Back to Journals » Journal of Inflammation Research » Volume 16
Fashionable, but What is Their Real Clinical Usefulness? NLR, LMR, and PLR as a Promising Indicator in Colorectal Cancer Prognosis: A Systematic Review
Authors Misiewicz A , Dymicka-Piekarska V
Received 1 October 2022
Accepted for publication 16 December 2022
Published 7 January 2023 Volume 2023:16 Pages 69—81
DOI https://doi.org/10.2147/JIR.S391932
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam D Bachstetter
Aleksandra Misiewicz,1,* Violetta Dymicka-Piekarska2,*
1Medical Diagnostic Laboratory Lab 110, Bialystok, Poland; 2Department of Clinical Laboratory Diagnostics, Medical University of Bialystok, Białystok, Poland
*These authors contributed equally to this work
Correspondence: Violetta Dymicka-Piekarska, Department of Clinical Laboratory Diagnostics, Medical University of Bialystok, Waszyngtona Str. 15, Bialystok, 15-276, Poland, Tel +48 85 746 85 84, Email [email protected]
Abstract: The link between inflammation and cancer is still an attractive subject of many studies because systemic inflammatory response has been proven to play a pivotal role in cancer progression and metastasis. The strongest relationship between chronic inflammation and cancer development is observed in colorectal cancer (CRC). The evaluation of ratios derived from the routinely performed inflammatory biomarkers shows limited performances and limited clinical utility when individually used as prognostic factors for patients with CRC. In this review, we would like to summarize the latest knowledge about the diagnostic utility of systemic inflammatory ratios: neutrophil/lymphocyte (NLR), lymphocyte/monocyte (LMR), and platelet/lymphocyte (PLR) in CRC. We focused on the papers that assessed the diagnostic utility of blood cell parameters on the basis of the area under the ROC curve published in the recent 6 years. Identification of biomarkers that are significantly associated with prognostic in cancer would help the selection of patients with a high risk of poor outcomes.
Keywords: biomarkers, colorectal cancer, inflammation, prognosis
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and women in the world (according to 2018 statistics by the World Cancer Research Found), despite significant advances in diagnosis and treatment. They account for 11% of all diagnosed cancers.1,2 In recent years, CRC incidence has been increasing in developed countries, with the highest prevalence observed in Australia, and New Zealand, as well as in Western Europe. The lowest incidence is observed in Africa and South-Central Asia.3
In spite of systemic improvement, the prognosis of affected patients, due to local recurrences or metastasis, is still a cause for concern in many countries. The 5-year survival rate is approximately 65% in Australia, Canada, the United States, and Western Europe. According to the Polish National Cancer Registry, the 5-year survival rate among patients with colorectal cancer in the first decade of the 21st century has increased slightly: in men from 43.3% to 47.6%, and in women from 44.1% to 49, 1%.3 That is why it is so important to understand the key molecular elements leading to malignant invasion and metastasis, as well as to identify effective diagnostic and prognostic biomarkers.2,4
The link between inflammation and the development of neoplastic diseases continues to be the subject of many studies. The first evidence of the involvement of chronic inflammation in the development of a neoplastic disease was provided by the works of the French surgeon Jean Nicholas Marjolin, who in 1828 observed the development of squamous cell carcinoma around an open wound accompanied by chronic inflammation.5,6
Up to 25% of cancers are thought to be linked to chronic inflammation, whether caused by chemical or physical factors, or by infections.7 The strongest relationship between chronic inflammation and cancer is observed in inflammatory diseases of the large intestine, ie ulcerative colitis (colitis ulcerosa) and Crohn’s disease.8 Their occurrence increases the risk of colorectal cancer up to 10 times.9 In contrast, tumors that do not arise from inflammation are characterized by the presence of inflammatory cells and mediators. In this case, inflammation is the result of tumor growth.
Tumor-Associated Cells
Tumors are not solely made out of mutant cells. The tumor microenvironment consists of numerous cells, for instance, the tumor stroma contains fibroblasts, endothelial cells, pericytes and mesenchymal cells among others.10 These cells produce pro-inflammatory cytokines (IL-1, −6, −8, −10, −17, −23, TNF-α, TGF-β) and chemokines (CCL2, CXCL8, CCL11) that attract immune cells such as macrophages, neutrophils, mast cells, dendritic cells, T and B lymphocytes, and natural killer (NK) cells. Leukocytes may constitute up to 50% of the total tumor mass.11,12 They all constitute the so-called tumor-associated systemic inflammatory response (SIR) – pivotal elements of tumor progression and can lead to the formation of micro-metastasis lesions. SIR is also associated with patient’s cachexia and malnutrition which all together can promote tumor progression.13 Homeostasis is maintained through the interaction of all cell types. Tumor growth is increased when mutated cells use their ability to communicate directly or by producing various mediators, which promotes their survival. Excessive and chronically produced pro-inflammatory mediators contribute to the promotion and progression of the tumor.14–17
Inflammation-Related Biomarkers
The search for new biomarkers, useful in diagnostics and prognostic terms, is still an attractive research topic in neoplastic diseases. Moreover, the relationship between inflammation and tumors is still an attractive subject and is pivotal for micro-metastasis lesions. SIR can cause cachexia and aggravate patients’ malnutrition, which promote and favor tumor progression.18
As neutrophils, lymphocytes, monocytes, and platelets play a key role in tumor-induced SIR,19 their quantification may provide an indicator of SIR in cancer patients. Blood morphology, due to its low cost and availability, is one of the most frequently performed routine laboratory tests. It provides a great deal of information about the quantitative composition of the major blood cells. It provides the basic parameters describing the population of leukocytes, erythrocytes, and platelets. It also makes the separation of leukocytes into individual fractions possible. Neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) are closely correlated with systemic inflammation and are promising biomarkers not only in systemic diseases such as SLA (systemic lupus erythematosus) or RA (rheumatoid arthritis),20–22 but also in acute coronary syndrome,23 type 1 diabetes24 and more recently in patients suffering from Covid-19.22,25 These markers are used also as prognostic biomarkers in various malignancies, including esophageal,26 lung cancer,27 head and neck,28 breast cancer,29 and colorectal cancer.30
These indicators reflect the interaction taking place between cancer cells and cells of the immune system (Figure 1). It has been found that NLR and PLR reflect the size and stage of cancer and can be used for early diagnosis and prognosis in patients affected by CRC.31 Conversely, lymphocytes play mainly an anti-tumor defense role. They induce cytotoxic cell death and produce cytokines that inhibit cancer cell proliferation and metastatic activity.32 Studies have shown that LMR is capable of providing valuable information on the diagnosis and prognosis of colorectal cancer as it reflects the degree of tumor differentiation and metastasis.33 Lymphocytes inhibit tumor growth and spread, in contrast to neutrophils and platelets, which have the ability to change their anti-neoplastic phenotype into a pro-carcinogenic and pro-metastatic phenotype.34
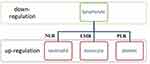 |
Figure 1 Inflammation-related parameters can be divided into two groups: the down-regulation variable – lymphocytes, and the up-regulation variable – neutrophils, platelets and monocytes. The combination of both can be used as a marker reflecting the inflammatory response in the course of cancer. Data from Ciocan et al.54 |
Thus far, there is disagreement as to which inflammatory biomarkers are the most clinically useful and offer the best prognostic indicators for CRC. The best combination of biomarkers and the optimal cut-off value may vary depending on the type of cancer. Moreover, anti-neoplastic therapy may influence the state of systemic inflammation in various ways. Some of the biomarkers have high prognostic value for a variety of cancer types.35 According to Herold et al retrospective study, abnormal LMR and NLR are associated with shorter overall and progression free survival times in CRC patients.36
In our study, we review the available literature about diagnostic significance of NLR, LMR, and PLR as the inflammation-biomarkers in colorectal cancer, on the basis of the area under the ROC curve published in the recent 6 years.
Neutrophile-to-Lymphocyte Ratio (NLR)
Neutrophils constitute the major component of the leukocyte population. They secrete cytokines, chemokines, and enzymes including neutrophil elastase, matrix metalloprotein 9 (MMP9), and vascular endothelial growth factor (VEGF). Regularly observed higher neutrophil numbers are involved in remodeling the extracellular matrix, which promotes angiogenesis and contributes to tumor development.37–39 Lymphocytes, in turn, constitute an important component of the host’s immune system and can attack cancer cells, and infiltration of these cells on the tumor is considered to be an anti-neoplastic immune response correlated with improved survival.40,41 Lymphopenia is often observed in advanced stages of cancer and may result in a weak and insufficient immunological response. Earlier reports have indicated that a decrease in the number of serum lymphocytes has a negative impact on the prognosis of CRC patients and can accelerate the development and progression of tumor cells.42–46
The neutrophil-lymphocyte ratio (NLR) is one of the most promising biomarkers in the prognosis in several types of cancer, including CRC. Several studies have proposed various cut-offs for NLR for OS (overall survival).36,47 The normal value of NLR in healthy people is around 1–3, but more recent reports by Forget et al38 reported the NLR value for the healthy adult population (21–66 years; mean age 38 years) between 0.78–3.53 (mean NLR value 1.65 ± 1.96). In CRC the most common cut-off value of NLR was from 2 to 5. The higher NLR usually is associated with poor prognosis in CRC patients.30
Chan et al48 analyzed the results of 2280 patients with CRC who had undergone tumor resection and found that the combination of preoperative and postoperative NLR was a significant predictor of OS. Patients with high pre- and postoperative NLR (> 3.75) exhibited the worst OS, while those with low pre- and postoperative NLR (≤3.75) exhibited the best OS.
The potential prognostic role of NLR concerning colorectal cancer remains controversial depending on tumor staging (TNM). However, the authors showed similar cut-off values regardless of tumor stage. Li et al49 conducted studies on a large group of patients suffering from colorectal cancer (n = 5336) after surgical resection in stage I–III and found that NLR with cut-off > 2.72 was an independent predictor of overall survival (OS) and disease-free survival (DFS). Jiang et al50 divided 684 CRC patients with stage II–III according to NLR cut-off, which was obtained by use of receiver-operating characteristic (ROC) curve. The optimal cut-off was 3.0. The higher NLR patients (> 3.0) were older, and also a presented higher ratio of colonic cancer and stage III, than patients with lower NLR (<3.0). The 5-year OS rates of high NLR and low NLR patients were 59.6% and 73.2% respectively (p = 0.001). Multivariate analysis revealed that older age (> 65 years), poor differentiation grade, high TNM stage and higher NLR were independent risk factors of prognosis for patients with locally advanced colorectal cancer (LACRC). Similarly, Kim et al39 conducted a retrospective study involving 1868 CRC patients who showed that NLR at cut-off ≥ 3.0 and PLR ≥160 were independent prognostic factors in predicting long-term outcomes in patients with stage III and IV CRC, however, not in stage I and II CRC. The authors suggest, that the assessment of NLR and PLR should be interpreted depending on tumor stage CRC and it should be taken under consideration during the planning of treatment strategy. It appears that increased NLR or PLR are associated with relative reduction in lymphocytes and lymphocyte-mediated immune response, which plays a crucial role in cytotoxic cell death. The cut-off of NLR was 3.0 (sensitivity 51% and specificity 62%) and for PLR 160 (sensitivity 53% and specificity 55%). Although the sensitivity and specificity were rather low, the NLR and PLR values were relatively reliable due to the large number of patients qualified for the study. The authors observed that with later stages of CRC and patients’ older age, higher NLR and thus worse OS occurred.39
Research conducted by Ying et al2 and Choi et al51 is convergent and demonstrated that NLR was superior in comparison to dNLR (derived neutrophil-to-lymphocyte ratio), PLR, and LMR as a prognostic predictor in CRC. The reason why NLR was superior to other inflammatory markers as a prognostic biomarker in CRC remains unclear. Also, Chen et al52 investigated the prognostic value of NLR in patients suffering from colorectal cancer. The authors divided the patients into two groups due to cut-off for high NLR group ≥ 2.03 and low NLR group <2.03 (AUC was 0.650). They concluded, that the survival rate of low NLR was significantly higher than of the high NLR group. NLR can reflect the preoperative inflammation and immune status of CRC patients.
Several recent studies assessed the prognostic value of NLR in CRC patients by means of ROC/AUC analysis (Table 1, Figure 2A) The results are inconsistent. Some authors reported NLR as a satisfactory test for differentiation between healthy and unhealthy patients, but others report it as unreliable.
 |
Table 1 Diagnostic Utility of Neutrophile-Lymphocyte Ratio (NLR) in Colorectal Cancer (CRC) Patients |
Zou et al59 conducted a retrospective study on 216 patients with CRC and found an AUC for NLR of 0.748 with a high cut-off 4.98. The results demonstrated that the risk of developing CRC was increased in patients with high NLR compared to patients with high PLR. High NLR and PLR were associated with poor tumor differentiation and a larger tumor diameter, respectively (P<0.05). In addition, patients with high NLR (≥4.98) had a significantly worse 5-year OS rate in comparison to patients with low NLR.
A similar result was obtained by Xia et al58 reported an AUC of 0.711, with a cut-off value of 2.8, with low sensitivity (53.0%) and high specificity (84.0%). Patients with high NLR exhibited a worse 3-year OS rate than patients with low NLR (p <0.001).
Ming-Sheng et al53 performed univariate and multivariate analyses to assess independent predictors of survival. Patients were divided into two groups based on the optimal cut-off value: low NLR <3.03 and high NLR ≥ 3.03. They demonstrated that the risk of death in the group with high NLR was 1.38 times higher than in the group with low NLR (p = 0.112). In addition, high NLR significantly influenced the survival of patients in CRC stages I–II and is an independent risk factor for poor prognosis in these patients.
Kim et al39 decided to divide the patients depending on the cut-off in a similar fashion (NLR ≥ 3.0 and NLR <3.0). The OS of patients with low NLR was better than that of patients with high NLR (p <0.001). However, in a multivariate analysis, they observed that in patients at stage I and II CRC, NLR was not a significant factor influencing long-term treatment outcomes, and in patients at stage III and IV CRC, high NLR was a significant prognostic factor influencing long-term prognosis. In patients at stage III and IV CRC, high NLR was an independent factor influencing OS (HR = 1.44, 95% CI: 1.14–1.83, P = 0.003).39
NLR is a systemic inflammatory response (SIR) biomarker. Numerous studies have proven that NLR is a cheap and easy-to-obtain parameter that can be used as a significant predictor of survival in patients suffering from CRC. This may help direct the benefits of adjuvant therapy, despite the fact that it may be applied to different patient populations at different stages of the disease. Furthermore, most studies chose the cut-off of 2.8160–62 which may help with faster calculation of the potential biomarker associated with poor prognosis in CRC. The univariate analysis showed that NLR was related to the postoperative and chemotherapy prognosis and can be used as a predictor of death.54 NLR also correlated with age, sex, location, death, T stage, N and M stage of CRC. On the other hand, some authors indicate that evaluation NLR and other ratios derived from morphology, show limited performance and clinical utility upon the ROC analysis.54
Based on the literature available in recent years, the mean AUC value for NLR was 0.742, cut-off = 3.31, while sensitivity = 63.03%, and specificity = 62.55%, and these values differed depending on the tumor stage, metastasis, or OS prognosis.
Lymphocyte-to-Monocyte-Ratio (LMR)
In the tumor microenvironment, monocytes may have different functions at different stages of tumor growth and progression.37,63 Pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 1 (IL-1) secreted by monocytes are associated with poor prognosis in cancer patients. In the course of neoplastic disease monocytes differentiate into tumor-associated macrophages (TAMs) and may promote tumor cell death.64 On the other hand, they exhibit pro-cancerous properties, eg suppression of acquired immunity, facilitating angiogenesis, invasion, and migration.65,66 Monocyte activity in cancer is associated with two phenotypes: M1 and M2. M1 macrophages through, among other methods, the production of IL-1, −6, −12, TNF-α, ROS and RNI enhance the anti-cancer response.67 Cells with the M2 phenotype produce IL-12, −23, and IL-10, which contribute to extinguishing the inflammatory process and weakening the anti-cancer immune response of the body.68,69
Thus, macrophages create a favorable immune microenvironment for the development of cancer and, playing a central role in it, are a significant drug target in anti-cancer therapy. It follows from the above that decreased LMR may be associated with poor prognosis in CRC patients66,70 and generally, increased monocytes have been associated with shorter rectal cancer survival rates.71
Chan et al72 studied a group of 1623 patients with CRC who underwent therapeutic surgical tumor resection (all stages) and found that elevated LMR before surgery (> 2.38) is an independent predictor of OS. They established that LMR is a more reliable predictor of OS in this patient group than NLR and PLR. They also demonstrated that the proportion of tumors with a high degree of histological malignancy was higher in patients with low LMR than in those with high LMR, and that tumors with low LMR were more common in the left side of the colon. Similar observations were presented by Wu et al,70 who studied 8648 patients and also showed that patients with low LMR have worse OS after treatment. In an analysis of subgroups divided by TNM stage, the prognostic role of LMR was observed for stage I CRC in comparison to stage III and IV (HR 1.70, 95% CI 1.30–2.23, P <0.001; and HR 1.45, 95% CI 1.06–1.99, P = 0.021, respectively). The cut-off values ranged from 2.14 to 3.78, therefore the studies were conducted in 2 groups according to the cut-off value: <3.00 and ≥3.00. Subgroup analysis showed that low LMR was associated with worse OS both in the <3.00 (HR 1.60, 95% CI 1.43–1.80) and ≥3.00 groups (HR 1.47, 95% CI 1.19–1.80, p <0.001). The other authors proposed LMR as a prognostic factor for metastasis in CRC, but did not prove such clinical reliability as NLR.73 Whereas Ciocan et al54 indicated that LMR proved to be an important predictor for T and M stage, independent of age and gender. The authors showed lower LMR values in patients with CRC who died (median=3.00) than those who survived (median=3.55).54
Several studies have been published, in recent years, that assess the prognostic potential of the LMR ratio in patients with CRC using the ROC/AUC analysis (Table 2, Figure 2B). The LMR cut-off ranged from 2.13 to 16.210 and varied depending on the study group, stage, and the presence or absence of metastases. A lower LMR indicates a reduced number of lymphocytes and an increased number of monocytes, which may indicate active inflammation, in this case promoting tumor development.
 |
Table 2 Diagnostic Utility of Lymphocyte-Monocyte Ratio (LMR) in Colorectal Cancer (CRC) Patients |
The discrepancies can also be found in the size of the area under the ROC curve. Ciocan et al54 analyzed the results of 1688 patients suffering from CRC, indicating the presence of lower values of LMR with advanced stage (T3 and T4) as compared to early stage (T1-T2). The authors showed very low, lower than 0.5 AUC values (0.418), with a very high cut-off of 16,210. The AUC = 0.634 of LMR indicates significantly lower values among those patients with chemo- or radiotherapy compared to those without. The AUCs of LMR (0.406) also indicate the existence of significantly lower values among those with metastasis, as compared to those without metastasis. On the other hand, AUC of LMR supports the existence of lower values of death (AUC = 0.429; cut-off = 5.465) as compared to living subjects. In this study, the multivariable regression models showed that NLR and LMR exhibit predictive potential for T stage, independent of patient’s age.54
Slightly different values for LMR were published by Xia et al.58 They enrolled 154 patients with CRC and reported an AUC value of 0.68, with a cut-off of 3.9, with high sensitivity (73.0%) but low specificity (65.0%). However, according to the research by Li et al31 LMR exhibits valuable diagnostic utility (AUC = 0.865) in differentiating healthy people from patients in the early stage of CRC.
The relevant literature from the last 6 years suggests that the mean AUC value for LMR was 0.629 and cut-off = 5.46, while sensitivity = 67.97%, and specificity = 71.60%. These values differed depending on the tumor stage, tumor diameter, and OS prognosis.
Platelet-to-Lymphocyte-Ratio (PLR)
Blood platelets are one of the first cells to accumulate at the site of damage and by locally releasing the content of their granules, they initiate what is known as an inflammatory cascade. Together with other immunologically competent cells, they form the so-called “tumor microenvironment”. Thrombocytosis, a platelet count (PLT) > 450,000/µL, is often observed in patients with solid tumors and chronic inflammation, and in combination with increased activation, it may result in an increased risk of thrombosis.75–77 An elevated platelet count is a negative predictor of survival in several cancers, including CRC.78 Thrombocytosis is not only an epiphenomenon of malignancy, but rather, a paraneoplastic abnormality.79 This increase can develop for several reasons, such as bleeding from the tumor-reactive thrombocytosis or metabolic changes caused by the tumor itself, called paraneoplastic thrombocytosis.80
The way platelets interact with tumor cells is complex. In the tumor microenvironment, platelets promote angiogenesis and, consequently, the formation of metastatic foci, eg, by releasing vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGF-β).81 In turn, platelet-derived growth factor (PDGF) plays an important role in promoting tumor growth and invasion. In addition, cytokines (IL-1β) and chemokines: CXCL1 (β-thromboglobulin), CXCL4 (PF4, platelet factor 4), CCL5 (RANTES, regulated upon activation and normal T cell ex- pressed and secreted), CXCL12 (SDF-1, stromal cell-derived factor-1) produced by platelets may promote the development of cancer-related inflammation.81,82 Also tumor cells themselves have the ability to alter platelet activity to best manage tumor growth, proliferation, metastasis, and survival.83
Paraneoplastic thrombocytosis appears to involve a “positive feedback loop”. Malignant tumors can increase PLT production, by increasing TPO (thrombopoietin) and interleukin 6 (IL-6) production, leading to secondary (reactive) thrombocytosis (Lin).79 At the same time, the cancer cells themselves directly or indirectly activate platelets. In turn, an increased number of activated platelets promotes further tumor growth and metastasis, leading to an even greater stimulation of the number and level of platelet activity.
Like NLR and LMR, PLR also becomes a useful, although the least researched, predictive marker (Table 3, Figure 2C). PLR has been shown to be a prognostic factor for ovarian,84 breast,85 or lung cancer.86 The prognostic value of PLR in patients suffering from CRC is ambiguous.
 |
Table 3 Diagnostic Utility of Platelet to-Lymphocyte Ratio (PLR) in Colorectal Cancer (CRC) Patients |
Pedrazzani et al89 conducted a study on 603 patients with CRC. The authors showed that an increased platelet count (>350 × 109 /l) may be a significant predictor of poor OS. Similar results were obtained by Ishizuka et al,77 who found that platelets >300 × 109 /l were associated with poor prognosis in patients with CRC at all stages.
A high PLR reflects both an increase in the platelet count (PLT) and a decrease in the number of lymphocytes, which is associated with a poor prognosis for the patient. Increased PLT in patients with CRC is associated with poor prognosis and an increased risk of distant metastasis.87 Thrombocytosis is related with systemic inflammation due to cancer, additionally, thrombosis can show systemic inflammatory and tumor activity. However, the assessment of the diagnostic power of PLR did not show that it is an indicator of high diagnostic utility in colorectal neoplasms.
Zou et al59 found that AUC of PLR is 0.690 with a high cut-off of 246.36. High PLR in patients was associated with a larger tumor diameter (P <0.05), poor primary tumor classification (T classification) (p = 0.006), and a significantly worse 5-year OS rate compared to patients with low PLR ≤ 246.36 (34.0 vs 79.2%; P <0.001).
Ackigoz et al87 investigated the prognostic and predictive value of PLR and the relationship between PLR and tumor location in 229 patients suffering from CRC. Based on the size of the area under the ROC curve (AUC=0.690), the PLR cut-off was 196.5 (sensitivity and specificity respectively 85.0% and 65.0%). Logistic regression analysis showed a significant association between PLR and BRAF mutation, treatment response, tumor location, and tumor progression. A significantly higher BRAF mutation rate was detected in patients with high PLR (> 196.5) compared to patients with low PLR (≤196.5). Moreover, PLR was significantly higher in tumors located in the proximal colon than in tumors in the distal colon. OS and DFS were significantly better in patients with low PLR compared to patients with high PLR.87
In a large-scale retrospective study of 1868 patients with CRC, Kim et al39 demonstrated a PLR AUC of 0.556, with a mean PLR of 200.17 ± 168.96 and a cut-off of 160. Interestingly, the prognosis based on NLR and PLR differed depending on the stage of the cancer. In contrast, the OS and DFS of patients with stage I and II CRC did not differ significantly depending on NLR and PLR, as compared to patients with stage III and IV CRC. The performed regression analysis showed that high PLR (≥ 160), as well as NLR ≥3.0 were independent risk factors predicting poor long-term outcomes in patients with more advanced CRC (stage III and IV) than in patients with stage I and II CRC.
Jia et al in the univariate analysis indicating that PLR, but not NLR, was related to OS and DFS in CRC patients. Also, high PLR (>154.31) may be of prognostic relevance in CRC patients receiving NAC (neoadjuvant chemotherapy).57
Similar results were obtained by Xia et al58 AUC = 0.64, with a cut-off value of 140. Patients with stage T1–2 rectal cancer with high PLR (≥ 140) had a significantly worse 3-year OS rate than patients with low-low PLR (≤140) (p = 0.001).
Recent studies show that the combination of inflammatory parameters and tumor markers has a higher diagnostic value in malignant tumors.90,91 Peng et al,92 after performing a comparative analysis of the ROC curves, demonstrated that the diagnostic efficacy of the combination of NLR, PLR, and CEA (AUC = 0.831, 95% CI = 0.807–0.852) for CRC was not only significantly higher than that of the NLR alone (AUC = 0.755, 95% CI = 0.728–0.780), PLR (AUC = 0.723, 95% CI = 0.696–0.749, P = 0.037) or CEA (AUC = 0.690, 95% CI = 0.662–0.717, P = 0.002), but also higher than any combination of two of these three biomarkers (p <0.05).
In the summary of the results acquired in the literature available in recent 6. years, the mean AUC value for PLR was 0.648, cut-off = 146.98, while the sensitivity and specificity were 67.83% and 60.65%, respectively. These values varied depending on the tumor stage, tumor size, metastasis, and OS prognosis.
Conclusion
The most important conclusion from this literature review is the need to develop norms or cut-off points for SIR biomarkers calculated from CBC (complete blood count) (NLR, LMR, and PLR). In recent years, a growing number of researchers have been dealing with this topic because these indicators are cheap, easily obtainable, and with a potential that is not yet fully understood. Further studies therefore, a large prospective multi-center study is recommended, eg randomized controlled trials (RCT), taking into account larger study populations, more homogeneous in terms of age, gender, and clinical stage - at different stages of advancement in order to establish values (cut-off) that allow patients to differentiate with a poor outcome. SIR biomarkers, especially NLR and PLR, also could be useful for evaluating treatment outcomes and survival. Each of the evaluated ratios possesses prognostic value for certain outcomes considered, but the reported models need external validation to recommend their utilization in clinical practice. Further studies to prove their clinical relevance are needed.
Disclosure
All authors declare that there is no possible conflict of interest.
References
1. Son W, Shin SJ, Park SH, et al. Clinical impact of combined modified glasgow prognostic score and c-reactive protein/albumin ratio in patients with colorectal cancer. Diagnostics. 2020;10(11):859. doi:10.3390/DIAGNOSTICS10110859
2. Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31(12):1–8. doi:10.1007/S12032-014-0305-0
3. Wojciechowska U, Didkowska J. Nowotwory Złośliwe w Polsce w 2018 [Malignant tumors in Poland in 2018]. Krajowy Rejestr Nowotworów; 2020.
4. Praktyce OW, Zyśk R, Wysocki P, Wyrwicz L. Colorectal cancer — the social significance of changes in the epidemiology and treatment options in Poland. Oncol Clin Pract. 2014;10(4):212–223.
5. Korniluk A, Koper O, Kemona H, Dymicka-Piekarska V. From inflammation to cancer. Ir J Med Sci. 2017;186(1):57–62. doi:10.1007/S11845-016-1464-0
6. Dymicka-Piekarska V, Koper-Lenkiewicz OM, Zińczuk J, Kratz E, Kamińska J. Inflammatory cell-associated tumors. Not only macrophages (TAMs), fibroblasts (TAFs) and neutrophils (TANs) can infiltrate the tumor microenvironment. The unique role of tumor associated platelets (TAPs). Cancer Immunol Immunother. 2021;70(6):1497–1510. doi:10.1007/S00262-020-02758-7
7. Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22(1):33–40. doi:10.1016/J.SEMCANCER.2011.12.005
8. Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39(1):87–92. doi:10.1136/GUT.39.1.87
9. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/NATURE01322
10. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. doi:10.1038/ONC.2008.271
11. Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–1183. doi:10.1172/JCI31537
12. de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi:10.1038/NRC1782
13. Kasprzak A. The role of tumor microenvironment cells in colorectal cancer (CRC) cachexia. Int J Mol Sci. 2021;22(4):1–34. doi:10.3390/IJMS22041565
14. Piskor BM, Pryczynicz A, Lubowicka E, et al. Immunohistochemical expression of Fascin-1 in colorectal cancer in relation to clinical and pathological parameters. Folia Histochem Cytobiol. 2018;56:106–112. doi:10.5603/FHC.a2018.0011
15. Zińczuk J, Maciejczyk M, Zaręba K, et al. Pro-oxidant enzymes, redox balance and oxidative damage to proteins, lipids and DNA in colorectal cancer tissue. is oxidative stress dependent on tumour budding and inflammatory infiltration? Cancers. 2020;12:1636. doi:10.3390/cancers12061636
16. Zińczuk J, Zaręba K, Kamińska J, et al. Association of tumour microenvironment with protein glycooxidation, DNA damage, and nitrosative stress in colorectal cancer. Cancer Manag Res. 2021;13:6329–6348. doi:10.2147/CMAR.S314940
17. Dorf J, Zaręba K, Matowicka-Karna J, et al. May the nitrosative and carbonyl stress promote inflammation in patients with colorectal cancer? J Inflamm Res. 2022;15:4585–4600. doi:10.2147/JIR.S374387
18. Zhang Q, Song MM, Zhang X, et al. Association of systemic inflammation with survival in patients with cancer cachexia: results from a multicentre cohort study. J Cachexia Sarcopenia Muscle. 2021;12(6):1466–1476. doi:10.1002/JCSM.12761
19. Zhang L, Wei Z, Xu A, Zang JH. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int J Surg. 2018;56:320–327. doi:10.1016/J.IJSU.2018.06.037
20. Mercan R, Bitik B, Tufan A, et al. The association between neutrophil/lymphocyte ratio and disease activity in rheumatoid arthritis and ankylosing spondylitis. J Clin Lab Anal. 2016;30(5):597–601. doi:10.1002/JCLA.21908
21. Koiwa M, Goto S, Takahashi K, Kamada T, Takai S, Nakamura H. Neutrophil/lymphocyte ratio in patients with rheumatoid arthritis treated with biological agents. J Nippon Med Sch. 2016;83(3):118–124. doi:10.1272/JNMS.83.118
22. Wang X, Qiu L, Li Z, Wang XY, Yi H. Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front Immunol. 2018;9. doi:10.3389/FIMMU.2018.02456
23. Angkananard T, Anothaisintawee T, McEvoy M, Attia J, Thakkinstian A. Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1–11. doi:10.1155/2018/2703518
24. Mertoglu C, Gunay M. Neutrophil-Lymphocyte ratio and Platelet-Lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndr. 2017;11(Suppl 1):S127–S131. doi:10.1016/J.DSX.2016.12.021
25. Yang AP, Liu J, Tao W, Li H. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi:10.1016/J.INTIMP.2020.106504
26. Xiao Q, Zhang B, Deng X, et al. The preoperative neutrophil-to-lymphocyte ratio is a novel immune parameter for the prognosis of esophageal basaloid squamous cell carcinoma. PLoS One. 2016;11(12):e0168299. doi:10.1371/JOURNAL.PONE.0168299
27. Winther-Larsen A, Aggerholm-Pedersen N, Sandfeld-Paulsen B. Inflammation scores as prognostic biomarkers in small cell lung cancer: a systematic review and meta-analysis. Syst Rev. 2021;10(1). doi:10.1186/S13643-021-01585-W
28. Kim DY, Kim IS, Park SG, Kim H, Choi YJ, Seol YM. Prognostic value of posttreatment neutrophil-lymphocyte ratio in head and neck squamous cell carcinoma treated by chemoradiotherapy. Auris Nasus Larynx. 2017;44(2):199–204. doi:10.1016/J.ANL.2016.05.013
29. Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19(1):217–224. doi:10.1245/S10434-011-1814-0
30. Hayama T, Ozawa T, Tsukamoto M, et al. Predicting overall survival using preoperative nutritional and inflammation status for colorectal cancer. In Vivo. 2022;36(1):450–457. doi:10.21873/INVIVO.12724
31. Li X, Guo D, Chu L, et al. Potential diagnostic value of combining inflammatory cell ratios with carcinoembryonic antigen for colorectal cancer. Cancer Manag Res. 2019;11:9631–9640. doi:10.2147/CMAR.S222756
32. Tan D, Fu Y, Tong W, Li F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: a meta-analysis. Int J Surg. 2018;55:128–138. doi:10.1016/J.IJSU.2018.05.030
33. Kostakis ID, Vaiopoulos AG, Garoufalia Z, et al. What can preoperative blood tests tell us about colorectal cancer? JBUON. 2018;23(1):84–95.
34. Sánchez JCS, Quiñones JLG, Muñoz-Velandia OM, Zambrano MA, Ramírez JRT. High mean platelet volume and neutrophil-to-lymphocyte ratio are predictors of mortality in patients with HIV-related non-Hodgkin’s lymphoma. Acta Haematol Pol. 2021;52(3):195–201. doi:10.5603/AHP.2021.0037
35. Heeke S, Mograbi B, Alix-Panabières C, Hofman P. Never travel alone: the crosstalk of circulating tumor cells and the blood microenvironment. Cells. 2019;8(7):714. doi:10.3390/CELLS8070714
36. Herold Z, Herold M, Lohinszky J, Szasz AM, Dank M, Somogyi A. Longitudinal changes in personalized platelet count metrics are good indicators of initial 3-year outcome in colorectal cancer. World J Clin Cases. 2022;10(20):6825–6844. doi:10.12998/wjcc.v10.i20.6825
37. Yamamoto T, Kawada K, Obama K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. 2021;22(15):8002. doi:10.3390/IJMS22158002
38. Forget P, Khalifa C, Defour JP, Latinne D, van Pel MC, de Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10(1):1–4. doi:10.1186/S13104-016-2335-5
39. Kim JH, Lee JY, Kim HK, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with stage III and IV colorectal cancer. World J Gastroenterol. 2017;23(3):505–515. doi:10.3748/wjg.v23.i3.505
40. He S, Lamers GEM, Beenakker JWM, et al. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J Pathol. 2012;227(4):431–445. doi:10.1002/PATH.4013
41. Klajman A, Cohen R, Bruderman I, Steiner Z, Klajman A. Functional analysis of mononuclear cells infiltrating into tumors: lysis of autologous human tumor cells by cultured infiltrating lymphocytes. Cancer Res. 1987;47(1):173–177.
42. Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi:10.1038/35077246
43. Yang J, Guo X, Wang M, Ma X, Ye X, Lin P. Pre-treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild-type RAS. Sci Rep. 2017;7(1). doi:10.1038/S41598-017-17130-6
44. Dou X, Wang R, Yan HJ, et al. Circulating lymphocytes as predictors of sensitivity to preoperative chemoradiotherapy in rectal cancer cases. Asian Pac J Cancer Prev. 2013;14(6):3881–3885. doi:10.7314/APJCP.2013.14.6.3881
45. Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 2010;5(1). doi:10.1186/1748-717X-5-47
46. Noh OK, Oh SY, Kim YB, Suh KW. Prognostic significance of lymphocyte counts in colon cancer patients treated with FOLFOX chemotherapy. World J Surg. 2017;41(11):2898–2905. doi:10.1007/S00268-017-4104-6
47. Li Y, Wu H, Xing C, et al. Prognostic evaluation of colorectal cancer using three new comprehensive indexes related to infection, anemia and coagulation derived from peripheral blood. J Cancer. 2020;11(13):3834–3845. doi:10.7150/jca.42409
48. Chan JCY, Diakos CI, Chan DLH, et al. A longitudinal investigation of inflammatory markers in colorectal cancer patients perioperatively demonstrates benefit in serial remeasurement. Ann Surg. 2018;267(6):1119–1125. doi:10.1097/SLA.0000000000002251
49. Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. 2016;139(1):220–231. doi:10.1002/IJC.30071
50. Jiang H, Li H, Li A, et al. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget. 2016;7(44):72076. doi:10.18632/ONCOTARGET.12271
51. Choi WJ, Cleghorn MC, Jiang H, Jackson TD, Okrainec A, Quereshy FA. Preoperative neutrophil-to-lymphocyte ratio is a better prognostic serum biomarker than platelet-to-lymphocyte ratio in patients undergoing resection for nonmetastatic colorectal cancer. Ann Surg Oncol. 2015;22(Suppl 3):603–613. doi:10.1245/S10434-015-4571-7
52. Zhan L, Liu Y, Cheng Y, Guo W, Yang J. Predictive value of neutrophil/lymphocyte ratio (NLR) on cardiovascular events in patients with COVID-19. Int J Gen Med. 2021;14:3899–3907. doi:10.2147/IJGM.S317380
53. Ming-Sheng F, Mei-Ling D, Xun-Quan C, Yuan-Xin H, Wei-Jie Z, Qin-Cong P. Preoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and CEA as the potential prognostic biomarkers for colorectal cancer. Can J Gastroenterol Hepatol. 2022;2022:1–9. doi:10.1155/2022/3109165
54. Ciocan A, Ciocan RA, Al Hajjar N, Gherman CD, Bolboacă SD. Abilities of pre-treatment inflammation ratios as classification or prediction models for patients with colorectal cancer. Diagnostics. 2021;11(3):566. doi:10.3390/DIAGNOSTICS11030566
55. Turhan VB, Ünsal A, Gök HF, et al. Predictive value of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in determining the stage of colon tumors. Cureus. 2021. doi:10.7759/cureus.18381
56. Chen W, Yi HJ, Chen XQ, et al. Prognostic value of the NLR combined with CIP2A in the serum of patients with colorectal cancer. BMC Surg. 2021;21(1). doi:10.1186/s12893-021-01273-5
57. Jia W, Yuan L, Ni H, Xu B, Zhao P. Prognostic value of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and lymphocyte-to-white blood cell ratio in colorectal cancer patients who received neoadjuvant chemotherapy. Technol Cancer Res Treat. 2021;20:153303382110342. doi:10.1177/15330338211034291
58. Xia LJ, Li W, Zhai JC, Yan CW, Chen JB, Yang H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer. 2020;20(1). doi:10.1186/s12885-020-6698-6
59. Zou ZY, Liu HL, Ning N, Li SY, Du XH, Li R. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11(3):2241–2248. doi:10.3892/OL.2016.4216
60. Okamura Y, Sugiura T, Ito T, et al. Neutrophil to lymphocyte ratio as an indicator of the malignant behaviour of hepatocellular carcinoma. Br J Surg. 2016;103(7):891–898. doi:10.1002/BJS.10123
61. Liu Y, Wang ZX, Cao Y, Zhang G, Chen WB, Jiang CP. Preoperative inflammation-based markers predict early and late recurrence of hepatocellular carcinoma after curative hepatectomy. Hepatobiliary Pancreat Dis Int. 2016;15(3):266–274. doi:10.1016/S1499-3872(16)60094-2
62. Lu SD, Wang YY, Peng NF, et al. Preoperative ratio of neutrophils to lymphocytes predicts postresection survival in selected patients with early or intermediate stage hepatocellular carcinoma. Medicine. 2016;95(5). doi:10.1097/MD.0000000000002722
63. Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106(2):309–322. doi:10.1002/JLB.4RI0818-311R
64. Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267(2):204–215. doi:10.1016/J.CANLET.2008.03.028
65. Rumba R, Cipkina S, Cukure F, Vanags A. Systemic and local inflammation in colorectal cancer. Acta Med Litu. 2018;25(4):185–196. doi:10.6001/ACTAMEDICA.V25I4.3929
66. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi:10.1016/J.CELL.2006.01.007
67. Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6(3):1670–1690. doi:10.3390/CANCERS6031670
68. Singh N, Baby D, Rajguru J, Patil P, Thakkannavar S, Pujari V. Inflammation and cancer. Ann Afr Med. 2019;18(3):121. doi:10.4103/AAM.AAM_56_18
69. Dwyer AR, Greenland EL, Pixley FJ. Promotion of tumor invasion by tumor-associated macrophages: the role of CSF-1-activated phosphatidylinositol 3 kinase and src family kinase motility signaling. Cancers. 2017;9(6):68. doi:10.3390/CANCERS9060068
70. Wu Q, Hu T, Zheng E, Deng X, Wang Z. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: an up-to-date meta-analysis. Medicine. 2017;96(22). doi:10.1097/MD.0000000000007051
71. Zhang Y, Liu X, Xu M, Chen K, Li S, Guan G. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci Rep. 2020;10(1). doi:10.1038/S41598-020-64684-Z
72. Chan JCY, Chan DL, Diakos CI, et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2017;265(3):539–546. doi:10.1097/SLA.0000000000001743
73. Basile D, Garattini SK, Corvaja C, et al. The MIMIC study: prognostic role and cutoff definition of monocyte-to-lymphocyte ratio and lactate dehydrogenase levels in metastatic colorectal cancer. Oncologist. 2020;25(8):661–668. doi:10.1634/THEONCOLOGIST.2019-0780
74. Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative lymphocyte-to-monocyte ratio in patients with colorectal cancer. Oncol Lett. 2017;13(2):1000–1006. doi:10.3892/OL.2016.5487
75. Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2005;25(7):1321–1324. doi:10.1161/01.ATV.0000166521.90532.44
76. Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618. doi:10.1056/NEJMOA1110352
77. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol. 2012;106(7):887–891. doi:10.1002/JSO.23163
78. Rao XD, Zhang H, Xu ZS, Cheng H, Shen W, Wang XP. Poor prognostic role of the pretreatment platelet counts in colorectal cancer: a meta-analysis. Medicine. 2018;97(23):e10831. doi:10.1097/MD.0000000000010831
79. Lin RJ, Afshar-Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood. 2014;124(2):184–187. doi:10.1182/blood-2014-03-562538
80. Herczeg G, Somogyi A, Herold M, et al. Does diabetes affect paraneoplastic thrombocytosis in colorectal cancer? Open Med. 2022;17(1):160–173. doi:10.1515/med-2021-0407
81. Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017;36(2):249–262. doi:10.1007/S10555-017-9673-1
82. Flad HD, Brandt E. Platelet-derived chemokines: pathophysiology and therapeutic aspects. Cell Mol Life Sci. 2010;67(14):2363–2386. doi:10.1007/S00018-010-0306-X
83. Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229(8):1005–1015. doi:10.1002/JCP.24539
84. Zhao Z, Zhao X, Lu J, Xue J, Liu P, Mao H. Prognostic roles of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in ovarian cancer: a meta-analysis of retrospective studies. Arch Gynecol Obstet. 2018;297(4):849–857. doi:10.1007/S00404-018-4678-8
85. Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524–2530. doi:10.1038/BJC.2014.163
86. Ding N, Pang Z, Shen H, Ni Y, Du J, Liu Q. The prognostic value of PLR in lung cancer, a meta-analysis based on results from a large consecutive cohort. Sci Rep. 2016;6. doi:10.1038/SREP34823
87. Acikgoz O, Cakan B, Demir T, et al. Platelet to lymphocyte ratio is associated with tumor localization and outcomes in metastatic colorectal cancer. Medicine. 2021;100(44):e27712. doi:10.1097/MD.0000000000027712
88. Mo C, Hu Z, Qin S, et al. Diagnostic value of platelet-lymphocyte ratio and hemoglobin-platelet ratio in patients with rectal cancer. J Clin Lab Anal. 2020;34(4). doi:10.1002/jcla.23153
89. Pedrazzani C, Mantovani G, Fernandes E, et al. Assessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after R0 resection for colorectal cancer. Sci Rep. 2017;7(1). doi:10.1038/S41598-017-01652-0
90. Wu Y, Jiang M, Qin Y, Lin F, Lai M. Single and combined use of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and carcinoembryonic antigen in diagnosing gastric cancer. Clin Chim Acta. 2018;481:20–24. doi:10.1016/J.CCA.2018.02.027
91. Liu XF, Zhou LY, Wei ZH, et al. The diagnostic role of circulating inflammation-based biomarker in gallbladder carcinoma. Biomark Med. 2018;12(10):1095–1103. doi:10.2217/BMM-2018-0049
92. Peng HX, Yang L, He BS, et al. Combination of preoperative NLR, PLR and CEA could increase the diagnostic efficacy for I-III stage CRC. J Clin Lab Anal. 2017;31(5):e22075. doi:10.1002/JCLA.22075
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

