Back to Journals » Journal of Inflammation Research » Volume 16
An Easy-to-Use Nomogram Based on SII and SIRI to Predict in-Hospital Mortality Risk in Elderly Patients with Acute Myocardial Infarction
Authors Chen Y , Xie K , Han Y, Xu Q, Zhao X
Received 13 July 2023
Accepted for publication 2 September 2023
Published 13 September 2023 Volume 2023:16 Pages 4061—4071
DOI https://doi.org/10.2147/JIR.S427149
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Yan Chen,1,* Kailing Xie,2,* Yuanyuan Han,1 Qing Xu,1 Xin Zhao1
1Department of Cardiology, the Second Hospital of Dalian Medical University, Dalian, People’s Republic of China; 2Department of Second Clinical College, China Medical University, Shenyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xin Zhao, Department of Cardiology, the Second Hospital of Dalian Medical University, No. 467 Zhongshan Road, Shahekou District, Dalian, 116023, People’s Republic of China, Tel +86-0411-84671291-3097, Email [email protected]
Aim: Inflammatory response is closely associated with poor prognosis in elderly patients with acute myocardial infarction (AMI). The aim of this study was to develop an easy-to-use predictive model based on medical history data at admission, systemic immune inflammatory index (SII), and systemic inflammatory response index (SIRI) to predict the risk of in-hospital mortality in elderly patients with AMI.
Methods: We enrolled 1550 elderly AMI patients (aged ≥ 60 years) with complete medical history data and randomized them 5:5 to the training and validation cohorts. Univariate and multivariate logistic regression analyses were used to screen risk factors associated with outcome events (in-hospital death) and to establish a nomogram. The discrimination, calibration, and clinical application value of nomogram were evaluated based on receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA), respectively.
Results: The results of multivariate logistic regression showed that age, body mass index (BMI), previous stroke, diabetes, SII, and SIRI were associated with in-hospital death, and these indicators will be included in the final prediction model, which can be obtained by asking the patient’s medical history and blood routine examination in the early stage of admission and can improve the utilization rate of the prediction model. The areas under the ROC curve for the training and validation cohorts nomogram were 0.824 (95% CI 0.796 to 0.851) and 0.809 (95% CI 0.780 to 0.836), respectively. Calibration curves and DCA showed that nomogram could better predict the risk of in-hospital mortality in elderly patients with AMI.
Conclusion: The nomogram constructed by combining SII, SIRI, and partial medical history data (age, BMI, previous stroke, and diabetes) at admission has a good predictive effect on the risk of in-hospital death in elderly patients with AMI.
Keywords: coronary artery disease, elderly, systemic inflammatory markers, nomogram, prediction model
Introduction
Acute myocardial infarction (AMI) is one of the common subtypes of coronary heart disease and has been of great concern because of its high mortality and morbidity.1 Mortality within 12 months in patients with AMI is approximately 10%,2,3 and the risk of death during hospitalization is about 4%~12%.4 Data from provincial, municipal, and county-level hospitals in China from 2013 to 2014 indicate in-hospital mortality rates for AMI patients of 3.1%, 5.3%, and 10.2%, respectively.5 Importantly, the risk of death in patients with AMI increases significantly with age, with about 80% of in-hospital death in patients with AMI occurring in the elderly population.6,7 Early identification of the risk of in-hospital mortality in elderly patients with AMI may significantly improve their short-term prognosis.
Atherosclerosis is described as a continuous, dynamic, and inflammatory process in blood vessels.8 Neutrophils, monocytes, platelets, and lymphocytes all play important roles in causing plaque rupture, which may involve complex interactions between innate and adaptive immunity.9–13 Studies have shown that SII and SIRI are superior to traditional risk factors in predicting future adverse cardiovascular events in AMI patients.8,14 In addition, several studies have demonstrated that novel inflammatory markers, such as NLR, PLR, and SII, are strongly associated with the risk of in-hospital mortality in elderly AMI patients.15–18 SII and SIRI have advantages over NLR and PLR because they include more indicators associated with cardiovascular disease outcomes, and the cumulative effects of interactions among three different blood cells synergistically increase the predictive power for cardiovascular disease outcomes.19
The GRACE risk score is currently a valid tool for risk stratification during hospitalization in patients with unstable angina (UA) and non-ST-segment elevation myocardial infarction (NSTEMI),20 but this score does not include inflammation-related indexes. Considering the excellent performance of SII and SIRI in predicting adverse outcomes in AMI patients,8,14,18 we wanted to construct an easy-to-use predictive model based on patient medical history data at admission and novel inflammatory indexes (SII and SIRI) to predict the risk of death during hospitalization in elderly patients with AMI.
Methods
Data Source and Definition
We consecutively collected a total of 1550 elderly patients with complete data who were hospitalized due to AMI in the Second Hospital of Dalian Medical University from December 2015 to December 2021. Since this study did not involve any intervention on patients, the informed consent of patients could be exempted and has been approved by the ethics committee of the Second Hospital of Dalian Medical University (2023–181). This study complied with the ethical requirements of the declaration of Helsinki. Inclusion criteria: 1. Patients aged ≥60 years and diagnosed with AMI at admission.21,22 Exclusion criteria: 1. Patients with incomplete medical history in electronic medical record system; 2. Patients receiving continuous treatment for hematologic diseases or with severe uncontrolled systemic infection; 3. The expected survival time was less than 6 months; 4. Patients with severe coronary artery disease, requiring coronary-artery-bypass-grafting (CABG) or having previously undergone CABG less than half a year.
Data Collection and Outcome
Demographic and clinical data were collected, including age, gender, BMI, past medical history (stroke, hypertension, and diabetes), type of myocardial infarction (NSTEMI or STEMI), blood routine, lipid, and in-hospital medication. Blood routine test results were generally obtained within 2 hours after admission. SII is defined as neutrophil count × platelet count/lymphocyte count. SIRI was defined as neutrophil count × monocyte count/lymphocyte count. Outcome events were defined as all-cause death that occurred while the patient was hospitalized.
Statistical Analysis
Continuous variables were described as mean ± standard deviation if they conformed to normal distribution. Otherwise, Median (IQR) was used for description. Categorical variables were described as frequencies or percentages. Differences between groups were compared by t-test if continuous variables followed a normal distribution; nonparametric tests were used if a normal distribution was not met. Chi-square test was used for comparison between groups for categorical variables. SPSS 23.0, MedCalc 15.0, Stata 15, and R 4.2.1 were used for statistical analysis.
Using the method of random sampling, the patients were randomly divided into the training group and the validation group at the ratio of 5 : 5, and the baseline characteristics of the two groups were compared. SII and SIRI were analyzed based on ROC curves, and the optimal cutoff values for SII and SIRI were used as criteria for their conversion to dichotomous variables. Logistic regression model was used to evaluate the association between included variables and outcome events. Variables with P < 0.05 in the multivariate logistic regression were used to construct the final prediction model. Collinearity analysis was performed on the final included variables to identify whether there was collinearity between the variables.
The discrimination of the predictive model was firstly evaluated by the ROC curve, then the calibration of the predictive model was evaluated using H–L (Hosmer–Lemeshow) test, and the results were visualized by the calibration curve. Finally, the clinical application value of the predictive model was evaluated by decision curve analysis (DCA). P < 0.05 was considered statistically significant.
Results
Sample Characteristics
Patients were randomly assigned to the training cohort (n = 765) and validation cohort (n = 785) in a ratio of 5 to 5. There was no significant statistical difference in the incidence of outcome events between the training (n = 61, 8.0%) and validation (n = 71, 9.0%) cohorts (P = 0.45). In addition, there were no significant statistical differences in gender, age, BMI, SII, SIRI, and the number of patients with diabetes and previous stroke between the training and validation cohorts (P > 0.05), as detailed in Table 1.
 |
Table 1 Basic Characteristics of Enrolling Patients |
Factors Associated with in-Hospital Mortality in the Training Cohort
Before performing logistic regression analysis, we firstly converted age and BMI into dichotomous variables according to whether the patient ‘s age was ≥75 years and BMI was ≥28 kg/m2.23 Subsequently, we determined the optimal cutoff values of SII and SIRI by ROC curve analysis, and converted SII (≤1043.42 vs >1043.42) and SIRI (≤2.32 vs >2.32) into categorical variables according to their optimal cutoff values, and the results of ROC curve analysis are shown in Supplementary Figure 1. Converting the above variables into categorical variables can not only facilitate the use of the prediction model but also avoid collinearity between variables to a certain extent.
In order to construct a predictive model that could quickly assess the risk of in-hospital mortality at the beginning of a patient ‘s admission and was easy to use, we performed univariate logistic regression analysis of factors that made it possible to obtain patients quickly and easily at admission, including age, gender, BMI, hypertension, diabetes, previous stroke, SII, and SIRI. Subsequently, we included factors with P < 0.1 in univariate logistic regression analysis into multivariate logistic regression, and the results showed that age (≥75 years), BMI (≥28 kg/m2), diabetes, previous stroke, SII (>1043.42), and SIRI (>2.32) were associated with the risk of in-hospital death in elderly AMI patients, as detailed in Table 2.
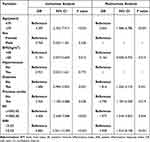 |
Table 2 Univariable and Multivariable Logistic Regression Analysis in the Training Cohort |
Finally, we performed a collinearity analysis of variables included in the final model, which showed that all variables had tolerance >0.2 and VIF (variance inflation factor) <10, indicating that there was no collinearity between the included variables, as detailed in Supplementary Table 1.
Nomogram Development and Validation
Based on the results of multivariate logistic regression analysis, we constructed an easy-to-use nomogram using variables available at admission (age, BMI, and medical history) and systemic inflammatory markers (SII and SIRI) to predict the risk of death during hospitalization in elderly patients with AMI (Figure 1). We evaluated the predictive power of nomogram by ROC curves and showed that the AUC of nomogram was 0.824 (95% CI 0.796 to 0.851) and 0.809 (95% CI 0.780 to 0.836) in the training and validation cohorts, respectively. We also analyzed the AUC of the model that was not included in the systemic inflammatory markers (SII and SIRI), and the results showed that when SII and SIRI were included, the AUC of nomogram was significantly improved and the predictive power was significantly increased, as detailed in Figure 2. The calibration curve also revealed good agreement between the nomogram ‘s predictions and the actual outcomes, as detailed in Figure 3.
 |
Figure 3 Calibration curve analysis of the nomogram. (A) training cohort; (B) validation cohort. |
Clinical Application Value
The results of the decision curve analysis (DCA) for the training and validation cohorts nomogram were presented in Figure 4. We found that the nomogram with the systemic inflammatory index (SII and SIRI) had a significantly higher net benefit compared to model 1 without the systemic inflammatory index. The results of DCA showed that within a certain threshold probability range, using this prediction model to assess the in-hospital mortality risk of elderly patients with AMI might help to improve their short-term prognosis.
Discussion
In this study, we performed a retrospective analysis of data from 1550 elderly patients with AMI. The results of multivariate logistic regression analysis showed that age, BMI, diabetes, previous stroke, SII, and SIRI were associated with the risk of in-hospital death in elderly AMI patients. We combined these variables together by nomogram to construct a predictive model. Results showed an area under the ROC curve of 0.824 (95% CI 0.796 to 0.851) and 0.809 (95% CI 0.780 to 0.836) for the training and validation cohorts, respectively. The results of calibration curve and DCA show that the model has good consistency and clinical application value.
Age, diabetes, and previous stroke are extremely closely related to the risk of in-hospital mortality in AMI patients. The risk of death increases significantly with age in patients with AMI.6 Most elderly patients with AMI often have comorbid conditions, such as hypertension, diabetes, and hyperlipidemia, which increase the risk of in-hospital mortality in elderly patients with AMI. Notably, senescent cells in older adults contribute to the production of pro-inflammatory factors, collectively referred to as the senescence-related secretory phenotype.24 In addition, with aging, the immune system degrades misfolded proteins and organelles less efficiently and senescent cells accumulate, thereby causing systemic inflammation,19,25 which may be one of the reasons for the increased risk of in-hospital death in elderly AMI. Almost two-thirds of AMI patients have diabetes or impaired glucose tolerance.26 When AMI patients have diabetes, their risk of death is twice that of AMI patients without diabetes.26 Previous studies have suggested that the risk of excess mortality may increase with age in patients with diabetes.27 Surgical risk and mortality risk are also significantly increased in AMI patients with poor glycemic control or long duration of diabetes at admission. Prosser et al found that fatal or non-fatal cardiac events occur in approximately 19% of patients within the first trimester of ischemic stroke.28 Stroke-induced damage to the central nervous system may alter the balance of sympathetic and parasympathetic tone, and the most serious consequence of this change is a significant increase in the risk of sudden death, especially in the elderly population.29–31 In our study, age, diabetes, and previous stroke were all positively associated with the risk of in-hospital mortality in elderly AMI patients. Interestingly, our findings suggest that obesity (BMI ≥ 28) is a protective factor for the risk of in-hospital mortality in older patients with AMI, which has also been reported in previous studies and is called the “obesity paradox”.32 Previous studies have suggested that the “obesity paradox” may be related to the protective effects of excessive body fat, additional muscle strength, and metabolic reserve in acute stress,33,34 as well as to aggressive clinical management programs in overweight patients.35
Previous studies have shown that multiple immune cells and pro-inflammatory factors are associated with plaque instability in patients with acute coronary syndrome (ACS).9,15,36 Neutrophils, monocytes, and platelets are inextricably linked to the development of atherosclerosis.15 Activation of neutrophils releases highly reactive oxygen species to kill pathogens, and the extracellular traps (NETs) they form can also help eliminate pathogens, but at the same time they can also damage the vessel wall and promote thrombosis and coagulation processes.37,38 It is worth noting that neutrophils can aggravate and maintain a chronic inflammatory environment in the later stages of atherosclerosis.39,40 Monocytes, as one of the leukocyte subtypes, are also closely associated with atherosclerosis progression, and their secretion of pro-inflammatory cytokines, proteolytic enzymes, and reactive oxygen species can promote atherosclerosis progression.41,42 These substances can also cause atherosclerotic dysfunction and lead to plaque instability.10,39 Platelets adhere to the vessel wall to promote plaque formation, and upon activation release inflammatory mediators that enrich the inflammatory environment.43 Lymphocytes, unlike these pro-inflammatory cells, are thought to have anti-atherosclerotic effects and tend to be associated with worse cardiovascular outcomes when lymphocyte counts are low.44,45 Neutrophils, monocytes, platelets, and lymphocytes are all directly or indirectly involved in the process of atherosclerosis, which provides a pathophysiological rationale for the link between SII and SIRI and coronary artery disease (CAD). Yang et al8 found that SII was associated with future major adverse cardiovascular events (MACEs) in CAD patients undergoing percutaneous coronary intervention (PCI). Li et al14 came to the same conclusion and also found that SIRI was most strongly associated with later MACEs in CAD patients undergoing PCI compared to other markers of systemic inflammation (NLR, PLR, MLR, and SII). These findings suggest that SII and SIRI are strongly associated with future cardiovascular events in CAD patients. To our knowledge, only one study18 has reported an association between SII and risk of in-hospital mortality in elderly patients with AMI treated with PCI, and another study, although reporting SII as a predictor of length of hospital stay in ACS patients, did not investigate the association between SII and risk of in-hospital mortality.46 In our study, we found that both SII and SIRI were associated with the risk of all-cause mortality during hospitalization in elderly AMI patients, and SIRI showed a stronger association with outcome events than SII, which may be due to different mechanisms of platelets and monocytes in plaque instability. Interestingly, no association was found between SII and the future occurrence of coronary heart disease (CHD) or myocardial infarction in healthy people,47,48 suggesting that SII or SIRI may only be associated with cardiovascular outcome events when the body is in a state of enhanced systemic or local inflammation and chronic low-grade inflammation, but this hypothesis needs to be confirmed by larger cohort studies in the future.
The factors included in our predictive model are very easy to obtain. Except for SII and SIRI, which need to be obtained through laboratory tests, all other indicators can be obtained by inquiring about the patient’s medical history and physical examination, which makes the use of the predictive model more convenient. Before conducting more complex and accurate clinical examinations, the risk of death during hospitalization can be quickly and roughly evaluated. It is worth mentioning that some factors related to PCI are closely associated to the risk of death and death during hospitalization in AMI patients, such as the type of stent, the experience of the operator, and PCI-related complications.49,50 Our constructed predictive model performed well in our data, but PCI-related factors were not included, and its predictive power still needs to be tested on a larger cohort. However, our research also has some limitations: 1. This study is a single-center retrospective study with a small sample size and may require large-scale, multicenter studies for validation in the future; 2. Our prediction model lacks external validation and has not been compared with other classic prediction models (such as the GRACE risk score); 3. Failure to obtain the outcome status of patients who did not die during hospitalization within 30 days after discharge; 4. We excluded patients who died just after admission (and also those who died on the way to admission) as well as those who had difficulty obtaining their accurate BMI data during hospitalization, so our results and conclusions may not apply to such patients.
Conclusion
We constructed a nomogram to predict the risk of in-hospital mortality in elderly patients with AMI, in which the predictors were age, BMI, presence of diabetes, previous stroke, SII, and SIRI, and these medical history data were easily available. Our data suggest that this nomogram shows good discrimination and clinical availability and can help clinicians quickly and roughly assess the risk of in-hospital mortality in such patients and improve their short-term prognosis.
Data Sharing Statement
The data that support the results of this study are available from the corresponding author upon reasonable request.
Statement of Ethics
The study protocol has been reviewed and approved by the Ethics Committee of the Second Hospital of Dalian Medical University. The Ethics Committee of the Second Hospital of Dalian Medical University waived the need for informed consent based on the following reasons: (1) The purpose of the study was important; (2) The possible risk to patients was not higher than the minimum one; (3) The waiver of informed consent would not adversely affect the rights and health of patients; (4) The patients’ privacy and personal identity information were well protected. We have desensitized the patient ‘s personal identity to protect patient privacy. The protocol of the study is compliant with the Declaration of Helsinki.
Patient Privacy Protection Statement
We desensitized all the data that can be used to identify patient personal information, such as their names, hospitalization ID, and telephone numbers, to protect the privacy of patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology. 急性ST段抬高型心肌梗死诊断和治疗指南(2019) [2019 Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of patients with ST-segment elevation myocardial infarction]. Zhonghua xin xue guan bing za zhi. 2019;47(10):766–783. Chinese. doi:10.3760/cma.j.issn.0253-3758.2019.10.003
2. Pedersen F, Butrymovich V, Kelbæk H, et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol. 2014;64(20):2101–2108. doi:10.1016/j.jacc.2014.08.037
3. Fokkema ML, James SK, Albertsson P, et al. Population trends in percutaneous coronary intervention: 20-year results from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry). J Am Coll Cardiol. 2013;61(12):1222–1230. doi:10.1016/j.jacc.2013.01.007
4. Kristensen SD, Laut KG, Fajadet J, et al. Reperfusion therapy for ST elevation acute myocardial infarction 2010/2011: current status in 37 ESC countries. Eur Heart J. 2014;35(29):1957–1970. doi:10.1093/eurheartj/eht529
5. Xu H, Yang Y, Wang C, et al. Association of Hospital-Level Differences in Care With Outcomes Among Patients With Acute ST-Segment Elevation Myocardial Infarction in China. JAMA Netw Open. 2020;3(10):e2021677. doi:10.1001/jamanetworkopen.2020.21677
6. Xia TL, Huang FY, Li YM, et al. The impact of age on the implementation of evidence-based medications in patients with coronary artery disease and its prognostic significance: a retrospective cohort study. BMC Public Health. 2018;18(1):150. doi:10.1186/s12889-018-5049-x
7. Alexander KP, Newby LK, Cannon CP, et al. Acute coronary care in the elderly, part I: non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–2569. doi:10.1161/CIRCULATIONAHA.107.182615
8. Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5):e13230. doi:10.1111/eci.13230
9. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–1866. doi:10.1161/CIRCRESAHA.114.302721
10. Nording H, Baron L, Langer HF. Platelets as therapeutic targets to prevent atherosclerosis. Atherosclerosis. 2020;307:97–108. doi:10.1016/j.atherosclerosis.2020.05.018
11. Pamukcu B, Lip GY, Devitt A, Griffiths H, Shantsila E. The role of monocytes in atherosclerotic coronary artery disease. Ann Med. 2010;42(6):394–403. doi:10.3109/07853890.2010.497767
12. Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91(4):281–291. doi:10.1161/01.RES.0000029784.15893.10
13. Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 2014;9:73–102. doi:10.1146/annurev-pathol-020712-163936
14. Li Q, Ma X, Shao Q, et al. Prognostic Impact of Multiple Lymphocyte-Based Inflammatory Indices in Acute Coronary Syndrome Patients. Front Cardiovasc Med. 2022;9:811790. doi:10.3389/fcvm.2022.811790
15. Ji Z, Liu G, Guo J, et al. The Neutrophil-to-Lymphocyte Ratio Is an Important Indicator Predicting In-Hospital Death in AMI Patients. Front Cardiovasc Med. 2021;8:706852. doi:10.3389/fcvm.2021.706852
16. Li L, Ma Y, Geng XB, et al. Platelet-to-lymphocyte ratio relates to poor prognosis in elderly patients with acute myocardial infarction. Aging Clin Exp Res. 2021;33(3):619–624. doi:10.1007/s40520-020-01555-7
17. Chen Y, Chen S, Han Y, Xu Q, Zhao X. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio are Important Indicators for Predicting in-Hospital Death in Elderly AMI Patients. J Inflamm Res. 2023;16:2051–2061. doi:10.2147/JIR.S411086
18. Huang J, Zhang Q, Wang R, et al. Systemic Immune-Inflammatory Index Predicts Clinical Outcomes for Elderly Patients with Acute Myocardial Infarction Receiving Percutaneous Coronary Intervention. Med Sci Monit. 2019;25:9690–9701. doi:10.12659/MSM.919802
19. Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic Immune Inflammation Index (SII), System Inflammation Response Index (SIRI) and Risk of All-Cause Mortality and Cardiovascular Mortality: a 20-Year Follow-Up Cohort Study of 42,875 US Adults. J Clin Med. 2023;12(3):1128. doi:10.3390/jcm12031128
20. de Araújo Gonçalves P, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J. 2005;26(9):865–872. doi:10.1093/eurheartj/ehi187
21. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177. doi:10.1093/eurheartj/ehx393
22. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi:10.1093/eurheartj/ehaa575
23. Li T, Yuan D, Wang P, et al. Associations of lipid measures with total occlusion in patients with established coronary artery disease: a cross-sectional study. Lipids Health Dis. 2022;21(1):118. doi:10.1186/s12944-022-01733-8
24. Guan Y, Zhang C, Lyu G, et al. Senescence-activated enhancer landscape orchestrates the senescence-associated secretory phenotype in murine fibroblasts. Nucleic Acids Res. 2020;48(19):10909–10923. doi:10.1093/nar/gkaa858
25. Yousefzadeh MJ, Flores RR, Zhu Y, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594(7861):100–105. doi:10.1038/s41586-021-03547-7
26. Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34(39):3035–3087. doi:10.1093/eurheartj/eht108
27. Gholap NN, Achana FA, Davies MJ, Ray KK, Gray L, Khunti K. Long-term mortality after acute myocardial infarction among individuals with and without diabetes: a systematic review and meta-analysis of studies in the post-reperfusion era. Diabetes Obes Metab. 2017;19(3):364–374. doi:10.1111/dom.12827
28. Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. 2007;38(8):2295–2302. doi:10.1161/STROKEAHA.106.471813
29. Tokgözoglu SL, Batur MK, Topçuoglu MA, Saribas O, Kes S, Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke. 1999;30(7):1307–1311. doi:10.1161/01.STR.30.7.1307
30. Pfeifer MA, Weinberg CR, Cook D, Best JD, Reenan A, Halter JB. Differential changes of autonomic nervous system function with age in man. Am J Med. 1983;75(2):249–258. doi:10.1016/0002-9343(83)91201-9
31. Sörös P, Hachinski V. Cardiovascular and neurological causes of sudden death after ischaemic stroke. Lancet Neurol. 2012;11(2):179–188. doi:10.1016/S1474-4422(11)70291-5
32. Patlolla SH, Gurumurthy G, Sundaragiri PR, Cheungpasitporn W, Vallabhajosyula S. Body Mass Index and In-Hospital Management and Outcomes of Acute Myocardial Infarction. Medicina. 2021;57(9). doi:10.3390/medicina57090926
33. Fukuoka S, Kurita T, Dohi K, et al. Untangling the obesity paradox in patients with acute myocardial infarction after primary percutaneous coronary intervention (detail analysis by age). Int J Cardiol. 2019;289:12–18. doi:10.1016/j.ijcard.2019.01.011
34. Mei X, Hu S, Mi L, Zhou Y, Chen T. Body mass index and all-cause mortality in patients with percutaneous coronary intervention: a dose-response meta-analysis of obesity paradox. Obes Rev. 2021;22(2):e13107. doi:10.1111/obr.13107
35. Steinberg BA, Cannon CP, Hernandez AF, Pan W, Peterson ED, Fonarow GC. Medical therapies and invasive treatments for coronary artery disease by body mass: the “obesity paradox” in the Get With The Guidelines database. Am J Cardiol. 2007;100(9):1331–1335. doi:10.1016/j.amjcard.2007.06.019
36. Massberg S, Schulz C, Gawaz M. Role of platelets in the pathophysiology of acute coronary syndrome. Semin Vasc Med. 2003;3(2):147–162. doi:10.1055/s-2003-40673
37. Gómez-Moreno D, Adrover JM, Hidalgo A. Neutrophils as effectors of vascular inflammation. Eur J Clin Invest. 2018;48(Suppl 2):e12940. doi:10.1111/eci.12940
38. Döring Y, Soehnlein O, Weber C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ Res. 2017;120(4):736–743. doi:10.1161/CIRCRESAHA.116.309692
39. Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122(18):1837–1845. doi:10.1161/CIRCULATIONAHA.110.961714
40. Urbanowicz T, Olasińska-Wiśniewska A, Michalak M, et al. The Prognostic Significance of Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR) and Platelet to Lymphocyte Ratio (PLR) on Long-Term Survival in Off-Pump Coronary Artery Bypass Grafting (OPCAB) Procedures. Biology. 2021;11(1). doi:10.3390/biology11010034
41. Gratchev A, Sobenin I, Orekhov A, Kzhyshkowska J. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiology. 2012;217(5):476–482. doi:10.1016/j.imbio.2012.01.008
42. Dziedzic EA, Gąsior JS, Tuzimek A, et al. Investigation of the Associations of Novel Inflammatory Biomarkers-Systemic Inflammatory Index (SII) and Systemic Inflammatory Response Index (SIRI)-With the Severity of Coronary Artery Disease and Acute Coronary Syndrome Occurrence. Int J Mol Sci. 2022;23(17):9553. doi:10.3390/ijms23179553
43. Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost. 2011;106(5):827–838. doi:10.1160/TH11-08-0592
44. Ammirati E, Moroni F, Magnoni M, Camici PG. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin Exp Immunol. 2015;179(2):173–187. doi:10.1111/cei.12477
45. Núñez J, Miñana G, Bodí V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18(21):3226–3233. doi:10.2174/092986711796391633
46. Gur DO, Efe MM, Alpsoy S, et al. Systemic Immune-Inflammatory Index as a Determinant of Atherosclerotic Burden and High-Risk Patients with Acute Coronary Syndromes. Arq Bras Cardiol. 2022;119(3):382–390. doi:10.36660/abc.20210416
47. Xu M, Chen R, Liu L, et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: the Dongfeng-Tongji cohort study. Atherosclerosis. 2021;323:20–29. doi:10.1016/j.atherosclerosis.2021.02.012
48. Jin Z, Wu Q, Chen S, et al. The Associations of Two Novel Inflammation Indexes, SII and SIRI with the Risks for Cardiovascular Diseases and All-Cause Mortality: a Ten-Year Follow-Up Study in 85,154 Individuals. J Inflamm Res. 2021;14:131–140. doi:10.2147/JIR.S283835
49. Kupó P, Tornyos D, Bálint A, Lukács R, Jánosi A, Komócsi A. Use of drug-eluting stents in elderly patients with acute myocardial infarction: an analysis of the Hungarian Myocardial Infarction Registry. Int J Clin Pract. 2021;75(1):e13652. doi:10.1111/ijcp.13652
50. Tokarek T, Dziewierz A, Plens K, et al. Radial Approach Expertise and Clinical Outcomes of Percutanous Coronary Interventions Performed Using Femoral Approach. J Clin Med. 2019;8(9):1484. doi:10.3390/jcm8091484
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



