis via NK1R/PKCδ pathway in a mouse model of intracerebral hemorrhage. J Neuroinflammation. 2022;19(1):198. doi:10.1186/s12974-022-02558-z
21. Zhang X, Huang T, Lang L, Yu L. Effects of lysophosphatidic acid receptor 5 on NLRC4 inflammasome in brain tissues of transient cerebral ischemia/reperfusion rat. Hum Exp Toxicol. 2022;41:9603271221078870. doi:10.1177/09603271221078870
22. Habib P, Harms J, Zendedel A, Beyer C, Slowik A. Gonadal hormones E2 and P mitigate cerebral ischemia-induced upregulation of the AIM2 and NLRC4 inflammasomes in rats. Int J Mol Sci. 2020;21(13):4795. doi:10.3390/ijms21134795
23. Ding W, Cai C, Zhu X, Wang J, Jiang Q. Parthenolide ameliorates neurological deficits and neuroinflammation in mice with traumatic brain injury by suppressing STAT3/NF-κB and inflammasome activation. Int Immunopharmacol. 2022;108:108913. doi:10.1016/j.intimp.2022.108913
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.