Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Cardiovascular Events According to Inhaler Therapy and Comorbidities in Chronic Obstructive Pulmonary Disease
Authors Kim EK , Lee E, Park JE, Lee JS , Choi HS , Park B, Sheen SS , Park KJ, Rhee CK , Lee SY, Yoo KH , Park JH
Received 30 August 2023
Accepted for publication 26 December 2023
Published 19 January 2024 Volume 2024:19 Pages 243—254
DOI https://doi.org/10.2147/COPD.S433583
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Zhang
Eun Kyung Kim,1,* Eunyoung Lee,2,* Ji Eun Park,3,* Jae Seung Lee,4 Hye Sook Choi,5 Bumhee Park,6,7 Seung Soo Sheen,3 Kwang Joo Park,3 Chin Kook Rhee,8 Sang Yeub Lee,9 Kwang Ha Yoo,10 Joo Hun Park3
1Department of Pulmonology, Allergy and Critical Care Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Republic of Korea; 2Department of Neurology, McGovern Medical School at UTHealth, Houston, TX, USA; 3Department of Pulmonary and Critical Care Medicine, Ajou University School of Medicine, Suwon, Republic of Korea; 4Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; 5Department of Internal Medicine, Kyung Hee University Medical Center, Seoul, Republic of Korea; 6Office of Biostatistics, Medical Research Collaborating Center, Ajou Research Institute for Innovative Medicine, Ajou University Medical Center, Suwon, Republic of Korea; 7Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Republic of Korea; 8Department of Internal Medicine, Seoul St. Mary’s Hospital, Catholic University of Korea, Seoul, Republic of Korea; 9Department of Internal Medicine, Korea University Anam Hospital, Seoul, Republic of Korea; 10Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Republic of Korea
*These authors contributed equally to this work
Correspondence: Joo Hun Park, Department of Pulmonary and Critical Care Medicine, Ajou University School of Medicine, Worldcup Road 164, Suwon, Gyeonggi-do, 16499, Republic of Korea, Tel +82-31-219-5116, Fax +82-31-219-5124, Email [email protected]; [email protected]
Background: COPD coexists with many concurrent comorbidities. Cardiovascular complications are deemed to be major causes of death in COPD. Although inhaler therapy is the main therapeutic intervention in COPD, cardiovascular events accompanying inhaler therapy require further investigation. Therefore, this study aimed to investigate new development of cardiovascular events according to each inhaler therapy and comorbidities.
Methods: This study analyzed COPD patients (age ≥ 40 years, N = 199,772) from the Health Insurance Review and Assessment Service (HIRA) database in Korea. The development of cardiovascular events, from the index date to December 31, 2020, was investigated. The cohort was eventually divided into three arms: the LAMA/LABA group (N = 28,322), the ICS/LABA group (N = 11,812), and the triple group (LAMA/ICS/LABA therapy, N = 6174).
Results: Multivariable Cox analyses demonstrated that, compared to ICS/LABA therapy, triple therapy was independently associated with the development of ischemic heart disease (HR: 1.22, 95% CI: 1.04– 1.43), heart failure (HR: 1.45, 95% CI: 1.14– 1.84), arrhythmia (HR: 1.72, 95% CI: 1.41– 2.09), and atrial fibrillation/flutter (HR: 2.31, 95% CI: 1.64– 3.25), whereas the LAMA/LABA therapy did not show a significant association. Furthermore, emergency room visit during covariate assessment window was independently associated with the development of ischemic heart disease, heart failure, arrhythmia, and atrial fibrillation/flutter (p < 0.05).
Conclusion: Our data suggest that cardiovascular risk should be considered in COPD patients receiving triple therapy, despite the confounding bias resulting from disparities in each group.
Keywords: COPD, inhaler therapy, cardiovascular event, comorbidities
Introduction
Chronic obstructive pulmonary disease (COPD) is a global health issue with increasing prevalence, morbidity, and mortality.1,2 COPD coexists with many concurrent co-morbidities, including hypertension, ischemic heart disease, chronic heart failure, osteoporosis, muscle weakness, depression, and lung cancer.3–5 Cardiovascular complications are deemed to be major causes of death in COPD.3–5 The development of cardiovascular comorbidities in COPD is explained by several mechanisms, including lung hyperinflation, hypoxemia, pulmonary hypertension, systemic inflammation, oxidative stress, and exacerbations.6,7
Current guidelines recommend the use of inhaled long-acting bronchodilators in symptomatic COPD patients because beneficial effects of inhaled long-acting bronchodilators including long-acting β2-agonists (LABA) and long-acting muscarinic antagonists (LAMA) in COPD have been documented in several clinical trials.8–10 Inhaled long-acting bronchodilators can reduce the frequency and severity of acute exacerbations, improve exercise tolerance, and ameliorate health status.9,10 Recent studies have suggested that triple therapy with LAMA/LABA/inhaled corticosteroids (ICS) is superior to LABA/ICS due to a lower rate of acute exacerbations and a better quality of life in moderate-to-severe COPD with a history of acute exacerbations.11,12
Despite the pivotal role of inhaler therapy in COPD, concerns have been raised over adverse events, especially cardiovascular complications associated with exposure to LAMA or LABA.13–16 Whether LABA/LAMA therapy is related to cardiovascular events is still under debate. Some multiple observational studies have reported an increased risk of adverse cardiovascular events from LABA/LAMA therapy,13–16 while others have reported contradictory results.8,10,17 Accordingly, cardiovascular outcomes associated with inhaler therapy require further investigation since previous large randomized clinical trials (RCTs) have reported no evidence of increased cardiovascular events when excluding patients with a history of recent cardiovascular disease and life-threatening cardiovascular events.8,10 Incomplete medication records and a dropout rate of more than 50% among eligible patients might have weakened causality or generalizability.14,16,18 So far, few studies have examined the development of cardiovascular comorbidities concerning new therapeutic interventions of inhaler therapy, including LABA/LAMA/ICS combination, in a real-world setting.
Hence, the objective of this study was to investigate the development of new cardiovascular events according to each inhaler treatment and comorbidities in COPD using a Korean Health Insurance Review and Assessment Service (HIRA) database.
Methods
Data Sources
HIRA is an agency that evaluates all medical claims data in South Korea. It has accumulated all medical reimbursement records, which can be used to assess nationwide impact of an illness and subsequent health care.19,20 In this study, we retrospectively analyzed data registered in the HIRA between January 1, 2015, and December 31, 2020.
Study Population
Similar to the criteria used in a previous report including HIRA data, COPD was defined with the following criteria: 1) age ≥ 40 years old, 2) International Classification of Disease, Tenth Revision (ICD-10) codes for COPD or emphysema (J43-J44), and 3) use of one or more COPD medications at least twice per year. COPD medications included 1) LAMA; 2) LABA; 3) LAMA + LABA; 4) ICS; 5) ICS plus LABA; 6) triple therapy (LAMA + LABA + ICS); 7) phosphodiesterase-4 (PDE-4) inhibitors; 8) theophylline; and 9) mucolytics.20,21
Study Design
Among COPD patients identified from a retrospective cohort study based on HIRA database, we analyzed those who were followed up from January 1, 2016, to December 31, 2020. Among them, COPD patients who had received inhaler prescriptions three or more times during the observation period were included (≥40 years of age, N = 199,772). The date of the first prescription of inhaled respiratory medication was defined as an index date. The enrolled patients had no prior history of inhaler therapy before the index date.
The washout window as well as the covariate assessment window was 1 year before the index date. Patients were excluded by the following criteria : previous histories of cardiovascular complications during covariate assessment window (the period focused on comorbidity examination); usage of inhaler during washout window (the period investigated for the medication exposure); switch of inhaler therapy, intermittent triple treatment, LABA only therapy, LAMA only therapy, or ICS only therapy during observation period; age less than 40 years (Figure 1). The final enrolled cohort consisted of 46,308 patients in three arms: LAMA/LABA group (N = 28,322), ICS/LABA group (N = 11,812), and triple-based group (LAMA/ICS/LABA therapy, N = 6,174). The development of new cardiovascular events as primary outcomes from the index date until December 31, 2020, was investigated (Figure 2).
 |
Figure 1 Flow diagram of the study population. |
 |
Figure 2 Study design. |
Definition of Co-Morbidities
Cardiovascular outcomes were identified when COPD patients made an inpatient or emergency department visit with a primary diagnosis of ischemic heart disease (ICD-10 codes I20-I25), myocardial infarction (I21-I22), heart failure (I50), arrhythmia (I44-I49), atrial fibrillation or atrial flutter (I48), hemorrhagic stroke (I60-I62), or ischemic stroke (I63-I66).
Other co-morbidities were defined based on ICD-10 codes: asthma (J45-46), hypertension (I10-15), diabetes mellitus (E10–E14), tuberculosis (A15, A19, and B90), diffuse interstitial lung disease (J84), and lung cancer (C33-C34). The Charlson Comorbidity index was analyzed as described previously.22
Statistical Analyses
Baseline characteristics are presented as mean ± standard deviation. A one-way analysis of variance for continuous variables and a chi-squared test for categorical variables were used. Differences in medication use and prevalence of cardiovascular complications among three groups were tested by chi-squared test and adjusted by Bonferroni correction for multiple comparisons. Proportional hazard assumption was assessed using Schoenfeld residuals for the Cox proportional hazards regression model. Multivariable Cox proportional hazards regression analyses were performed to identify significant risk factors predicting the development of cardiovascular events after adjusting for age, sex, and comorbidities such as asthma, hypertension, diabetes mellitus, tuberculosis, diffuse interstitial lung disease, lung cancer, the Charlson Comorbidity index score, history of hospitalization, and emergency room visit. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for the risk of cardiovascular events. A threshold of p < 0.05 was deemed significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Ethics Statement
The present study was approved by the Institutional Review Board of Ajou University Hospital (AJOUIRB-EXP-2021-582). The data accessed complied with relevant data protection and privacy regulations. The requirement for informed consent from patients analyzed was waived by the ethical review board.
Results
Baseline Characteristics of This Cohort
A total of 46,308 COPD patients were enrolled for the final analysis. In this final cohort, 45.9% had hypertension, 23.3% had diabetes mellitus, and 46.7% had a Charlson Comorbidity index score ≥ 3 (Table 1). This cohort was classified into three groups: LAMA/LABA therapy, ICS/LABA therapy, and triple (ICS/LABA/LAMA) therapy (Figure 1). The triple therapy group had a higher proportion of men, older age, higher Charlson Comorbidity Index score, and more frequent hospitalizations and emergency room visits during the covariate assessment window (all p < 0.001) (Table 1 and Table 2). Medication status is presented in Supplementary Table 1.
 |
Table 1 Baseline Characteristics of Cohort |
 |
Table 2 Hospital Visit During Covariate Assessment Window |
Cumulative and Annual Incidences of Cardiovascular Events
During the observation period, ischemic heart disease developed significantly (p < 0.001) higher in the triple therapy group (4.3%) than in the LAMA/LABA group (3.7%) and the ICS/LABA group (3.1%) (Table 3). The development of arrhythmias and atrial fibrillation/flutter was also higher in the triple therapy group (p < 0.001) (Table 3). Ischemic stroke also occurred more frequently in the triple therapy group (2.8%) than in the LAMA/LABA group (2.2%) (p < 0.05) (Table 3). The annual incidence of ischemic heart disease, heart failure, arrhythmia, and atrial fibrillation/flutter occurred most frequently in the first year of inhaler therapy, and the frequency decreased as therapeutic duration became longer (Figure 3, Supplementary Table 2).
 |
Table 3 Cumulative Incidence of Cardiovascular Events During Observation Period |
Independent Variables for the Development of Cardiovascular Events
Univariable Cox regression analysis showed that, compared to ICS/LABA therapy, LAMA/LABA therapy was significantly associated with the occurrence of ischemic heart disease (HR: 1.32, 95% CI: 1.17–1.48) and atrial fibrillation/flutter (HR: 1.39, 95% CI: 1.03–1.87) (Supplementary Tables 3 and 4).
Another univariable Cox regression analysis showed that triple therapy was significantly associated with the occurrence of ischemic heart disease (HR: 1.50, 95% CI: 1.28–1.75), heart failure (HR: 1.84, 95% CI: 1.45–2.33), arrhythmia (HR: 2.07, 95% CI: 1.70–2.51), and atrial fibrillation/flutter (HR: 2.87, 95% CI: 2.05–4.03), compared to ICS/LABA therapy (p < 0.05) (Supplementary Tables 3 and 4).
Multivariable Cox analyses demonstrated that compared to ICS/LABA therapy, triple therapy was independently associated with the development of ischemic heart disease (HR: 1.22, 95% CI: 1.04–1.43), heart failure (HR: 1.45, 95% CI: 1.14─1.84), arrhythmia (HR: 1.72, 95% CI: 1.41─2.09), and atrial fibrillation/flutter (HR: 2.31, 95% CI: 1.64─3.25), whereas the LAMA/LABA therapy did not show a significant association (Table 4 and Table 5). In addition, emergency room visit was independently associated with the development of ischemic heart disease, heart failure, arrhythmia, and atrial fibrillation/flutter (all p < 0.05) (Table 4L and Table 5). Multivariable analyses also found that tuberculosis (HR: 1.43, 95% CI: 1.10 ─ 1.85), diffuse interstitial lung disease (HR: 2.00, 95% CI: 1.24 ─ 3.24), older age, and hypertension were independently associated with the development of arrhythmia (all p < 0.05) (Table 5).
 |
Table 4 Multivariable Analysis of Risk Factors for the Development of Ischemic Heart Disease and Heart Failure in COPD |
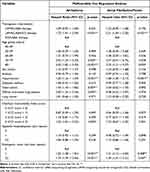 |
Table 5 Multivariable Analysis of Risk Factors for the Development of Arrhythmia in COPD |
Discussion
This observational study based on real-world data performed a comprehensive analysis of cardiovascular events in COPD according to each inhaler therapy and comorbidities in Korean population. Our data showed that triple therapy, compared to ICS/LABA therapy, was independently associated with cardiovascular events in a Korean COPD population. The current study also found an independent association between emergency room visit during covariate assessment window and cardiovascular events, in addition to other well-known risk factors such as diabetes mellitus, hypertension, and old age.
Our study has several interesting findings. First, our multivariable analysis demonstrated that triple therapy, compared to ICS/LABA therapy, independently contributed to the development of cardiovascular events, including ischemic heart disease, heart failure, arrhythmia, and atrial fibrillation/flutter. In contrast, multivariable analysis revealed no differences in cardiovascular outcomes between LAMA/LABA and ICS/LABA. This study aimed to investigate the independent effect of triple therapy on cardiovascular outcomes through multivariate analysis, adjusting for major risk factors of cardiovascular disease, hospitalization, and Charlson Comorbidity index. However, this study could still harbor the risk of confounding bias due to disparities among the three groups and the potential impact of missing confounders, as the triple therapy group had higher rates of co-morbidities, more frequent hospitalizations, and emergency room visits.
Despite these limitations, the current study thoroughly examined major cardiovascular events, including ischemic heart disease, heart failure, and cardiac arrhythmias, as primary outcomes in COPD patients newly initiated on inhalers.
Our findings are supported by previous studies reporting that LAMA-based therapy is associated with cardiovascular complications.15,23,24 A recent meta-analysis of 51 RCTs on combination therapies in COPD has reported that LAMA/LABA or triple therapy can increase cardiovascular risk compared to ICS/LABA.23 A meta-analysis of five RCTs has shown that tiotropium mist inhaler is significantly associated with cardiovascular and all-cause mortality.24 Several studies have also reported that new initiation of long-acting β-agonists and anticholinergics is associated with an increased risk of serious cardiovascular events.15,18,25 However, large RCTs including TORCH, UPLIFT, and FLAME studies suggested otherwise.8,10,26 Recent RCTs comparing ICS/LABA/LAMA with LAMA/LABA or ICS/LABA combinations have provided no evidence of an excess cardiovascular risk by triple therapy.12,27,28 A recent IMPACT study demonstrated that triple therapy could result in a significantly lower annual rate of COPD exacerbations, greater improvements in both lung functions, and a significant reduction in all-cause mortality without any cardiovascular risk, although that study excluded severe cardiac disease or abnormal electrocardiography at baseline.12 Even observational studies have shown contradicting results.15,29 In a large observational study conducted by Suissa et al, long-acting bronchodilator treatment of COPD in the real-world-setting did not increase the risk of most cardiovascular events.29 However, a study by Wang et al showed that new initiation of LABA or LAMA in COPD was associated with an approximate 1.5-fold increase of severe cardiovascular risk, irrespective of prior cardiovascular disease status or history of exacerbations.15 The study of Wang et al might explain why previous relevant RCTs did not find an increased cardiovascular risk with LABA or LAMA therapy.15 The greatest risk of cardiovascular disease emerged around the 30th day following initiation of LABA and LAMA therapy.15 However, a substantial proportion of participants with prior LABA or LAMA usage was included in previous RCTs.8,10,30 Moreover, highly strict inclusion criteria set by RCTs can result in a limited external validity to real-world application, since less than 20% of patients in real-life settings would meet the selection criteria commonly adopted by RCTs on COPD.31 Accordingly, the issue remains unclear due to limitations of both study types, such as limited generalizability, inclusion of patients already using the study medications in RCTs, and a lack of randomization in observational studies.32
Hence, our study excluded COPD patients with a history of cardiovascular comorbidities during covariate assessment window and observed the development of new cardiovascular events after the initiation of inhaler therapy. This study found that triple therapy, comprising LAMA, LABA, and ICS in a real-world setting, was associated with more cardiovascular events, which could be explained by several mechanisms. The suppression of parasympathetic control by long-acting anticholinergics and the stimulation of sympathetic tone by LABA might lead to tachyarrhythmia, myocardial ischemia, stroke, and death.16,33 The potential cardiovascular risk of LAMA is attributable to antagonizing M3 receptor-mediated cardiac functions, whereas ICS therapy may reduce the risk of cardiovascular events because atherosclerosis is a pro-inflammatory condition.34–36 The systemic anti-inflammatory effect of ICS can modulate the risk of cardiovascular events and ameliorate cardiovascular disease by reducing exacerbations.37 However, considering the limitation that the triple therapy group had higher rates of co-morbidities, which may make them more susceptible to cardiovascular events, further verification appears to be necessary.
Second, our data found that COPD patients with tuberculosis or diffuse interstitial lung disease during covariate assessment window had a higher incidence of arrhythmia during the observation period. Although firm evidence linking arrhythmia and COPD with tuberculosis or diffuse interstitial lung disease has not yet been reported, this finding is supported by a study conducted by Yakar et al.38 According to their study, patients with a history of tuberculosis had an earlier onset of COPD, lower FEV1 values, more severe hypercapnia, more frequent hospitalizations, and a lower survival rate, compared to those without a history of tuberculosis.38
Additionally, a recent study has demonstrated that COPD patients with diffuse interstitial lung disease have a high mortality rate and a rapid decline in lung function.39 Further investigation with a larger cohort is necessary to confirm these results.
Third, this study discovered that emergency room visit during covariate assessment window was independently associated with the development of ischemic heart disease, heart failure, arrhythmia, and atrial fibrillation/flutter. The increased cardiovascular risk associated with an emergency room visit can be explained by the correlation between cardiovascular risk and COPD exacerbation, because severe COPD exacerbations often necessitate hospitalization or emergency room visits.40,41
Several limitations of this study should be acknowledged. First, due to an observational design of this study, the causal link could not be firmly suggested as in a randomized control study. Second, because this study was conducted with a claim database, some potential confounders including pulmonary function test, hypercholesterolemia, laboratory tests, and air pollution that might contribute to the occurrence of cardiovascular disease were not analyzed. Third, the definition of COPD and cardiovascular disease by ICD code can harbor a potential risk of overdiagnosis or misdiagnosis because these diseases should be confirmed by tests such as spirometry, electrocardiogram, echocardiography, or coronary artery angiography. Fourth, information about smoking status, respiratory symptoms, and family history were not available in this cohort. Fifth, this study could have a confounding bias, as co-morbidities and hospitalizations were not evenly matched among the three arms. Sixth, the precise therapeutic duration of each inhaler could not be obtained due to the limitations of the HIRA data source. Seventh, social factors such as occupation and income level were not investigated as causal factors for the development of cardiovascular outcomes. Seventh, medication adherence was not measured.
In conclusion, our data demonstrated that the development of cardiovascular events in COPD was associated with triple therapy compared to ICS/LBA therapy, suggesting cardiovascular risk should be considered in COPD patients receiving triple therapy. However, further verification is necessary, considering the confounding bias resulting from disparities in each group.
Abbreviations
COPD, Chronic obstructive pulmonary disease; CI, confidence intervals; PDE-4 inhibitor, phosphodiesterase-4 inhibitor; HIRA, Health Insurance Review and Assessment Service; HR, Hazard ratio; ICS, inhaled corticosteroid; LABA, long-acting beta-2 agonist; LAMA, long-acting muscarinic antagonist; RCT, randomized clinical trial.
Data Sharing Statement
HIRA is open and public data to which any researcher can get access through the website (https://www.hira.or.kr).
Ethics Approval and Consent to Participate
The present study was approved by the Institutional Review Board of Ajou University Hospital (AJOUIRB-EXP-2021-582). The requirement for informed consent from patients analyzed was waived by the ethical review board.
Acknowledgment
This work was supported by the research program funded from Korea National Institute of Health (Funding code 2016ER670100, 2016ER670101, 2016ER670102, 2018ER67100, 2018ER67101, 2018ER67102, and 2021ER120500), National Research Foundation from Korean Government (NRF-2021R1I1A3056129), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR16C0001).
The abstract of this paper was presented at the American thoracic society 2023 international conference as a poster presentation with interim findings.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the research program funded from Korea National Institute of Health (Funding code 2016ER670100, 2016ER670101, 2016ER670102, 2018ER67100, 2018ER67101, 2018ER67102, and 2021ER120500), National Research Foundation from Korean Government (NRF-2021R1I1A3056129), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR16C0001).
Disclosure
All authors have nothing to declare regarding relevant financial activities and relationships/conditions/circumstances that present a potential conflict of interest.
References
1. Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1248–1256. doi:10.1056/NEJMra1900475
2. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. doi:10.1016/S0140-6736(07)61380-4
3. Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi:10.1164/rccm.201201-0034OC
4. Müllerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144(4):1163–1178. doi:10.1378/chest.12-2847
5. Sidney S, Sorel M, Quesenberry CP Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: kaiser Permanente Medical Care Program. Chest. 2005;128(4):2068–2075. doi:10.1378/chest.128.4.2068
6. Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128(4):2640–2646. doi:10.1378/chest.128.4.2640
7. Singh S, Loke YK, Enright P, Furberg CD. Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Thorax. 2013;68(1):114–116. doi:10.1136/thoraxjnl-2011-201275
8. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
9. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5). doi:10.1183/13993003.00164-2019
10. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. New Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800
11. Koarai A, Yamada M, Ichikawa T, Fujino N, Kawayama T, Sugiura H. Triple versus LAMA/LABA combination therapy for patients with COPD: a systematic review and meta-analysis. Respir Res. 2021;22(1):183. doi:10.1186/s12931-021-01777-x
12. Lipson DA, Barnhart F, Brealey N, et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. New Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
13. Au DH, Lemaitre RN, Randall Curtis J, Smith NL, Psaty BM. The risk of myocardial infarction associated with inhaled β-adrenoceptor agonists. Am J Respir Crit Care Med. 2000;161(3):827–830. doi:10.1164/ajrccm.161.3.9904006
14. Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173(13):1175–1185. doi:10.1001/jamainternmed.2013.1016
15. Wang M-T, Liou J-T, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178(2):229–238. doi:10.1001/jamainternmed.2017.7720
16. Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. Bronchodilator use and the risk of arrhythmia in COPD: part 1: Saskatchewan cohort study. Chest. 2012;142(2):298–304. doi:10.1378/chest.10-2499
17. Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817–1826. doi:10.1016/S0140-6736(16)30069-1
18. Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest. 2012;142(2):305–311. doi:10.1378/chest.11-1597
19. Kim J-A, Yoon S, Kim L-Y, Kim D-S. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32(5):718–728. doi:10.3346/jkms.2017.32.5.718
20. Lee J, Lee JH, Kim J-A, Rhee CK. Trend of cost and utilization of COPD medication in Korea. Int J Chronic Obstr. 2017;12:27. doi:10.2147/COPD.S121687
21. Lee CH, Kim K, Hyun MK, Jang EJ, Lee NR, Yim JJ. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013;68(12):1105–1113. doi:10.1136/thoraxjnl-2012-203175
22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
23. Yang M, Li Y, Jiang Y, Guo S, He J-Q, Sin DD. Combination therapy with long-acting bronchodilators and the risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Eur Respir J. 2022.
24. Singh S, Loke YK, Enright PL, Furberg CD. Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2011;342.
25. Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(12):1439–1450. doi:10.1001/jama.300.12.1439
26. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. New Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
27. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi:10.1164/rccm.201703-0449OC
28. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084. doi:10.1016/S0140-6736(18)30206-X
29. Suissa S, Dell’Aniello S, Ernst P. Concurrent use of long-acting bronchodilators in COPD and the risk of adverse cardiovascular events. Eur Respir J. 2017;49(5):1602245. doi:10.1183/13993003.02245-2016
30. Wedzicha JA, Zhong N, Ichinose M, et al. Indacaterol/glycopyrronium versus salmeterol/fluticasone in Asian patients with COPD at a high risk of exacerbations: results from the FLAME study. Int J Chronic Obstr. 2017;12:339. doi:10.2147/COPD.S125058
31. Scichilone N, Basile M, Battaglia S, Bellia V. What proportion of chronic obstructive pulmonary disease outpatients is eligible for inclusion in randomized clinical trials? Respiration. 2014;87(1):11–17. doi:10.1159/000355082
32. Suissa S, Ernst P, Vandemheen KL, Aaron S. Methodological issues in therapeutic trials of COPD. Eur Respir J. 2008;31(5):927–933. doi:10.1183/09031936.00098307
33. Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of β-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309–2321. doi:10.1378/chest.125.6.2309
34. Wang H, Lu Y, Wang Z. Function of cardiac M3 receptors. Auton. Autacoid Pharmacol. 2007;27(1):1–11. doi:10.1111/j.1474-8673.2006.00381.x
35. Kistemaker LE, Bos IS, Hylkema MN, et al. Muscarinic receptor subtype-specific effects on cigarette smoke-induced inflammation in mice. Europ resp J. 2013;42(6):1677–1688. doi:10.1183/09031936.00112412
36. Libby P. Inflammation in atherosclerosis. Arteriosclerosis Thrombosis Vasc Biol. 2012;32(9):2045–2051. doi:10.1161/ATVBAHA.108.179705
37. Sin DD, Man SP, Marciniuk DD, et al. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(11):1207–1214. doi:10.1164/rccm.200709-1356OC
38. Yakar HI, Gunen H, Pehlivan E, Aydogan S. The role of tuberculosis in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:323–329. doi:10.2147/COPD.S116086
39. Guiot J, Henket M, Frix A-N, et al. Combined obstructive airflow limitation associated with interstitial lung diseases (O-ILD): the bad phenotype? Respir Res. 2022;23(1):1–9. doi:10.1186/s12931-022-02006-9
40. Jang S, Kim Y, Cho WK. A Systematic Review and Meta-Analysis of Telemonitoring Interventions on Severe COPD Exacerbations. Int J Environ Res Public Health. 2021;18(13). doi:10.3390/ijerph18136757
41. Goto T, Shimada YJ, Faridi MK, Camargo CA, Hasegawa K. Incidence of Acute Cardiovascular Event After Acute Exacerbation of COPD. J Gen Intern Med. 2018;33(9):1461–1468. doi:10.1007/s11606-018-4518-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

