Back to Journals » Journal of Pain Research » Volume 15
Development and Validation of a Prediction Model for Chronic Post-Surgical Pain After Thoracic Surgery in Elderly Patients: A Retrospective Cohort Study
Authors Wu XD, Zeng FF, Yu XX, Yang PP, Wu JP, Xv P, Wang HT , Pei YM
Received 31 March 2022
Accepted for publication 5 August 2022
Published 29 September 2022 Volume 2022:15 Pages 3079—3091
DOI https://doi.org/10.2147/JPR.S368295
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jonathan Greenberg
Xiao-Dan Wu,1 Fan-Fang Zeng,1 Xiao-Xuan Yu,1 Pan-Pan Yang,1 Jun-Peng Wu,1 Ping Xv,2 Hai-Tang Wang,1 You-Ming Pei1
1Department of Anesthesiology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, 510515, People’s Republic of China; 2Department of Anesthesiology, Zhujiang Hospital, Southern Medical University, Guangzhou, Guangdong, 510280, People’s Republic of China
Correspondence: Hai-Tang Wang; You-Ming Pei, Department of Anesthesiology, Nanfang Hospital, Southern medical university, 1838 Guangzhou North Road, Guangzhou, Guangdong, 510515, People’s Republic of China, Tel +86-18718911653 ; +86-15889942610, Fax +86 20-62787271 ; +86 20-62787271, Email [email protected]; [email protected]
Purpose: Chronic post-surgical pain (CPSP) is one of the adverse outcomes after surgery, especially in thoracotomy. However, the prevalence of CPSP in elderly adults (≥ 65 years), is still limited. Therefore, the present study was undertaken to establish and validate the prediction model of CPSP in those patients after thoracic surgery, including thoracotomy and video-assisted thoracoscopic surgery.
Patients and Methods: This retrospective, observational single-center cohort study was conducted in Nanfang Hospital, Southern Medical University, which randomly and consecutively collected 577 elderly patients who underwent thoracic surgery between January 1, 2017, and December 31, 2020. According to the Akaike information criterion, the prediction model was built based on all the data and was validated by calibration with 500 bootstrap samples.
Results: The mean age of participants was 69.09± 3.80 years old, and 63.1% were male. The prevalence of CPSP was 26.9%. Age more than 75 years, BMI, blood loss, longer length of hospital stays, and higher pre-operative neutrophil count were associated with CPSP. Except for these factors, we incorporated history of drinking to build up the prediction model. The areas under the curve (AUCs) of the prediction models were 0.66 (95% CI, 0.61– 0.71) and 0.64 (95% CI, 0.59– 0.69) in the observational and validation cohorts, respectively. And the calibration curve of the predictive model showed a good fit between the predicted risk of CPSP and observed outcomes in elderly patients.
Conclusion: The present developed model may help clinicians to find high-risk elderly patients with CPSP after thoracic surgery and take corresponding measures in advance to reduce the incidence of CPSP and improve their life quality.
Keywords: chronic post-surgical pain, the elderly, thoracic surgery, prediction model, nomogram
Introduction
Chronic pain is one of the most common and consequential diseases in older adults, aged over 65 years.1 In 2016, a global survey about the prevalence of chronic pain in 50 million adults over the age of 18 revealed a higher prevalence of chronic pain in adults over the age of 65, compared to those less than 65. Besides, the elderly persons suffered from chronic pain that limited life activities or work as high as 26.5%.2 For older persons, fear of pain prevents individuals from exercising, taking care of themselves in daily life, or even impairing their memory.3–6 Therefore, chronic pain is a significant health hazard and needs to arise more attention.
As the standard of living and medical conditions improve, elderly patients account for an increasing proportion of the overall surgical patient population. Chronic post-surgical pain (CPSP), as one of the adverse outcomes after surgery, has a prevalence of about 30–40% and seriously affects the normal physical function, emotion and quality of life.7,8 Current studies on CPSP are for adults over 18 years, but fewer epidemiological surveys have been conducted on aged adults who were older than 65 years.9,10 And other studies about chronic pain in the elderly mainly focused on patients suffering from osteoarthritis and rheumatoid arthritis, yet fewer researchers investigated chronic pain in elderly patients after surgery.4,11,12 Therefore, it is crucial to understand and address chronic postoperative pain in elderly patients.
CPSP is common in thoracotomy.8 Thus, the aim of our study was to conduct a retrospective study to establish a prediction model of CPSP as an easy-to-use tool. Then, clinicians could give appropriate intervention and attention at the early stage to promote suitable postoperative care to improve life quality and reduce mortality in the elderly after surgery.
Materials and Methods
Patient Selection
This retrospective, observational single-center study was conducted in Nanfang Hospital, Southern Medical University. Patients who underwent thoracic surgery between January 1, 2017 and December 31, 2020 were eligible. And the study was approved by the Institutional Ethics Committee of Nanfang Hospital, Southern Medical University and registered at the Chinese Clinical Trial Register (ChiCTR2000035312). Because patients had been discharged at the time of study, a verbal questionnaire survey was conducted by telephone in March 2021 to collect the data of CPSP. At the beginning of the interview, we would seek for patients’ consent of participating in this study and allowing us to use the collected data for scientific purposes. Then the other data were gathered from medical records. The feasibility and reliability of this verbal informed consent were approved by the Institutional Ethics Committee of Nanfang Hospital, Southern Medical University. All patient identifiers were deleted before data analysis and the study was performed conforming to the Declaration of Helsinki.
Patients were eligible if they met the following criteria: 1. aged 65 years or over, 2. ASA status I–II, 3. intercostal nerve blocks with ropivacaine (7.5 mg diluted in 20 mL of 0.9% saline) were applied at the end of the surgery, 4. the post-operative intravenous analgesia pump was used continuously for 24–48 hours. The exclusion criteria included: 1. emergency surgery, 2. ASA status III-Ⅴ, 3. existing pain associated with CPSP before surgery, 4. losing contact, 5. tumor recurrence or metastasis at follow-up visit, 6. death, 7. psychiatric abnormalities and unable to communicate.
Data Collection
Demographic Data Collection
Variables that might have an association with CPSP were selected based on literature review and our previous study.9,13–18 Information collected via medical records included sex, age, BMI, history of smoking and drinking, presence of hypertension and diabetes mellitus, history of operation, approach of surgery, duration of surgery, type of anesthesia, blood loss, duration of chest tube drainage, additional needs for analgesic after surgery, length of hospital stay, pre-operative white blood cell count, preoperative neutrophil count, preoperative monocyte count. The operational methods included video-assisted thoracic surgery (VATS) and thoracotomy. Duration of surgery was the time spanning from the start of operation to the completion of incision suturing. Type of anesthesia included intravenous and inhalational combined anesthesia and total intravenous anesthesia. Additional needs for analgesics after surgery represented that some patients had required additional analgesics even if they received intercostal nerve block and intravenous patient-controlled analgesia after the operation.
CPSP Data Collection
Patients were assessed through telephone interviews. The 11-point numeric rating scale (NRS-11) was applied to assess self-reported pain levels for at least 3 months after thoracic surgery. If the NRS was greater than or equal to 1 point, patients were further asked to answer some questions according to the questionnaire designed by our research group previously.19 This questionnaire covered briefs about pain intensity, sensory characteristics, affective qualities, and activities limited by pain. At the end of the follow-up, patients would provide the names and therapeutic effects of any pharmacologic medications or other treatments they used to alleviate pain. If 0 point was chosen, the following contents would not be assessed.
Outcomes
Developing and validating a prediction model of chronic pain after thoracic surgery for at least 3 months was the most important result of this study. According to the International Association for the Study of Pain (IASP),20 CPSP is a pain that develops or increases in intensity after a surgical procedure and persists beyond the healing process for at least 3 months after surgery. This type of pain has to be localized to the surgical field and projected to the innervation territory of a nerve situated in this area. Other causes, such as pre-existing pain conditions, infections, malignancy, etc. have to be excluded in all cases of CPSP.
Sample Size
There have been no generally accepted approaches to estimate the sample size for studies of risk prediction models until recently.21 Thus, we tried to enroll as many patients as possible to ensure the stability of the prediction model. To maximize statistical power and minimize bias that might occur if patients with missing data were excluded from analyses, the missing data were imputed by the mean of continuous variables.
Statistical Analysis
Baseline demographic and clinical characteristics of all participants were presented as means ± standard deviations or medians (quartiles) for continuous variables, and as frequencies (percentage) for categorical variables. Continuous variables were compared by unpaired t-test for normally distributed continuous variables and Kruskal–Wallis rank test for non-normally distributed continuous variables. Categorical variables were compared by chi-square test or Fisher exact test.
The relationship between each predicted variable and chronic pain after thoracic surgery was assessed by univariate analysis. In order to improve the accuracy of the model, all the variables were used for CPSP model construction. Following collinearity screening, logistic regression model was applied to assess the significance of each variable to search for the independent risk factors for CPSP. Then for the sake of identifying a simple and reliable risk prediction model, we established three models for comparison. First, we chose the iterative fashion of the multivariable fractional polynomials (MFP) algorithm to determine the significant variables and functional form by backward elimination to establish a stable model (MFP model) in the real world.22 Second, we applied all risk factors to create a full model. Third, the Akaike information criterion (AIC) was used to perform a backward step-down selection process to create a parsimonious model (stepwise model).23 To assess and compare the differences among these three models, we plotted the receiver operating characteristic (ROC) curve and calculated the area under the ROC curve (AUC) with 95% confidence intervals (CI). Considering that there were fewer variables in the stepwise model and the prediction performance might be relatively close, the stepwise model was selected for further analysis. Receiver operating characteristic curve analysis was used to calculate the optimal cutoff values that were determined by maximizing the Youden index. The accuracy of the optimal cutoff value was estimated by the sensitivity, specificity, predictive values, and likelihood ratios. Given nomogram was an intuitive graphical prediction model which might provide personalized risk predictions to individuals, we further constructed the nomogram of the stepwise model.24 Finally, we would obtain a chronic pain prediction formula after thoracic surgery for practical application. After that, the predictive performance of the nomogram was measured by calibration with 500 bootstrap samples to decrease the overfit bias.
The analyses were performed by package R (http://www.R-project.org, The R Foundation) and Empower-Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). Two-tailed P values of less than 0.05 were considered to indicate statistical significance.
Results
From January 1, 2017 to December 31, 2020, 929 patients, age≥65 years, underwent thoracic surgery in our hospital. After telephone interviews, only 577 patients were finally enrolled in this study for analysis, and 352 patients were excluded from the exclusion criteria. The basic characteristics of the participants were listed in Table 1, and the flow chart of the study participants was shown in Figure 1.
 |
Table 1 Characteristic of Participants (n=577) |
 |
Figure 1 Flow chart of the study participants. |
As a result, 155 elderly patients suffered from CPSP in this study. The incidence rate of CPSP was 26.9% in these patients, which was lower than that in adults (≥18 years old).25 In these patients, there were 58.7% (91) of patients with mild pain, 32.9% (51) with moderate pain, and 7.7% (12) with severe pain according to NRS-11. The most frequent complaints were numbness (39 /155, 25.2%) and pruritus (35/155, 22.6%), stabbing pain (23/155, 14.8%), squeezing pain (20/155, 12.9%), light pressure pain (19/155, 12.3%), oppression (12/155, 7.7%), piercing pain (9/155, 5.8%), light touch of pain (7/155, 4.5%), electric-shock pain (7/155, 4.5%), burning pain (5/155, 3.2%), brushing pain (4/155, 2.6%), and cold/heat pain (2/155, 1.3%) at rest. Among these patients with CPSP, 16.1% (25) of patients reported that CPSP had severely affected normal life, characterized by limited activities and movement. In terms of impact on mood, 22.6% (35) of patients with mild impact on mood would feel unhappy due to CPSP and 18.1%(28) of patients with heavy impact on mood, may feel distressed, anxious or even depressed for CPSP. Because of the occurrence of CPSP, patients’ sleep time was significantly reduced (p < 0.001). However, only 31 patients (20.0%) visited the clinic for pain or taking drugs by themselves, and only 17 received pharmacological treatment, including analgesics, Chinese herbal therapy and functional exercise. After treatment, 15 patients reported relief slightly and only 2 patients were very satisfied with the results.
In the univariate analysis, age, BMI, blood loss, length of hospital stay and pre-operative neutrophil count were significantly associated with CPSP (p<0.05), but no significant correlation was found in other variables (Table 2). Then, based on all variables, we established three prediction models based on all variables, including the MFP model, full model and stepwise model. The AUCs of the MFP model, full model and stepwise model were 0.651, 0.663 and 0.659 respectively (Table 3). The AUCs of the three models were relatively close (Figure 2). Given that the stepwise model incorporated fewer risk factors and was simpler than MFP and full models, we chose the stepwise model as the optimal risk prediction model for CPSP. Table 4 showed that 6 variables were selected by stepwise model, including age, BMI, history of drinking, blood loss, length of hospital stay and pre-operative neutrophil count. And participants with age≥75 years (OR:2.744;95% CI:1.255–6.000) and longer lengths of hospital stay (OR:1.044;95% CI:1.011–1.077) were more likely to develop CPSP. In contrast, participants with BMI≥30 kg/m2 (OR:0.162;95% CI:0.039–0.681) and more blood loss (OR:0.885;95% CI:0.814–0.963) were less likely to develop CPSP. In the model, the ORs of age ≥75years, BMI≥30 kg/m2 and blood loss were statistically significant (p < 0.05) (Table 4). We further draw a corresponding nomogram to provide a quantitative and simple tool for predicting the risk of CPSP by using age, BMI, history of drinking, blood loss, length of hospital stays and pre-operative neutrophil count (Figure 3). Each variable in the nomogram was assigned a specific point, and the points from each variable value are summed to obtain the total points, which were used to obtain the probability of predicting CPSP. And the algorithm of CPSP risk in stepwise model was logit (CPSP)= 0.310 +0.262*(70≤age<75years) +1.009*(age≥75years) +0.417*(25≤BMI<30 kg/m2) −1.820*(BMI≥30 kg/m2) −0.531*(history of drinking) −0.122* blood loss (mL kg-1) +0.043* length of hospital stay (days)+0.118* preoperative neutrophil count (× 109/L). The calibration curve of the predictive model showed a good fit between the predicted risk of CPSP and observed outcomes in elderly patients (Figure 4).
 |
Table 2 Univariate Logistic Regression Analysis and Odds Ratio of Variables |
 |
Table 3 Prediction Performance of Four Models: MFP Model, Full Model, Stepwise Model and Bootstrap Stepwise Model |
 |
Table 4 Variables Selected Using Stepwise Logistic Regression |
 |
Figure 2 The ROC curves of the MFP model, full model, stepwise model and BS stepwise model. |
 |
Figure 3 The predictive nomogram of the stepwise model of CPSP. |
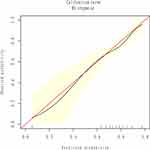 |
Figure 4 The calibration plots between observed probability and model-predicted proportion of CPSP. |
Discussion
In this retrospective study, we established a CPSP prediction model for elderly patients and drew a personalized prediction nomogram to help clinicians to identify elderly individuals with a high risk of developing CPSP after thoracic surgery. The stepwise model included six parameters: age, BMI, history of drinking, blood loss, length of hospital stays and pre-operative neutrophil count, of which, age, BMI, blood loss and length of hospital stay were statistically associated with CPSP. Besides, the AUCs of the stepwise model and the bootstrap stepwise model were 0.659 and 0.643 respectively, indicating the good accuracy of this predicted model. Meanwhile, the optimal calibration curves demonstrated the agreement between prediction and actual observation.
The incidence of CPSP was 26.9% in this study, which was lower than the incidence in adults less than 65 years old and was similar to what was previously reported.14,15 Changes in the pain perception in elderly patients, including higher pain thresholds, greater pain acceptance and self-efficacy with age might be involved. In addition, the weaker correlation between pain catastrophizing and depression compared to younger people might be related to the underestimation of pain in elderly patients.26,27
CPSP is a common problem but often difficult to treat. In order to find individuals who were more prone to develop CPSP, studies9,16,17,28,29 had identified some perioperative risk factors and developed some prediction models for CPSP in adults (≥18 years); however, the data remained unclear for elderly patients over 65 years old. Based on previous reports, we selected eighteen variables as candidates and the final model included 6 variables. In addition to age and BMI as common predictors, the prediction model also incorporated history of drinking, blood loss, length of hospital stay and preoperative neutrophil count.
Studies have shown that the incidence of CPSP decreased with age, yet the exact extent of the effect is unclear. In former investigations,19,30 the incidence of CPSP was higher in patients aged <60 years than in those aged ≥60 years, representing that younger age was a risk predictor of persistent pain after surgery. However, in this study, we found that the incidence rate of CPSP increased significantly with age (OR:2.744;95% CI:1.255–6.000, p<0.05), especially in those patients over 75 years, where the incidence was 3.015 times higher than those younger than 75 years (OR 4.232, 95% CI 1.392 to 6.534, p < 0.05). Interestingly, Shi et al reported similar results by investing 18,439 patients troubled by pain, whose ages were over 50 years and revealed that patients older than 80 years were more likely to experience pain than patients with ages younger than 50.31 These findings reminded us that elderly patients might be more tended to develop CPSP after thoracic surgery.
Besides, we divided the patients into three groups according to WHO’s BMI grading criteria: standard weight group (BMI <25 kg/m2), super group (25 ≤ BMI <30 kg/m2), and obese group (BMI ≥30 kg/m2). Results showed that the risk of CPSP in the obese group was 0.84 times lower than that in the standard weight group, which showed that obesity would not increase the incidence of CPSP in patients ≥ 65 years. The relationship between high BMI and CPSP in breast cancer surgery in 18–75 years old women has been previously reported;32 on the contrary, we found that the incidence of CPSP was lower in elderly obese people. The relationship between BMI and CPSP was not linear, especially for patients with BMI≥30 kg/m2, indicating that age might have a greater effect on pain perception than obesity itself.
Our study also revealed that intraoperative bleeding was negatively associated with the occurrence of CPSP. A former study analyzed the relationship between bleeding volume and CPSP undergoing hip arthroplasty in elderly patients aged >65 years, but the result was no statistical correlation (OR 1.001,95% CI1.000 to 1.002, p=0.094).16 Thus the relationship between pain and intraoperative bleeding needs to be further studied and explored.
Although VATS is considered more nerve injury than thoracotomy, increasing data showed that the incidence of CPSP between VATS and thoracotomy was similar. Consistent with the previous research,33,34 CPSP for VATS patients was not different compared to those of thoracotomy patients in the elderly, which suggests that its mechanism is not just owing to nerve injury. Severe acute postoperative pain is considered a predictor of chronic postoperative pain.35–37 In this study, approximately 50% of thoracic surgery patients used additional analgesics for acute pain relief, allowing for timely resolution of pain and reducing the risk of developing CPSP. In prognostic prediction models for chronic postsurgical pain, the length of hospital stay has often been overlooked.17 Length of hospital stay was closely related to the cost of hospitalization for patients. It contained information on patients’ comorbidities and postoperative complications that could affect the outcome. And the other predictors did not fully include this information, thus the length of hospital stay was used in the final prediction model. Serum inflammatory markers are easy to evaluate from the complete blood count. The status of inflammatory markers could represent the host’s chronic inflammatory status and host immune response to the tumor. As mentioned in the previous reports, preoperative inflammatory biomarkers had been independent predictors of prognostic markers who undergo thoracic surgery, potentially linked to smoking.38–40 In addition, there were found that preoperative inflammatory parameters were associated with postoperative pain in many operations,41–45 but the relationship between them was still controversial. In this study, we found the relevance between neutrophils and CPSP in elderly patients.
Developing a predictive model may help to identify variables that are effective in predicting patient outcomes and could be used by clinicians to guide patients’ treatment. However, the prediction model established for predicting CPSP in elderly patients has been seldom reported. Constructing suitable prediction models for the elderly, can complement the reliability and clinical utility of existing prediction models and reduce their bias. Even so, this study still had several limitations. First, we could not assess the patient’s psychological state before surgery, because our study was retrospective. Second, due to the strict inclusion criteria, the sample size was relatively small. Therefore, we plan to validate our results with a larger prospective, multicenter cohort study to obtain a more stable and reliable model in the future.
Conclusion
The present developed model for the prediction of CPSP in the elderly was reliable and, may help clinicians to find elderly patients who will be more likely to develop CPSP and take some actions in advance to reduce the incidence of CPSP and improve patients’ life.
Data Sharing Statement
There are no additional data from the study available for sharing.
Funding
This study was supported by the Guangdong Provincial Science and technology project (2015A020210050).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Domenichiello AF, Ramsden CE. The silent epidemic of chronic pain in older adults. Prog Neuropsychopharmacol Biol Psychiatry. 2019;93:284–290. doi:10.1016/j.pnpbp.2019.04.006
2. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic Pain among adults — United States. MMWR Morb Mortal Wkly Rep. 2016;67(36):1001–1006. doi:10.15585/mmwr.mm6736a2
3. Li X, Zhu W, Li J, Huang C, Yang F. Prevalence and characteristics of chronic pain in the Chinese community-dwelling elderly: a cross-sectional study. BMC Geriatr. 2021;21(1). doi:10.1186/s12877-021-02432-2
4. Stompór M, Grodzicki T, Stompór T, Wordliczek J, Dubiel M, Kurowska I. Prevalence of chronic pain, particularly with neuropathic component, and its effect on overall functioning of elderly patients. Med Sci Monitor. 2019;25:2695–2701. doi:10.12659/MSM.911260
5. Bernfort L, Gerdle B, Rahmqvist M, Husberg M, Levin L. Severity of chronic pain in an elderly population in Sweden—impact on costs and quality of life. Pain. 2015;156(3):521–527. doi:10.1097/01.j.pain.0000460336.31600.01
6. van der Leeuw G, Ayers E, Leveille SG, Blankenstein AH, van der Horst HE, Verghese J. The effect of pain on major cognitive impairment in older adults. J Pain. 2018;19(12):1435–1444. doi:10.1016/j.jpain.2018.06.009
7. Niknejad B, Bolier R, Henderson CR, et al. Association between psychological interventions and chronic pain outcomes in older adults. JAMA Intern Med. 2018;178(6):830. doi:10.1001/jamainternmed.2018.0756
8. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi:10.1016/S0140-6736(06)68700-X
9. Hegarty D, Shorten G. Multivariate prognostic modeling of persistent pain following lumbar discectomy. Pain Physician. 2012;15(5):421–434. doi:10.36076/ppj.2012/15/421
10. Wylde V, Dennis J, Beswick AD, et al. Systematic review of management of chronic pain after surgery. Br J Surg. 2017;104(10):1293–1306. doi:10.1002/bjs.10601
11. Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: findings from the 2011 national health and aging trends study. Pain. 2013;154(12):2649–2657. doi:10.1016/j.pain.2013.07.029
12. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287. doi:10.1016/j.ejpain.2005.06.009
13. Peng Z, Li H, Zhang C, Qian X, Feng Z, Zhu S. A retrospective study of chronic post-surgical pain following thoracic surgery: prevalence, risk factors, incidence of neuropathic component, and impact on qualify of life. PLoS One. 2014;9(2):e90014. doi:10.1371/journal.pone.0090014
14. Luo F, Cai XJ, Li ZY. Effects of untreated preoperative essential hypertension on post-operative pain after major abdominal surgery. Eur J Pain. 2013;17(1):94–100. doi:10.1002/j.1532-2149.2012.00156.x
15. Wildgaard K, Ringsted TK, Hansen HJ, Petersen RH, Kehlet H. Persistent postsurgical pain after video-assisted thoracic surgery - an observational study. Acta Anaesthesiol Scand. 2016;60(5):650–658. doi:10.1111/aas.12681
16. Lu Y, Hu B, Dai H, Wang B, Yao J, Yao X. Predictors of chronic postsurgical pain in elderly patients undergoing hip arthroplasty: a multi-center retrospective cohort study. Int J Gen Med. 2021;14:7885–7894. doi:10.2147/IJGM.S337170
17. Papadomanolakis-Pakis N, Uhrbrand P, Haroutounian S, Nikolajsen L. Prognostic prediction models for chronic postsurgical pain in adults: a systematic review. Pain. 2021;162(11):2644–2657. doi:10.1097/j.pain.0000000000002261
18. Lim J, Chen D, McNicol E, et al. Risk factors for persistent pain after breast and thoracic surgeries: a systematic literature review and meta-analysis. Pain. 2022;163(1):3–20. doi:10.1097/j.pain.0000000000002301
19. Wang HT, Liu W, Luo AL, Ma C, Huang YG. Prevalence and risk factors of chronic post-thoracotomy pain in Chinese patients from Peking Union Medical College Hospital. Chin Med J. 2012;125(17):3033–3038.
20. Schug SA, Lavand’Homme P, Barke A, Korwisi B, Rief W, Treede R. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160(1):45–52. doi:10.1097/j.pain.0000000000001413
21. Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73. doi:10.7326/M14-0698
22. Roh J, Jung J, Lee Y, et al. Risk stratification using multivariable fractional polynomials in diffuse large B-cell lymphoma. Front Oncol. 2020;10. doi:10.3389/fonc.2020.00329
23. Collignon O, Monnez J. Clustering of the values of a response variable and simultaneous covariate selection using a stepwise algorithm. Appl Math. 2016;7(15):1639–1648. doi:10.4236/am.2016.715141
24. Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus–related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151(4):356. doi:10.1001/jamasurg.2015.4257
25. Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain. 2014;15(9):887–897. doi:10.1016/j.jpain.2014.06.005
26. Oliver JB, Kashef K, Bader AM, Correll DJ. A survey of patients’ understanding and expectations of persistent postsurgical pain in a preoperative testing center. J Clin Anesth. 2016;34:494–501. doi:10.1016/j.jclinane.2016.06.008
27. Murray CB, Patel KV, Twiddy H, Sturgeon JA, Palermo TM. Age differences in cognitive–affective processes in adults with chronic pain. Eur J Pain. 2021;25(5):1041–1052. doi:10.1002/ejp.1725
28. Meretoja TJ, Andersen KG, Bruce J, et al. Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. J Clin Oncol. 2017;35(15):1660–1667. doi:10.1200/JCO.2016.70.3413
29. Kalkman JC, Visser K, Moen J, Bonsel JG, Grobbee ED, Moons MKG. Preoperative prediction of severe postoperative pain. Pain. 2003;105(3):415–423. doi:10.1016/S0304-3959(03)00252-5
30. Pluijms WA, Steegers MAH, Verhagen AFTM, Scheffer GJ, Wilder-Smith OHG. Chronic post-thoracotomy pain: a retrospective study. Acta Anaesthesiol Scand. 2006;50(7):804–808. doi:10.1111/j.1399-6576.2006.01065.x
31. Shi Y, Hooten MW, Roberts RO, Warner DO. Modifiable risk factors for incidence of pain in older adults. Pain. 2010;151(2):366–371. doi:10.1016/j.pain.2010.07.021
32. Sipilä R, Estlander A-M, Tasmuth T, Kataja M, Kalso E. Development of a screening instrument for risk factors of persistent pain after breast cancer surgery. Br J Cancer. 2012;107(9):1459–1466. doi:10.1038/bjc.2012.445
33. Bayman EO, Parekh KR, Keech J, Selte A, Brennan TJ. A prospective study of chronic pain after thoracic surgery. Anesthesiology. 2017;126(5):938–951. doi:10.1097/ALN.0000000000001576
34. Rizk NP, GhanieM AA, Hsu MM, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using either thoracoscopy or thoracotomy. Ann Thorac Surg. 2014;98(4):1160–1166. doi:10.1016/j.athoracsur.2014.05.028
35. Niraj G, Kelkar A, Kaushik V, et al. Audit of postoperative pain management after open thoracotomy and the incidence of chronic postthoracotomy pain in more than 500 patients at a tertiary center. J Clin Anesth. 2017;36:174–177. doi:10.1016/j.jclinane.2016.10.011
36. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537–1546. doi:10.1016/S0140-6736(19)30352-6
37. Liu CW, Page MG, Weinrib A, et al. Predictors of one year chronic post-surgical pain trajectories following thoracic surgery. J Anesth. 2021;35(4):505–514. doi:10.1007/s00540-021-02943-7
38. Takada K, Takamori S, Matsubara T, et al. Clinical significance of preoperative inflammatory markers in non-small cell lung cancer patients: a multicenter retrospective study. PLoS One. 2020;15(11):e0241580. doi:10.1371/journal.pone.0241580
39. Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non–small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):425–428. doi:10.1016/j.jtcvs.2008.05.046
40. Kobayashi N, Usui S, Kikuchi S, et al. Preoperative lymphocyte count is an independent prognostic factor in node-negative non-small cell lung cancer. Lung Cancer. 2012;75(2):223–227. doi:10.1016/j.lungcan.2011.06.009
41. Daoudia M, Decruynaere C, Le Polain De Waroux B, Thonnard J, Plaghki L, Forget P. Biological inflammatory markers mediate the effect of preoperative pain-related behaviours on postoperative analgesics requirements. BMC Anesthesiol. 2015;15(1). doi:10.1186/s12871-015-0167-9
42. Ülger G, Baldemir R, Zengin M, Sazak H, Alagöz A. Is there a correlation between preoperative neutrophil-to-lymphocyte, platelet-to-lymphocyte, and lymphocyte-to-monocyte ratios and postoperative pain in video-assisted thoracoscopic surgery? Medicine. 2022;101(21):e29472. doi:10.1097/MD.0000000000029472
43. Turgut HC, Alkan M, Ataç MS, et al. Neutrophil lymphocyte ratio predicts postoperative pain after orthognathic surgery. Niger J Clin Pract. 2017;20(10):1242. doi:10.4103/1119-3077.181399
44. Oner K, Okutan AE, Ayas MS, Paksoy AE, Polat F. Predicting postoperative pain with neutrophil/ lymphocyte ratio after arthroscopic rotator cuff repair. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2020;20:24–27. doi:10.1016/j.asmart.2020.03.001
45. Bozkurt H, Arac D, Cigdem B. The effect of the preoperative uric acid level and neutrophil lymphocyte ratio on preoperative and postoperative visual pain scores in patients with lumbar disc hernia: a cross-sectional study. Turk Neurosurg. 2019. doi:10.5137/1019-5149.JTN.25897-19.2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
