Back to Journals » Risk Management and Healthcare Policy » Volume 16
Risk Factors for Granulomatous Mastitis and Establishment and Validation of a Clinical Prediction Model (Nomogram)
Authors Zeng Y, Zhang D, Fu N, Zhao W, Huang Q, Cui J, Chen Y, Liu Z, Zhang X, Zhang S, Mansoor KM
Received 18 July 2023
Accepted for publication 13 October 2023
Published 20 October 2023 Volume 2023:16 Pages 2209—2222
DOI https://doi.org/10.2147/RMHP.S431228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gulsum Kubra Kaya
Yifei Zeng,1,2 Dongxiao Zhang,1 Na Fu,1 Wenjie Zhao,1 Qiao Huang,1 Jianchun Cui,3 Yunru Chen,4 Zhaolan Liu,4 Xiaojun Zhang,5 Shiyun Zhang,6 Khattak Mazher Mansoor3
1Department of Galactophore, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, People’s Republic of China; 2Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 3Liaoning Provincial People’s Hospital (Department of Thyroid and Breast Surgery, People’s Hospital of China Medical University), Shenyang, People’s Republic of China; 4Centre for Evidence-Based Chinese Medicine, School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 5Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 6Guang’ Anmen Hospital, China Academy of Chinese Medical Science, Beijing, People’s Republic of China
Correspondence: Dongxiao Zhang, Department of Galactophore, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, No. 23, Art Museum Back Street, Dongcheng District, Beijing, 100010, People’s Republic of China, Tel +86 17862968916, Email [email protected]
Background: This study aimed to explore the risk factors and clinical characteristics of granulomatous mastitis (GM) using a case-control study and establish and validate a clinical prediction model (nomogram).
Methods: This retrospective case-control study was conducted in three hospitals in China from June 2017 to December 2021. A total of 1634 GM patients and 186 healthy women during the same period were included and randomly divided into the modeling and validation groups in a 7:3 ratio. To identify the independent risk factors of GM, univariate and multivariate logistic analyses were conducted and used to develop a nomogram. The prediction model was internally and externally validated using the Bootstrap technique and validation cohort. The receiver operating characteristic (ROC) curve and calibration curve were used to evaluate the discrimination and calibration of the prediction model. Decision curve analysis (DCA) and clinical impact curve (CIC) were used to evaluate the clinical significance of the model.
Results: The average age of GM patients was 33.14 years (mainly 20– 40). The incidence was high within five years from delivery and mainly occurred in the unilateral breast. The majority of the patients exhibited local skin alterations, while some also presented with systemic symptoms. On multivariate logistic analysis, age, high prolactin level, sex hormone intake, breast trauma, nipple discharge or invagination, and depression were independent risk factors for GM. The mean area under the curve (AUC) in the modeling and validation groups were 0.899 and 0.889. The internal and external validation demonstrated the model’s predictive ability and clinical value.
Conclusion: Lactation-related factors are the main risk factors of GM, leading to milk stasis or increased ductal secretion. Meanwhile, hormone disorders could affect the secretion and expansion of mammary ducts. All these factors can obstruct or injure the duct, inducing inflammatory reactions and immune responses. Additionally, blunt trauma, depressed mood, and diet preference can accelerate the process. The nomogram can effectively predict the risk of GM.
Keywords: granulomatous mastitis, risk factors, case-control study, nomogram, prediction model
Introduction
Granulomatous mastitis (GM), also known as idiopathic granulomatous mastitis clinically, is a benign inflammatory disease of the breast. The pathological features of GM are lobular-centered multifocal non-caseous granulomatous lesions of the breast. GM accounts for about 4–5% of clinical breast benign lesions,1 mainly occurring in women of childbearing age. The early clinical manifestations of GM are not specific, and it is easily misdiagnosed. Histopathological examination is the primary diagnostic method. The disease has a long course and is easy to relapse, seriously affecting patients’ breast appearance and quality of life.2 The occurrence of GM has been on the rise in recent years; however, the predisposing factors remain unclear, making it more challenging to prevent and treat GM.
Currently, three common hypotheses exist regarding the risk factors of GM, including autoimmune disorders, hormonal imbalances, and infections. Besides, multiple factors such as gestation, lactation, antitrypsin deficiency, smoking, and obesity may increase the risk of developing GM. However, limited conducted relevant research mostly consists of case reports or small sample retrospective analyses. Clinical prediction models for GM are also rarely reported or have not been thoroughly validated.
Based on the literature we reviewed, this is the largest case-control study of GM. We explored the risk factors for GM. The objective is to help make early clinical diagnosis, take preventive measures, and implement appropriate treatment strategies.
Materials and Methods
Clinical Data and Inclusion Criteria
This case-control study was conducted in the Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Xiyuan Hospital of China Academy of Chinese Medical Sciences, and Guang’anmen Hospital of China Academy of Chinese Medical Sciences from June 2017 to December 2020. Eligible GM patients were included in our study, and the healthy women who received physical examinations at the hospitals were recruited into the control group in the same period (all participants were female). The included participants were randomly divided into the modeling and validation groups in a ratio of 7:3 using the random numbers table method.
The inclusion criteria included: (1) GM patients diagnosed by pathology and control group participants with no history of GM; (2) patients with complete clinical data; (3) patients with normal communication ability and were able to cooperate with researchers to complete the questionnaire; (4) no severe heart, lung, liver and kidney dysfunctions; and (5) participants who voluntarily took part in this study, were not involved in other clinical trials in the same time, and signed the informed consent.
The exclusion criteria were people (1) incomplete information—missing more than 30% in the questionnaire; (2) malignant tumors or other breast diseases besides GM; (3) other autoimmune diseases, such as systemic lupus erythematosus, and rheumatism.
Sample Size
Using the PASS 15.0 software, a minimum of 82 cases in each group was required to detect a minimum odds ratio (OR) of 2.5, assuming an exposure rate of 0.5 for the study factors in the control group. We accepted an α and β risk of 0.05 and 0.10 in a unilateral contrast according to the recommended sample size estimation method in the literature.3
Data Collection
The researchers designed a questionnaire including 47 possible risk factors of GM based on literature reports, clinical experience, and consultation results from experts in this area. The questionnaires were filled out by trained professional researchers mainly through face-to-face interviews, and the information was collected on a one-to-one basis. The data collected included: (1) demographic information: age (years), height (m), weight (kg), BMI (kg/m2), marital status, menstrual status, gestation history, abortion history, time after last delivery (years), and family history of breast disease; (2) lactation-related factors: breastfeeding or not, lactation time (months), nipple discharge, nipple invagination, prolactin level, and history of psychotropic drugs intake; (3) hormone-related factors: sex hormone intake (medication history of drugs, including estrogen and progesterone) and thyroid hormone (hyperthyroidism and hypothyroidism); (4) other factors: blunt breast trauma; emotional abnormality (anxiety or depression); sleep condition; diet preference, smoking, allergic or skin diseases history.
The family history of breast diseases included breast cancer and non-lactation mastitis. Nipple invagination was graded as follows. In Grade I, the inversion is corrected simply by manipulation, and the nipple protrusion is long-lasting. In Grade II, the inversion can be corrected by manipulation, but recurrence of the inversion is frequent. Finally, the inversion can only be corrected with a surgical procedure in Grade III. Considering that long-term medication intake would not have a considerable influence on the disease, the period of drug intake was restricted to current users and those who used a drug in the past three months to avoid recall bias. BMI was calculated as follows, BMI= weight(kg)/[height(m)]2. The Self-Rating Depression Scale (SDS) and Self-Rating Anxiety Scale (SAS) were used to evaluate emotional conditions.
The investigators and researchers in this study all received unified training to ensure the standardization of information. We had thorough communication with the participants. Then, we designed a data extraction table for this study, and two researchers extracted the information from the obtained questionnaires. The EpiData3.1 software was used to establish a database and data entry program. We checked the data twice and locked the database.
Statistical Analysis
IBM SPSS Statistics 26.0 software was used for the statistical analysis. Measurement data were expressed as mean ± standard deviation (Mean ± SD) and analyzed using the t-test. Countable data and univariate comparisons were analyzed using the χ2 test. A multivariable logistic regression model was used to explore the importance of the risk factors identified from the univariate analysis. The adjusted ORs with their 95% confidence intervals (Cis) were obtained using binary conditional multiple logistic regression analysis. Missing values were handled using the column deletion method. The results were statistically significant at P<0.05.
We established the clinical prediction model (nomogram) to predict the occurrence risk of GM using the R-4.2.2 software. The predictive power of the nomogram was tested using internally repeated sampling 1000 times (Bootstrap method) and externally validated with the validation group. Calibration curves were plotted, and the predictive value of the model was evaluated by the Hosmer-Lemeshow Goodness of Fit test and receiver operating characteristic (ROC) curve. The larger the area under the ROC curve (AUC), the stronger the model’s predictive power. The clinical decision curve (DCA) and clinical impact curve (CIC) were plotted to visually estimate the number of patients at risk for each risk threshold and to evaluate the model’s clinical predictive value.
Results
Clinical Characteristics
In this study, 1634 eligible GM patients and 186 healthy women (control group) were included. The participants were randomly divided into the modeling and validation groups in a 7:3 ratio (Figure 1).
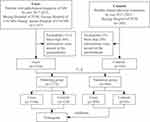 |
Figure 1 Flow chart of the study. |
Our results showed that the included GM patients were all female, and their median age at occurrence was 33.14. GM mainly occurred in women of childbearing age, between 20 and 40. The two groups showed significant differences in gestation history (91.78% vs 70.00%, P<0.01), which indicated that gestation might increase the risk of GM. However, there was no significant correlation between GM and the number of delivery and abortion history. Additionally, the results showed that GM incidence in menopausal women was significantly lower, suggesting that menopause status may be a protective factor for GM. The incidence of GM was highest within five years (with an average of 3.75 years) and significantly decreased after ten years of the last delivery. There was no significant difference in BMI and family history of breast disease (P>0.05) (Table 1).
 |
Table 1 Comparison of Basic Information Between the Two Groups |
Although most patients had a gestation history, and this disease mainly occurred during non-pregnancy and non-lactation periods. Most GM cases occurred in the unilateral breast (97.81%), and the most common onset locations were the areola areas (35.31%) and the outer upper quadrant of the breast (21.33%). Most patients (65.21%) had local skin changes, and the most common symptoms were skin redness and warmth (51.31%), swelling (32.78%), and the orange-peel sign (24.91%). The breast masses in 40.65% of the patients had festered and ruptured. In addition, 6.03% of the patients had fever, arthralgia, erythema nodosum, and other systemic manifestations, and 15.21% had axillary node enlargement (Table 2).
 |
Table 2 Clinical Characteristics of the Case Group |
Univariate Analysis
When comparing factors relevant to lactation, the results showed significant differences in nipple discharge and nipple invagination between the two groups (43.44% vs 17.69%, P<0.01; 31.00% vs 4.62%, P<0.01). In other words, women with nipple discharge and invagination were more likely to have GM. Besides, we found a significant difference in prolactin levels between the two groups (6.56% vs 0.77%, P<0.01). High prolactin levels may be an important factor in the occurrence of GM. Meanwhile, the intake of psychotropic drugs increased the risk of GM (P<0.01). These results showed that factors relevant to lactation were closely related to the occurrence of GM. The results also indicated that breastfeeding and lactation time were not relevant to the occurrence of GM (P>0.05) (Table 3).
 |
Table 3 Comparison of Factors Relevant to Lactation Between Two Groups |
Furthermore, we compared hormone-related factors, including sex hormone intake history and thyroid hormone, between the two groups. Significant differences in sex hormone intake were observed between the two groups (20.19% vs 8.46%, with P<0.01), suggesting that the intake of sex hormone medication may lead to hormonal disorders in the body and increase the risk of GM. Similarly, there were significant differences in thyroid hormone levels between the two groups (P<0.01), indicating a possible connection between thyroid hormone levels and GM.
Finally, we found significant differences in history of blunt breast trauma and emotional abnormalities (high SDS score) between the two groups (P<0.01; P<0.01), suggesting that blunt trauma and depressed mood might be the risk factors for GM. In addition, there were differences in diet preference between the two groups. The main preference was a spicy diet, which may promote the occurrence of GM (16.00% vs 6.92%, P<0.01). The results also showed that smoking, allergic history, skin diseases, and sleep disorders were not directly related to the incidence of GM (P>0.05) (Table 4).
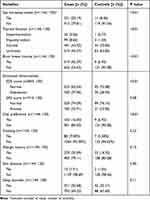 |
Table 4 Comparison of Other Factors Between the Two Groups |
Multivariate Logistic Analysis
Multivariate logistic analysis was performed for the factors obtained by univariate analysis. Our results showed that age, gestation history, sex hormone intake history, blunt breast trauma, nipple discharge history, nipple invagination, and high prolactin level were all independent risk factors for GM. Their ORs (95% CIs) values were 1.147 (1.076–1.223), 12.927 (4.027–38.590), 1.589 (1.058–1.991), 3.129 (2.675–5.216), 2.980 (1.206–7.365), 2.344 (1.010–6.194), 2.956 (1.915–5.999), 11.059 (2.667–45.863), respectively (Table 5).
 |
Table 5 Multivariate Logistic Analysis of Risk Factors for Granulomatous Mastitis |
Establishment and Validation of the Clinical Prediction Model (Nomogram)
Based on the logistic regression analysis results, we established the clinical prediction model (nomogram) to predict the risk of GM onset using the R software (Figure 2). Each variable in the graph corresponds to a point on the nomogram’s variable axis. The scores are obtained using this point perpendicular to the upper scoring scale. The total score was obtained by adding the scores of each variable, which corresponded to the risk score of GM.
 |
Figure 2 The clinical prediction model (Nomogram) for predicting the risk of granulomatous mastitis (*Indicates P<0.05; ***Indicates P<0.001). |
The C-index of the nomogram was 0.900 (95% CI=0.872–0.929), indicating the model had a excellent discriminatory ability to predict the risk of GM. The corrected C-index was 0.892 through internal verification using the Bootstrap method, showing that the prediction model had good accuracy. The P-value of the nomogram was 0.1046 (P>0.05) by the Hosmer-Lemeshow test, indicating good goodness of fit. In addition, we compared the differences in the above eight important variables between the modeling and validation groups, which showed no significant differences between the data of the two groups (P>0.05). This indicates that the validation group data sets can be used to validate the prediction model (Table 6). Furthermore, we drew the calibration curves of the prediction model in the modeling and validation groups to evaluate its uniformity. The results confirmed that the model had good predictive performance (Figure 3). Meanwhile, the ROC curves of the two groups were plotted using the R software. The AUC of the modeling and validation groups were 0.899 and 0.889; both were greater than 0.70, indicating that the model had high sensitivity and specificity (Figure 4).
 |
Table 6 Comparison of Important Variables Between the Modeling and Validation Groups |
 |
Figure 3 The calibration curves of the prediction model in modeling group (A) and validation group (B), indicating its uniformity. |
 |
Figure 4 The receiver operating characteristic curve of the prediction model in the modeling group (A) and validation group (B), indicating its discrimination. |
Finally, the R software was used to plot the DCA and CIC of the model in the modeling and validation groups to verify its clinical predictive value. The DCA of the simple clinical model (blue curve) was established using common risk factors of GM, including age, nipple discharge, and nipple invagination. The red DCA is the curve of the prediction model we established. Figure 5 shows that in both internal and external verification, the net benefit rate of the model constructed in this study was higher than that of the simple clinical model under each threshold probability. Meanwhile, the results of CIC indicate that when the threshold probability was greater than 0.3, the clinical high-risk population predicted by the model was close to the actual positive population. Our model is adequate for the clinical diagnosis of GM (Figure 6)
Discussion
GM is the most common pathological type of non-lactation mastitis. With the increasing incidence of GM, studies on its risk factors have sharply increased recently. They were mainly small sample studies or case reports based on the three hypotheses: autoimmunity, hormone disorders, and infection. The risk factors of GM remain controversial. Therefore, it is crucial to study the risk factors of GM using large clinical samples to explore the pathogenesis of the disease. Our study is the first case-control study with the largest sample size. We hope our results will help to develop reasonable preventive measures early to reduce the risk of susceptible people and improve their quality of life.
First, concerning the clinical features of GM, we found that it mostly occurs in the unilateral breast. The most common location was the areola and the outer upper quadrant of the breast. Skin changes were common in GM patients, mainly manifesting as redness, warmth, and rupture. Furthermore, some patients exhibited systemic symptoms like erythema nodosum, which aligns with the immunological hypothesis regarding GM etiology. Our results of the clinical characteristics analysis verified the reports of previous literature.4–6
According to the results, GM was most common in women of childbearing age between 20 and 40, and the median age was 33.14. Most patients had a gestation and lactation history, and women without a gestation history had a lower risk of GM. The incidence of GM was highest within five years after the last delivery and decreased significantly after ten years. Therefore, we believe there is a close correlation between lactation-related factors and the occurrence of GM. These factors include childbearing age, gestation history, time after the last delivery, and nipple invagination and discharge. These abnormal conditions could cause milk siltation or ductal secretion to increase. They can irritate and obstruct the mammary ducts, injuring the ductal epithelium and eventually triggering the local inflammatory reaction. It is worth mentioning that though nipple invagination may be one of the most important precursors of GM, based on our results, the severity of nipple invagination does not seem to have a clear causal relationship with the occurrence of GM. We speculate that the most likely reason is that patients with severe nipple invagination (Grade II & III) are relatively rare in the clinic, and it is difficult to identify significant differences in statistics. This still needs to be confirmed by further research. Moreover, the occurrence of GM appeared to be independent of breastfeeding status and duration, further indicating that we can prevent GM occurrence more effectively as long as the milk production or secretion is smooth in the mammary duct.
Another important factor relevant to lactation is hyperprolactinemia. Our results suggest that a high prolactin level is an independent risk factor for GM. Many studies have shown that hyperprolactinemia is closely relevant to the onset of GM in recent years. Lin7 found that high prolactin levels could increase the mammary duct’s secretion, promote the duct’s expansion, cause duct occlusion, destroy duct epithelial cells, and produce pro-inflammatory effects, ultimately promoting the occurrence of GM.8 Moreover, the high prolactin level in GM patients can also significantly increase the clinical recurrence rate.9,10 It is worth mentioning that our results showed that patients taking psychotropic drugs were at higher risk of GM. Studies have shown that some psychotropic drugs, such as risperidone, may be associated with the onset of GM. Our results showed that the related psychotropic drugs were risperidone, sertraline, ziprasidone, paroxetine, and aripiprazole. These drugs have side effects that increase secretions in the mammary duct, leading to the obstruction of the duct.11,12 It could trigger the inflammatory autoimmune reaction and eventually induce GM. Clinical trials13 have shown that the incidence of hyperprolactinemia is significantly increased in patients taking psychotropic drugs, suggesting that such psychotropic drugs can dramatically increase prolactin levels. Therefore, it can be inferred that the intake of psychotropic drugs may have a similar mechanism as high prolactin levels.
In addition, we investigated the relationship between hormone-related factors and the occurrence of GM. Some studies have suggested that sex hormone disorders may be an independent factor inducing GM,14 consistent with our findings which showed that the intake of sex hormones (estrogen and progesterone) increases the incidence of GM. Exogenous sex hormones could affect the secretion and expansion of mammary ducts. Excessive secretions can damage the duct epithelium and promote local duct inflammation.15 In other words, the menopausal state may be protective against GM. Besides, our study also found that thyroid dysfunction may be relevant to GM, which has not been reported previously. We speculate that breast cells are responsive to thyroid hormone signaling and are affected by altered thyroid hormone levels.16 However, further studies are needed to confirm the relevant mechanism.
Finally, we assessed other factors associated with the risk of GM. Our results suggest that blunt breast trauma, depressed mood (high SDS score), and diet preference could increase the risk of GM. Among them, blunt breast trauma and high SDS score were the independent risk factors for GM. The possible explanation is that blunt trauma can damage the local mammary duct, leading to a hypersensitivity reaction mediated by humoral or cellular immunity and inducing GM. The inflammatory response gradually spreads and eventually develops into a lump or abscess.17–20 We also found that the SDS score of GM patients was significantly higher than that of the normal population. Abnormal emotions can also affect immune response through the neuro-endocrine-immune network,21 promote the release of inflammatory factors, such as TNF-α and IL-6, and increase the risk of GM.22 It is also worth mentioning that diet preference could increase the inflammatory biomarkers in the blood and cause the occurrence or aggravation of chronic inflammation.23,24 Spicy diets can be considered one of the classic pro-inflammatory diets that could increase the expression of Th1-type chemokine receptors CXCR3 and CCR5, induce more Th1 cell aggregation, and mediate immune response.25 Thus, a spicy diet can increase the risk of GM to some extent.
Previous studies have found that the incidence of GM may be closely related to smoking26 and obesity.27 Interestingly, our study showed no significant correlation between smoking and the incidence of GM. We speculate that the main reason may be that the smoking rate in Chinese women is only 1.9%,28 which is significantly lower than that of some countries. For example, the smoking rate in American women is 14.2%.29 Therefore, smoking has a lower influence on Asian females. Meanwhile, there may be a racial difference in the adverse effects of smoking. A previous study30 suggested that obesity directly impacted local estrogen and inflammatory status in the breast, which may cause GM. However, our results did not show this tendency.
Meanwhile, based on the independent risk factors of GM obtained from this study, we established a clinical prediction model (nomogram) of GM. The nomogram can quantify and visualize the logistic regression results to predict the risk of GM. We confirmed the model’s good discrimination and accuracy through the internal validation method. Meanwhile, we further demonstrated the clinical predictive value of this model by proportional random selection of validation sets in the participants. The combination of internal and external validation makes our clinical model more convincing.
This clinical prediction model helps screen people at high risk of GM and take early prevention to reduce the incidence of GM. For instance, we can avoid exogenous breast injury and sex hormone intake to reduce the occurrence of GM. Moreover, we believe that therapies aimed at reducing elevated prolactin levels, correcting nipple invagination, promoting good mood, and replacing the drugs with side effects that increase duct secretion, could improve clinical effectiveness, shorten the disease course, and avoid relapse.
However, this study still needs some improvement. First, although this is the study with the largest sample size on GM, the patients were Asian and enrolled only from three hospitals. Therefore, racial differences in GM incidence could not be explored. Second, Corynebacterium infections were reported in some literature, and we discussed it. However, since we could not get biopsy samples from controls, it was impossible to compare Corynebacterium in both groups. So, infection-related factors were not analyzed in this study. Finally, we did not limit the abortion time before GM occurrence when collecting data on abortion history. Since a distant history of abortion has very limited influence on hormone disorders, this may result in false negative results.
Conclusion
In summary, this study explored the risk factors for GM using a case-control study with the largest sample size and established a clinical prediction model (nomogram). To the best of our knowledge, this is the first model validated internally and externally. The results suggest that lactation-related factors and hormone disorders are the most important factors for GM. This signifies that factors that cause increased milk siltation or ductal secretion, such as high prolactin level, nipple discharge, and invagination, could obstruct and injure the duct. Meanwhile, hormone disorders, including sex and thyroid hormones, may affect secretion and increase the risk of GM. Blunt breast trauma, depressed mood, and spicy food could participate in the processes and accelerate the onset of GM. Based on the results, we can take appropriate preventive and therapeutic measures to reduce the incidence and recurrence and improve the cure for GM. The nomogram we established and validated has high clinical predictive value, which can effectively evaluate the risk of GM in susceptible people. It valuable for early screening of high-risk populations.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics Approval and Consent to Participate
This study has been reviewed by the Ethics Committee of Beijing Hospital of Traditional Chinese Medicine, Capital Medical University (No: 2016BL-057-01). It complies with the World Medical Association Declaration of Helsinki in 1964 and subsequently amended versions. The participants voluntarily took part in this study and were not involved in other clinical trials in the same time, signed informed consent was received from all participants. No biological specimens were used in this study.
Funding
This study was supported by Young Doctor Scholar project (2022), Beijing talent project (2019), Capital research and transformation of clinical diagnosis and treatment technology (Z211100002921020), Gansu Provincial Science and Technology Program Subsidized Projects (21JRIRG303), and The Special Project on Traditional Chinese Medicine (TCM) Heritage of ancient books, literature and distinctive techniques (GZY-KJS-2022-035).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Pereira FA, Mudgil AV, Macias ES, et al. Idiopathic granulomatous lobular mastitis. Int J Dermatol. 2012;51(2):142–151. PMID: 22250621. doi:10.1111/j.1365-4632.2011.05168.x
2. Benson JR, Dumitru D. Idiopathic granulomatous mastitis: presentation, investigation and management. Future Oncol. 2016;12(11):1381–1394. PMID: 27067146. doi:10.2217/fon-2015-0038
3. Shi ZH, He Y. Statistics of Traditional Chinese Medicine and Software Application.
4. Mohammed S, Statz A, Lacross JS, et al. Granulomatous mastitis: a 10 year experience from a large inner city county hospital. J Surg Res. 2013;184(1):299–303. PMID: 23890401. doi:10.1016/j.jss.2013.06.047
5. Li J. Diagnosis and treatment of 75 patients with idiopathic lobular granulomatous mastitis. J Invest Surg. 2019;32(5):414–420. PMID: 29381437. doi:10.1080/08941939.2018.1424270
6. Centers for Disease Control and Prevention (CDC). Idiopathic granulomatous mastitis in Hispanic women - Indiana, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58(47):1317–1321. PMID: 19959984.
7. Lin CH, Hsu CW, Tsao TY, et al. Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia. Diagn Pathol. 2012;7(1):2. PMID: 22221904; PMCID: PMC3261802. doi:10.1186/1746-1596-7-2
8. Nikolaev A, Blake CN, Carlson DL. Association between hyperprolactinemia and granulomatous mastitis. Breast J. 2016;22(2):224–231. PMID: 26705962. doi:10.1111/tbj.12552
9. Sheybani F, Sarvghad M, Naderi H, et al. Treatment for and clinical characteristics of granulomatous mastitis. Obstet Gynecol. 2015;125(4):801–807. PMID: 25751209. doi:10.1097/AOG.0000000000000734
10. Tasci HI, Turk E, Erinanc OH, Erkan S, Gundogdu R, Karagulle E. Factors affecting recurrence of idiopathic granulomatous mastitis. J Coll Physicians Surg Pak. 2022;32(2):161–165. PMID: 35108784. doi:10.29271/jcpsp.2022.02.161
11. Wong SCY, Poon RWS, Chen JHK, et al.Corynebacterium kroppenstedtii is an emerging cause of mastitis especially in patients with psychiatric illness on antipsychotic medication. Open Forum Infect Dis. 2017;4(2):ofx096. PMID: 28852671; PMCID: PMC5570011. doi:10.1093/ofid/ofx096
12. Konan A, Kalyoncu U, Dogan I, et al. Combined long-term steroid and immunosuppressive treatment regimen in granulomatous mastitis. Breast Care. 2012;7(4):297–301. PMID: 23904832; PMCID: PMC3515783. doi:10.1159/000341388
13. Tollin SR. Use of the dopamine agonists bromocriptine and cabergoline in the management of risperidone-induced hyperprolactinemia in patients with psychotic disorders. J Endocrinol Invest. 2000;23(11):765–770. PMID: 11194712. doi:10.1007/BF03345068
14. Bi J, Li Z, Lin X, et al. Etiology of granulomatous lobular mastitis based on metagenomic next-generation sequencing. Int J Infect Dis. 2021;113:243–250. PMID: 34673215. doi:10.1016/j.ijid.2021.10.019
15. Amsterdam JD, Garcia-España F, Goodman D, et al. Breast enlargement during chronic antidepressant therapy. J Affect Disord. 1997;46(2):151–156. PMID: 9479619. doi:10.1016/s0165-0327(97)00086-4
16. Halada S, Casado-Medrano V, Baran JA, et al. Hormonal crosstalk between thyroid and breast cancer. Endocrinology. 2022;163(7):bqac075. doi:10.1210/endocr/bqac075
17. Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J. 2011;17(6):661–668. PMID: 21951547. doi:10.1111/j.1524-4741.2011.01162.x
18. Sarkar DK, Banerjee R, Gupta S, et al. Management of idiopathic granulomatous mastitis: a prospective study. Ann R Coll Surg Engl. 2022;105(3):218–224. PMID: 35638904. doi:10.1308/rcsann.2022.0017
19. Cserni G, Szajki K. Granulomatous lobular mastitis following drug-induced galactorrhea and blunt trauma. Breast J. 1999;5(6):398–403. PMID: 11348321. doi:10.1046/j.1524-4741.1999.97040.x
20. Lei X, Chen K, Zhu L, et al. Treatments for idiopathic granulomatous mastitis: systematic review and meta-analysis. Breastfeed Med. 2017;12(7):415–421. PMID: 28731822. doi:10.1089/bfm.2017.0030
21. Wang Y, Qiao J. Mechanisms by which cytokines influence pathological mood symptoms. Chin J Immunol. 2008;4:378–380+385.
22. Mihailova S, Ivanova-Genova E, Lukanov T, et al. A study of TNF-α, TGF-β, IL-10, IL-6, and IFN-γ gene polymorphisms in patients with depression. J Neuroimmunol. 2016;293:123–128. PMID: 27049572. doi:10.1016/j.jneuroim.2016.03.005
23. Shivappa N, Hébert JR, Rietzschel ER, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. 2015;113(4):665–671. doi:10.1017/S000711451400395X
24. Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51(5):794–811. doi:10.1016/j.immuni.2019.09.020
25. Lu C, Fang YH, Li ZN, et al. The expression of chemokine receptor in allergic contact dermatitis of cosmetics. Chin J Dermatovenereol. 2014;28(10):991–993+1003.
26. Asoglu O, Ozmen V, Karanlik H, et al. Feasibility of surgical management in patients with granulomatous mastitis. Breast J. 2005;11(2):108–114. PMID: 15730456. doi:10.1111/j.1075-122X.2005.21576.x
27. Bharat A, Gao F, Aft RL, Gillanders WE, Eberlein TJ, Margenthaler JA. Predictors of primary breast abscesses and recurrence. World J Surg. 2009;33(12):2582–2586. PMID: 19669231; PMCID: PMC3892669. doi:10.1007/s00268-009-0170-8
28. Liu Z, Li YH, Cui ZY, et al. Prevalence of tobacco dependence and associated factors in China: Findings from nationwide China Health Literacy Survey during 2018-19. Lancet Reg Health West Pac. 2022;24(100464). doi:10.1016/j.lanwpc.2022.100464
29. Rigotti NA, Kruse GR, Livingstone-Banks J, Hartmann-Boyce J. Treatment of tobacco smoking: a review. JAMA. 2022;327(6):566–577. PMID: 35133411. doi:10.1001/jama.2022.0395
30. Brown KA. Impact of obesity on mammary gland inflammation and local estrogen production. J Mammary Gland Biol Neoplasia. 2014;19(2):183–189. PMID: 24935438. doi:10.1007/s10911-014-9321-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


