Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Disease Progression and Age as Factors Underlying Multimorbidity in Patients with COPD: Results from COSYCONET
Authors Alter P , Kahnert K, Trudzinski FC , Bals R, Watz H, Speicher T, Söhler S , Andreas S , Welte T , Rabe KF , Wouters EFM, Sassmann-Schweda A, Wirtz H, Ficker JH , Vogelmeier CF, Jörres RA
Received 2 March 2022
Accepted for publication 21 July 2022
Published 29 July 2022 Volume 2022:17 Pages 1703—1713
DOI https://doi.org/10.2147/COPD.S364812
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Peter Alter,1 Kathrin Kahnert,2 Franziska C Trudzinski,3 Robert Bals,4 Henrik Watz,5 Tim Speicher,1 Sandra Söhler,1 Stefan Andreas,6 Tobias Welte,7 Klaus F Rabe,8 Emiel FM Wouters,9 Antonia Sassmann-Schweda,10 Hubert Wirtz,11 Joachim H Ficker,12,13 Claus F Vogelmeier,1 Rudolf A Jörres14
1Department of Medicine, Pulmonary and Critical Care Medicine, Philipps University of Marburg (UMR), Germany, Member of the German Center for Lung Research (DZL), Marburg, Germany; 2Department of Internal Medicine V, University Hospital, LMU Munich, Comprehensive Pneumology Center Munich (CPC-M), Member of the German Center for Lung Research (DZL), Munich, Germany; 3Department of Pneumology and Critical Care Medicine, Thoraxklinik, University of Heidelberg, Translational Lung Research Center Heidelberg (TLRC-H), Member of the German Center for Lung Research (DZL), Heidelberg, Germany; 4Department of Internal Medicine V - Pulmonology, Allergology, Intensive Care Medicine, Saarland University Hospital, Homburg, Germany; 5Pulmonary Research Institute at LungenClinic Grosshansdorf, Airway Research Center North (ARCN), Member of the German Center for Lung Research (DZL), Grosshansdorf, Germany; 6LungClinic Immenhausen and Department of Cardiology and Pneumology, University Medical Center Göttingen, Germany, Member of the German Center for Lung Research (DZL), Göttingen, Germany; 7Clinic for Pneumology, Hannover Medical School, Member of the German Center for Lung Research (DZL), Hannover, Germany; 8LungenClinic Grosshansdorf and Department of Medicine, Christian-Albrechts University, Kiel, Airway Research Center North (ARCN), Member of the German Center for Lung Research (DZL), Grosshansdorf, Germany; 9Department of Respiratory Medicine, Maastricht University Medical Centre, Maastricht, Netherlands and Ludwig Boltzmann Institute for Lung Health, Vienna, Austria; 10Center for Clinical Studies, Research Center Borstel, Borstel, Germany; 11Department of Internal Medicine I, Pneumology, University of Leipzig, Leipzig, Germany; 12Department of Respiratory Medicine, Allergology and Sleep Medicine, Klinikum Nuremberg, Nürnberg, Germany; 13Paracelsus Medical University Nuremberg, Nürnberg, Germany; 14Institute and Outpatient Clinic for Occupational, Social and Environmental Medicine, University Hospital, LMU Munich, Comprehensive Pneumology Center Munich (CPC-M), Member of the German Center for Lung Research (DZL), Munich, Germany
Correspondence: Peter Alter, Department of Medicine, Pulmonary and Critical Care Medicine, Philipps University of Marburg (UMR), Baldingerstrasse 1, Marburg, Germany, Email [email protected]
Background: Multimorbidity plays an important role in chronic obstructive pulmonary disease (COPD) but is also a feature of ageing. We estimated to what extent increases in the prevalence of multimorbidity over time are attributable to COPD progression compared to increasing patient age.
Methods: Patients with COPD from the long-term COSYCONET (COPD and Systemic Consequences - Comorbidities Network) cohort with four follow-up visits were included in this analysis. At each visit, symptoms, exacerbation history, quality of life and lung function were assessed, along with the comorbidities heart failure (HF), coronary artery disease (CAD), peripheral arterial disease (PAD), hypertension, sleep apnea, diabetes mellitus, hyperlipidemia, hyperuricemia and osteoporosis. Using longitudinal logistic regression analysis, we determined what proportion of the increase in the prevalence of comorbidities could be attributed to patients’ age or to the progression of COPD over visits.
Results: Of 2030 patients at baseline, 878 completed four follow-up visits (up to 4.5 years). CAD prevalence increased over time, with similar effects attributable to the 4.5-year follow-up, used as indicator of COPD progression, and to a 5-year increase in patients’ age. The prevalence of HF, diabetes, hyperlipidemia, hyperuricemia, osteoporosis and sleep apnea showed stronger contributions of COPD progression than of age; in contrast, age dominated for hypertension and PAD. There were different relationships to patients’ characteristics including BMI and sex. The results were not critically dependent on the duration of COPD prior to enrolment, or the inclusion of patients with all four follow-up visits vs those attending only at least one of them.
Conclusion: Analyzing the increasing prevalence of multimorbidity in COPD over time, we separated age-independent contributions, probably reflecting intrinsic COPD-related disease progression, from age-dependent contributions. This distinction might be useful for the individual assessment of disease progression in COPD.
Keywords: chronic obstructive pulmonary disease, comorbidities, multimorbidity, prognosis, disease progression
Introduction
Multimorbidity is frequent in patients with chronic obstructive pulmonary disease (COPD), and COPD itself is a comorbidity of other chronic diseases. Indeed, almost all patients with COPD exhibit at least one other chronic disease, the majority several,1–4 and their coexistence plays an important role for health status and prognosis.5 The comorbidity spectrum includes cardiovascular, metabolic, endocrine, and inflammatory disorders, often sharing common risk factors such as tobacco smoking. In all age groups, comorbid conditions are more frequent in patients with COPD than in individuals without.6 However, most of the disorders also become more frequent as age progresses, even in the absence of COPD.7
These observations raise the question of how age-related and COPD-related contributions to comorbidities are related to each other, and specifically, to what extent their prevalence increases over time due to factors intrinsic to COPD progression, compared to those attributable to increasing age. Answering this could improve the evaluation of disease progression and help to distinguish potentially treatable contributions arising from the lung disease from contributions primarily arising from advanced age.
The present study addressed this question by using longitudinal data from the German COPD cohort COSYCONET (COPD and Systemic Consequences - Comorbidities Network).
Materials and Methods
Study Population
COSYCONET is an ongoing, multi-center, long-term observational study focusing on the impact of comorbidities in patients with COPD. Details of the design and baseline (Visit 1) data have been previously published.3 Regular follow-up visits (Visits 2 to 5) were scheduled at 6, 18, 36 and 54 months after enrolment. In the present analysis, we included patients of initial Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric grades 1–4,8,9 who participated in the four follow-up visits. COSYCONET has been approved by the Ethics Committees of all study centers, and all patients provided written informed consent. It was performed in accordance with the declaration of Helsinki. ClinicalTrials.gov: NCT01245933.
Assessments
Patients were required to be clinically stable, ie no exacerbation for at least 4 weeks prior to each visit. COPD symptoms were determined using the modified Medical Research Council (mMRC) dyspnea scale, while exacerbation risk was based on patient-reported exacerbations in the previous year,10 allowing their allocation to GOLD groups A to D.8 To indicate increased symptoms, GOLD groups B and D were merged (BD), as were C and D for increased exacerbation risk (CD), and each was compared with the complement. For the assessment of quality of life, the visual analog scale (VAS) of the EuroQoL 5 dimension (EQ-5D-3L) questionnaire was used. Self-reported, physician-based comorbidities were captured using a structured interview.2 They comprised heart failure, coronary artery disease (CAD), peripheral artery disease (PAD), hypertension, diabetes mellitus, hyperlipidemia, hyperuricemia, osteoporosis and sleep apnea, the latter being chosen due to its known association with cardiovascular disease.11 Post-bronchodilator forced expiratory volume in 1 sec (FEV1) was measured following GOLD recommendations,9 using Global Lung Function Initiative (GLI) equations to express results as % predicted values.12
Statistical Analysis
For descriptive purposes, mean values and standard deviations (SD), median values, or numbers and percentages are given, depending on the type of data and distribution. Generalized linear models with repeated measures design and logit link equivalent to longitudinal logistic regression analysis were used to examine the relationship of each comorbidity to its predictors. Values of all visits of patients with four follow-up visits were included in the analysis (primary study population). Models included the COPD characteristics at each study visit as predictors, as well as four indicator variables reflecting the effects of Visits 2–5 relative to Visit 1 that served as reference. The demographic and clinical characteristics assessed at each visit comprised age, sex, body mass index (BMI), smoking status (active smoker versus never or ex-smoker), increased exacerbations (GOLD groups CD vs AB), increased symptoms (GOLD BD vs AC), EQ-5D-3L VAS and FEV1% predicted.
In the interpretation of the results, we compared the magnitude of the effect at Visit 5 (4.5 years after inclusion) vs baseline, as indicator of disease progression, with the magnitude of the effect of a 5-year increase in age. Since both time periods are similar, values could be compared. To detect the potential effect of selection bias resulting from loss during follow-up, we performed sensitivity analyses comparing the results between the primary study population and the larger population of patients having only at least one follow-up visit. Moreover, we compared the age-attributed contribution upon inclusion with that of the increase in age over the follow-up period. For this purpose, we repeated the analyses including only the age upon inclusion as predictor instead of the increasing age. There was also the possibility that the age-attributed contribution already comprised COPD-related factors as patients had a history of COPD prior to enrolment. To account for this, we also repeated the analyses with the addition of reported duration of COPD since diagnosis at inclusion as predictor.
P values <0.05 (two-sided) were considered statistically significant. All analyses were performed using procedures including GENLIN from the software package IBM SPSS Statistics (Version 26.0.0.0, Armonk, NY, US).
Results
Patients’ Baseline Characteristics
A total of 2741 patients were enrolled in COSYCONET,3 2291 of whom were spirometric GOLD grades 1–4. As would be expected for a follow-up study not newly recruiting patients beyond Visit 1, the number of patients attending the visits gradually decreased, with complete data on COPD characteristics and comorbidities at Visits 2–5 from 1961, 1683, 1162, and 878 patients, respectively. Reasons for drop-out included withdrawal of consent to participate, worsening of disease, and death. As a consequence, the primary study population comprised 878 patients, of which 111/437/281/49 were of GOLD grades 1–4. Median follow-up times were 0.53, 1.56, 3.06 and 4.55 years at Visits 2–5, respectively. Patients’ baseline demographics and COPD characteristics can be found in Table 1, and baseline comorbidities in Table 2. The prevalence of comorbidities increased with increasing follow-up duration, although to a varying degree (Figure 1).
 |
Table 1 Characteristics of the Primary Study Population at Visit 1 |
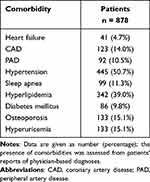 |
Table 2 Comorbidities of the Primary Study Population at Visit 1 |
Relationship of Age, Demographic, COPD Characteristics and Visit Number to Comorbidities
The regression coefficients for the relationship between patient characteristics and comorbidity prevalence are illustrated in Figure 2, with the numerical values in Table 3 (further details on confidence intervals are given in Supplemental Table S1).
 |
Table 3 Results from Generalized Logistic Models with Repeated-Measures Design and Logit Link |
 |
Figure 2 Results from generalized logistic regression models for the prevalence of comorbidities. Dependent variables are given in the headline and estimated regression coefficients in the columns. The effects of age are given per 5 years, of BMI per 5 units, of VAS per 20 units, and of FEV1 per 10% predicted. Scales are different among comorbidities. Significance levels are denoted as follows: +p<0.05, ++p<0.01 and +++p<0.001. Data refer to patients with all four follow-up visits, and numerical values are given in Table 3 (further details are given in Supplemental Table S1). Abbreviations: CAD, coronary artery disease; PAD, peripheral artery disease; HLP, hyperlipidemia; VAS, EQ-5D-3L Visual Analog Scale; FEV1, forced expiratory volume in 1 second. |
Cardiovascular and Respiratory Disease
The prevalence of heart failure increased across the study visits and was also associated with age, although the age effect was much smaller than the contribution at Visit 5. There was also an association with quality of life. For CAD, the prevalence also increased over the follow-up visits, and there was an association with age, with the age effect similar to that of Visit 5. CAD prevalence showed a correlation with male sex and was also was associated with exacerbations and symptoms. For PAD, again an increase in prevalence over the study visits was observed, although this was not statistically significant, in contrast to the much larger and statistically significant effect of age. In addition, PAD prevalence was associated with exacerbations, quality of life, and worsening of lung function. Hypertension prevalence also increased over follow-up and was associated with age and male sex, with the effect of age larger than that attributed to Visit 5. Similarly, the prevalence of sleep apnea increased over the study visits, and was associated with higher BMI and male sex – although not with age.
Metabolic Disease
The prevalence of diabetes mellitus increased over the follow-up period and was associated with age, but the effect of age was only about half of that attributable to Visit 5. Prevalence was also positively associated with male sex and increased BMI. Similarly, hyperlipidemia prevalence increased over the study period and with age, with the contribution of age smaller than that of Visit 5. Prevalence also increased with better lung function. Hyperuricemia became more prevalent over the observation period and was associated with higher BMI and male sex. There was a weak, not statistically significant relationship with age, the magnitude of which was much smaller than the effect attributed to Visit 5. The prevalence of osteoporosis also increased over the study visits, while the association with age was only about one-third of that attributed to Visit 5. Prevalence was higher in females.
Sensitivity Analyses
The longitudinal character of the study inevitably led to a reduction in the number of participants over consecutive visits with a potential differential loss of severely ill patients. In patients who continued in the study until Visit 5, mean FEV1 decreased from 57.5 to 53.6% predicted from Visit 1 to Visit 5, indicating a deterioration in lung function that corresponded to an average FEV1 decline of 45 mL per year. In the larger population of patients having at least one follow-up visit, FEV1% predicted was virtually unchanged over visits, with mean values of 53.3, 53.1, 52.3, 53.0 and 53.5% predicted at Visits 1–5. When the analyses were repeated using the data from these patients, the pattern of correlations was similar to the total population, especially for the relationship between the contributions of a 5-year increase in age versus visit 5. The only exception was PAD, in which the effect of age was similar in magnitude to that at visit 5.
Replacing age at each visit with the initial age in the regression analyses for the primary study population led to virtually the same results as the initial analysis, indicating the importance of the age upon entry. This was confirmed when the duration of COPD diagnosis on entry (mean ± SD: 8.0 ± 6.9 years, interquartile range 3, 11 years) was included as an additional predictor, which did not alter the magnitude or statistical significance of the contributions of follow-up versus age.
Discussion
The present study examined the prevalence of key comorbidities in patients with COPD, together with their changes over time, their associations with risk factors, and their mutual relationships. The analysis employed data from a large cohort of carefully characterized patients, and involved up to 4.5 years of follow-up. The prevalence of comorbidities increased over time and was associated with COPD progression over consecutive visits as well as patients’ age, although these two contributions differed markedly between comorbidities. Our analysis for the first time proposes to separate intrinsic, COPD-related from age-related factors in terms of the occurrence of comorbidities. This might be helpful in the understanding of COPD in the context of multimorbidity as well as the individual assessment of disease progress.
A large number of studies have analyzed the prevalence of comorbidities in COPD, most of cross-sectional design.1,2,13,14 In our study, the prevalence of comorbidities at baseline was similar to the values reported in these studies, especially that of cardiovascular diseases, suggesting that our data describe a typical COPD cohort. One of the previous cross-sectional analyses (conducted by Cazzola et al in an Italian primary care population) compared patients with COPD with non-COPD individuals.7 Prevalence values in patients with COPD were similar to our study, while those in the non-COPD individuals were lower, indicating an effect of COPD. Furthermore, the prevalence of ischemic heart disease and osteoporosis differed between men and women, consistent with our findings.
It is possible that an increase in the prevalence of comorbidities over time may have been partially masked by a selection bias due to increasing COPD severity. However, mean FEV1% predicted only slightly decreased over time in the primary study population (who attended all follow-up visits), whereas it did not decrease at all in the larger group of patients who attended at least one of the visits. This suggests that loss of patients to follow-up occurred in a homogeneous manner over time (at least in terms of their COPD severity). The slight discrepancy between the changes in prevalence in the raw data (Figure 1) and the adjusted estimates over consecutive visits (Figure 2) suggests that by the end of the follow-up there might have been an increased loss of patients due to comorbidities. However, after adjustment for changes in COPD characteristics to which comorbidities were linked, this bias seemed secondary, as indicated by the steady increase in the strengths of the regression coefficients over the visits in the adjusted results. Moreover, the results obtained in the primary population were similar to those obtained in the larger set of patients with at least one follow-up visit.
Age is a well-known risk factor for multimorbidity, while COPD mostly occurs in older individuals; thus, it is challenging to separate the two contributions. We estimated the contribution from COPD by comparing data collected at four follow-up visits to those collected at baseline, taking into account the time course of COPD characteristics. Therefore, potential changes in COPD severity were accounted for. We then compared the coefficients attributed to the last follow-up visit with those attributed to a 5-year increase in age; this seemed acceptable, as the follow-up time was 4.5 years. For the contribution of age, patients’ age at each visit was used. As this inevitably increased over consecutive visits, age and visits were correlated. This was supported by an additional analysis in which only baseline age was used in the regression analysis. Moreover, we introduced the time since COPD diagnosis as an additional covariate, since the effect of baseline age could be influenced by disease duration, thus intermingling the age and COPD effects. In 25% of patients, their COPD had been diagnosed at least 11 years before inclusion in COSYCONET, so that there had been more time for potential COPD effects on comorbidities than the 4.5-year observation period. The magnitude and statistical significance of the COPD and the age effects were, however, unaffected by this, again underlining that the distinction between the two effects had some credibility.
COPD and a range of comorbidities share not only hypothetical intrinsic risk factors but also extrinsic factors such as smoking. We accounted for this by using smoking status as a covariate; prior pack years were not significantly related to the course of prevalences. The role of age in the increased prevalence of chronic diseases is supported by the finding that many, especially cardiac disorders, are associated with increased expression of biological markers of ageing.15 It is likely that systemic and local premature ageing also plays an important role in COPD,16 although to what extent the observed age effects on comorbidities may be specific for coexisting COPD is unclear, as our study did not recruit non-COPD patients. Despite this, we have been able to provide estimates that clearly show different patterns for different comorbidities, and that therefore might help in the understanding of COPD in the context of multimorbidity.
The effects attributed to COPD progression versus age differed between comorbidities. While the presence of heart failure, diabetes, hyperlipidemia, hyperuricemia and sleep apnea was more strongly associated with COPD than age, in CAD estimates were similar, while hypertension and PAD were more strongly linked to age. Expressing the 5-year increase in age in our study as ratios relative to Visit 1 gives values of about 1.20–1.25 for cardiac diseases, ie, an increase in prevalence of 20–25%. The previous cross-sectional study by Cazzola et al mentioned above also permitted the estimation of age effects (in 10-year categories).7 As with our analyses, prevalence of a number of cardiac diseases increased with increasing age in patients with COPD. Although prevalence also increased in the non-COPD individuals, the effect of age was lower than in the patients with COPD. As a consequence, although prevalence was similar in the youngest age category (45–54 years of age), in the oldest age category (≥85 years) all cardiovascular comorbidities were more common in patients with COPD than in individuals without.
The multivariate, longitudinal regression models in our analysis showed associations between the prevalence of comorbidities and the presence of COPD symptoms and exacerbations. We also examined the relationship to the degree of airflow limitation, as associations between the prevalence of comorbidities and GOLD grades have been reported.13 Using FEV1% predicted, there was no significant, statistically independent effect on comorbidity prevalence, at least if symptoms, exacerbations and quality of life were simultaneously taken into account; the exception was hyperlipidemia. These confounders are known to be related to GOLD grades, which might explain the difference in results.
Although the present study combined cross-sectional with longitudinal data, the main limitation is that it does not permit the inference of causal relationships. In addition, there was a loss of participants over consecutive visits, although when the analyses were repeated with the larger population of patients having at least one follow-up visit, very similar results were obtained to the primary analysis. Moreover, we also had to rely on patients’ reports of physician-based diagnoses, and did not validate these by independent assessments. We also did not address the topic of occupational exposures which requires a separate, extensive assessment of prior occupations and potential exposures. The strengths of our study are the large sample size, the follow-up over several visits, and the detailed assessment of clinical data and comorbidities.3
Conclusion
The present findings indicate that in patients with COPD, age played a role in the occurrence of comorbidities, compared to the increase over visits that probably reflected the intrinsic risk from COPD. This risk was particularly important for heart failure and osteoporosis. Conversely, hypertension appeared to be primarily linked to age. The distinction between age- and COPD-associated factors may add a new dimension to the clinical evaluation and risk assessment of individual patients, as COPD-related factors that have an impact on comorbidities might be better amenable to treatment than age-related risk.
COSYCONET Study Group
Andreas, Stefan (Lungenfachklinik, Immenhausen); Bals, Robert Universitätsklinikum des Saarlandes); Behr, Jürgen and Kahnert, Kathrin (Klinikum der Ludwig-Maximilians-Universität München); Bahmer, Thomas (Universitätsklinikum Schleswig Holstein) and Bewig, Burkhard (Städtisches Krankenhaus Kiel); Ewert, Ralf and Stubbe, Beate (Universitätsmedizin Greifswald); Ficker, Joachim H. (Klinikum Nürnberg, Paracelsus Medizinische Privatuniversität Nürnberg); Grohé, Christian (Ev. Lungenklinik Berlin); Held, Matthias (Klinikum Würzburg Mitte gGmbH, Standort Missioklinik); Behr, Jürgen and Henke, Markus (Asklepios Fachkliniken München-Gauting); Herth, Felix (Thoraxklinik Heidelberg gGmbH); Kirsten, Anne-Marie and Watz, Henrik (Pneumologisches Forschungsinstitut an der Lungenclinic Grosshansdorf GmbH); Koczulla, Rembert (Schön Klinik Berchtesgadener Land); Kronsbein, Juliane (Berufsgenossenschaftliches Universitätsklinikum Bergmannsheil, Bochum); Kropf-Sanchen, Cornelia (Universitätsklinikum Ulm); Herzmann, Christian (Forschungszentrum Borstel); Pfeifer, Michael (Klinik Donaustauf); Randerath, Winfried J. (Wissenschaftliches Institut Bethanien e. V., Solingen); Seeger, Werner (Justus-Liebig-Universität Gießen); Studnicka, Michael (Uniklinikum Salzburg); Taube, Christian (Ruhrlandklinik gGmbH Essen); Timmermann, Hartmut (Hamburger Institut für Therapieforschung GmbH); Alter, Peter; Schmeck, Bernd and Vogelmeier, Claus (Universitätsklinikum Gießen und Marburg GmbH, Standort Marburg); Welte, Tobias (Medizinische Hochschule Hannover); Wirtz, Hubert (Universitätsklinikum Leipzig).
The study was based on patients recruited within the COSYCONET framework (ClinicalTrials.gov, Identifier: NCT01245933). For further information see Karch A, Vogelmeier C, Welte T, Bals R, Kauczor HU, Biederer J, Heinrich J, Schulz H, Glaser S, Holle R et al: The German COPD cohort COSYCONET: Aims, methods and descriptive analysis of the study population at baseline. Respir Med 2016, 114:27–37.
Abbreviations
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; COSYCONET, COPD and Systemic Consequences - Comorbidities Network; EQ-5D-3L VAS, visual analog scale of the EuroQoL 5 dimension; FEV1, forced expiratory volume in 1 sec; GLI, Global Lung Function Initiative; GOLD, Global Initiative for Chronic Obstructive Lung Disease; PAD, peripheral artery disease.
Data Sharing Statement
COSYCONET is an ongoing, long-term, multi-center observational study the data of which are not intended to be available without demand. If there is interest in the analysis of specific questions, however, there is a formalized procedure for submitting an application to the study office, which will be evaluated by the steering committee on scientific grounds. There is no limitation for this application except proven expertise in COPD studies.
Ethics Approval
The study protocol was approved by the central ethical committee in Marburg (Ethikkommission FB Medizin Marburg) and the respective local ethical committees: Bad Reichenhall (Ethikkommission Bayerische Landesärztekammer); Berlin (Ethikkommission Ärztekammer Berlin); Bochum (Ethikkommission Medizinische Fakultät der RUB); Borstel (Ethikkommission Universität Lübeck); Coswig (Ethikkommission TU Dresden); Donaustauf (Ethikkommission Universitätsklinikum Regensburg); Essen (Ethikkommission Medizinische Fakultät Duisburg-Essen); Gießen (Ethikkommission Fachbereich Medizin); Greifswald (Ethikkommission Universitätsmedizin Greifswald); Großhansdorf (Ethikkommission Ärztekammer Schleswig-Holstein); Hamburg (Ethikkommission Ärztekammer Hamburg); MHH Hannover/Coppenbrügge (MHH Ethikkommission); Heidelberg Thorax/Uniklinik (Ethikkommission Universität Heidelberg); Homburg (Ethikkommission Saarbrücken); Immenhausen (Ethikkommission Landesärztekammer Hessen); Kiel (Ethikkommission Christian-Albrechts-Universität zu Kiel); Leipzig (Ethikkommission Universität Leipzig); Löwenstein (Ethikkommission Landesärztekammer Baden-Württemberg); Mainz (Ethikkommission Landesärztekammer Rheinland-Pfalz); München LMU/Gauting (Ethikkommission Klinikum Universität München); Nürnberg (Ethikkommission Friedrich-Alexander-Universität Erlangen Nürnberg); Rostock (Ethikkommission Universität Rostock); Berchtesgadener Land (Ethikkommission Land Salzburg); Schmallenberg (Ethikkommission Ärztekammer Westfalen-Lippe); Solingen (Ethikkommission Universität Witten-Herdecke); Ulm (Ethikkommission Universität Ulm); Würzburg (Ethikkommission Universität Würzburg). The study was performed in accordance with the declaration of Helsinki, and all participants gave their written informed consent.
Acknowledgments
We are grateful to all COSYCONET study centers, especially to all study nurses, for their excellent and enduring work in data collection, as well as to all patients who were willing to participate in this study over an extended period of time. We also thank David Young for helpful comments on the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
We are grateful to all COSYCONET study centers, especially to all study nurses, for their excellent and enduring work in data collection, as well as to all patients who were willing to participate in this study. COSYCONET is supported by the German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET) and performed in collaboration with the German Center for Lung Research (DZL). The project is funded by the BMBF with grant number 01 GI 0881 and is supported by unrestricted grants from AstraZeneca GmbH, Bayer Schering Pharma AG, Boehringer Ingelheim Pharma GmbH & Co. KG, Chiesi GmbH, GlaxoSmithKline, Grifols Deutschland GmbH, MSD Sharp & Dohme GmbH, Mundipharma GmbH, Novartis Deutschland GmbH, Pfizer Pharma GmbH, Takeda Pharma Vertrieb GmbH & Co. KG, Teva GmbH for patient investigations and laboratory measurements. For the present study, an additional grant for data management was given by Novartis Pharma GmbH. The funding body had no involvement in the design of the study, or the collection, analysis or interpretation of the data.
Disclosure
Dr Franziska C Trudzinski reports personal fees from Novartis AG, non-financial support from CSL Behring, personal fees from Boehringer Ingelheim, personal fees from GlaxoSmithKline, personal fees from Chiesi, outside the submitted work. Professor Dr Robert Bals reports grants from BMBF, grants from Marburg University, during the conduct of the study; grants, personal fees from Various, outside the submitted work. Prof Dr Stefan Andreas reports grants from Boehringer, personal fees from Altana, Boehringer, AZ, GSK, Chiesi, GSK, Novartis, Menerini, outside the submitted work. Professor Dr Tobias Welte reports grants from German Ministry of Research and Education, during the conduct of the study. Dr Antonia Sassmann-Schweda reports Contractual payments for conduction of study visits; payments were made to Research Center Borstel, Center for Clinical Studies from Philipps University of Marburg, during the conduct of the study. Professor Dr Joachim H Ficker reports personal fees, non-financial support from CSL Behring, personal fees from Novartis, personal fees from AstraZeneca, personal fees from Boehringer, personal fees from Pfizer, outside the submitted work; Prof Dr Claus F Vogelmeier reports personal fees from Aerogen, grants, personal fees from AstraZeneca, grants, personal fees from Boehringer Ingelheim, grants, personal fees from CSL Behring, grants, personal fees from Chiesi, grants, personal fees from GlaxoSmithKline, grants, personal fees from Grifols, personal fees from Menarini, grants, personal fees from Novartis, personal fees from Nuvaira, personal fees from MedUpdate, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi:10.1164/rccm.201209-1665OC
2. Alter P, Mayerhofer BA, Kahnert K, et al. Prevalence of cardiac comorbidities, and their underdetection and contribution to exertional symptoms in COPD: results from the COSYCONET cohort. Int J Chron Obstruct Pulmon Dis. 2019;14:2163–2172. doi:10.2147/COPD.S209343
3. Karch A, Vogelmeier C, Welte T, et al. The German COPD cohort COSYCONET: aims, methods and descriptive analysis of the study population at baseline. Respir Med. 2016;114:27–37. doi:10.1016/j.rmed.2016.03.008
4. Alter P, Lucke T, Watz H, et al. Predictors of mortality and exacerbations in patients with stable COPD. Submitted. 2021;4:54.
5. Alter P, Baker JR, Dauletbaev N, et al. Update in chronic obstructive pulmonary disease 2019. Am J Respir Crit Care Med. 2020;202(3):348–355. doi:10.1164/rccm.202002-0370UP
6. Kahnert K, Alter P, Young D, et al. The revised GOLD 2017 COPD categorization in relation to comorbidities. Respir Med. 2018;134:79–85. doi:10.1016/j.rmed.2017.12.003
7. Cazzola M, Bettoncelli G, Sessa E, Cricelli C, Biscione G. Prevalence of comorbidities in patients with chronic obstructive pulmonary disease. Respiration. 2010;80(2):112–119. doi:10.1159/000281880
8. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. doi:10.1183/13993003.00214-2017
9. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2022. Available from: https://goldcopd.org/2022-gold-reports-2/
10. Trudzinski FC, Kahnert K, Vogelmeier CF, et al. Combined effects of lung function, blood gases and kidney function on the exacerbation risk in stable COPD: results from the COSYCONET cohort. Respir Med. 2019;154:18–26. doi:10.1016/j.rmed.2019.06.007
11. Tietjens JR, Claman D, Kezirian EJ, et al. Obstructive sleep apnea in cardiovascular disease: a review of the literature and proposed multidisciplinary clinical management strategy. J Am Heart Assoc. 2019;8(1):e010440. doi:10.1161/JAHA.118.010440
12. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312
13. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi:10.1186/1465-9921-11-122
14. Mullerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144(4):1163–1178. doi:10.1378/chest.12-2847
15. Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008;28(7):1379–1384. doi:10.1161/ATVBAHA.108.167049
16. Cherif H, Tarry JL, Ozanne SE, Hales CN. Ageing and telomeres: a study into organ- and gender-specific telomere shortening. Nucleic Acids Res. 2003;31(5):1576–1583. doi:10.1093/nar/gkg208
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Impact of Cardiovascular and Metabolic Comorbidities on Long-term Outcomes of Home-based Pulmonary Rehabilitation in COPD
Grosbois JM, Détrée A, Pierache A, Bautin N, Pérez T, Wallaert B, Chenivesse C, Le Rouzic O
International Journal of Chronic Obstructive Pulmonary Disease 2023, 18:155-167
Published Date: 23 February 2023
Prognostic Properties of the GOLD 2023 Classification System
Brat K, Svoboda M, Zatloukal J, Plutinsky M, Volakova E, Popelkova P, Novotna B, Dvorak T, Koblizek V
International Journal of Chronic Obstructive Pulmonary Disease 2023, 18:661-667
Published Date: 20 April 2023
Lack of Clinical Control in COPD Patients Depending on the Target and the Therapeutic Option
Soler-Cataluña JJ, Huerta A, Almagro P, González-Segura D, Cosío BG
International Journal of Chronic Obstructive Pulmonary Disease 2023, 18:1367-1376
Published Date: 6 July 2023
Obesity Prevalence and Association with Spirometry Profiles, ICU Admission, and Comorbidities Among Patients with COPD: Retrospective Study in Two Tertiary Centres in Saudi Arabia
Alqarni AA, Badr OI, Aldhahir AM, Alqahtani JS, Siraj RA, Naser AY, Alghamdi AS, Majrshi M, Alghamdi SM, Alyami MM, Alghamdi SA, Alwafi H
International Journal of Chronic Obstructive Pulmonary Disease 2024, 19:111-120
Published Date: 16 January 2024
Cardiovascular Events According to Inhaler Therapy and Comorbidities in Chronic Obstructive Pulmonary Disease
Kim EK, Lee E, Park JE, Lee JS, Choi HS, Park B, Sheen SS, Park KJ, Rhee CK, Lee SY, Yoo KH, Park JH
International Journal of Chronic Obstructive Pulmonary Disease 2024, 19:243-254
Published Date: 19 January 2024

