Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Development and Validation of a Nomogram for Patients Undergoing Transarterial Chemoembolization for Recurrent Hepatocellular Carcinoma After Hepatectomy
Authors Xie D, Li Z, Yuan J, Yin X, Chen R, Zhang L, Ren Z
Received 9 November 2023
Accepted for publication 26 March 2024
Published 4 April 2024 Volume 2024:11 Pages 693—705
DOI https://doi.org/10.2147/JHC.S444682
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmed Kaseb
Diyang Xie, Zhongchen Li, Jia Yuan, Xin Yin, Rongxin Chen, Lan Zhang, Zhenggang Ren
Department of Hepatic Oncology, Liver Cancer Institute, Zhongshan Hospital, Ministry of Education, Fudan University, Shanghai, 200032, People’s Republic of China
Correspondence: Zhenggang Ren, Department of Hepatic Oncology, Liver Cancer Institute, Zhongshan Hospital, Ministry of Education, Fudan University, Shanghai, 200032, People’s Republic of China, Tel/Fax +86-21-64037181, Email [email protected]
Purpose: This study aims to establish a prognostic nomogram for patients who underwent transarterial chemoembolization (TACE) for recurrent hepatocellular carcinoma (HCC) after hepatectomy.
Patients and Methods: Patients who underwent TACE for recurrent early- and middle-stage HCC after hepatectomy between 2009.01 and 2015.12 were included. Enrolled patients were randomly divided into training (n=345) and validation (n=173) cohorts according to a computer-generated randomized number. Independent factors for overall survival (OS) were determined and included in the nomogram based on the univariate and multivariate analyses of the training group. The nomogram was validated and compared to other prognostic models. Discriminative ability and predictive accuracy were determined using the Harrell C index (C-index), area under the receiver operating characteristic curve (AUROC), and calibration curve.
Results: The final nomogram was established based on four parameters including resection-to-TACE time interval, recurrent tumor diameter, recurrent tumor number, and AFP level. The C-indexes of the nomogram for predicting OS were 0.67 (95% CI 0.63– 0.70) and 0.71 (95% CI 0.68– 0.74) in the training and validation cohort respectively. The AUROCs for predicting the 1-year, 2-year and 3-year OS based on the nomogram were also superior to those of the other models. The calibration curve for 3-year survival showed a high congruence between the predicted and actual survival probabilities. According to the scores calculated by the nomogram, patients were stratified into three subgroups: high-risk (scoring ≥ 53 points), middle-risk (scoring ≥ 26 and < 53 points), and low-risk (scoring < 26 points) subgroups with a median OS of 10.1 (95% CI 0.63– 0.70), 20.3 (95% CI 17.5– 22.5) and 47.0 (95% CI 34.2– 59.8) months, respectively.
Conclusion: The proposed nomogram served as a new tool to predict individual survival in patients who underwent TACE for recurrent HCC after hepatectomy, with favorable performance and discrimination. For high-risk patients, treatment should be optimized beyond TACE alone based on the nomogram.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, nomogram
Introduction
Liver cancer is the sixth most prevalent and the third most lethal malignancy worldwide.1 Approximately 410 thousand patients were newly diagnosed with liver cancer in China in 2020 (https://gco.iarc.fr), accounting for 45.3% of new global new cases.2 Hepatocellular carcinoma (HCC) constitutes the majority of primary liver cancers, accounting for 85–90% of cases. Most HCC cases in China have a background of chronic hepatitis derived from hepatitis B virus (HBV) infection and are characterized by high invasiveness and poor differentiation.3,4 According to a previous report, only 36% of HCC cases were initially diagnosed at an early stage and qualified for radical treatment.5 Hepatectomy remains the mainstay radical treatment. However, recurrence occurred to 70% of patients within 5 years after hepatectomy.
Consistent with the treatment algorithm for newly diagnosed HCC patients, transarterial chemoembolization (TACE) is also indicated for patients with recurrent HCC after hepatectomy at an intermediate stage or at an early stage inappropriate for a second resection owing to tumor size, location, or complicated diseases.6 The prognosis of patients receiving TACE varies widely, depending on the heterogeneity of the target population. According to previous studies, the median overall survival (OS) for newly-diagnosed patients who underwent TACE for HCC varied from 13 to 43 months.7 Therefore, various stratification tools have been established to predict the long-term prognosis for patients after TACE, including the up-to-seven,8 four-and-seven,9 hepatoma arterial embolization prognostic (HAP),10 modified HAP (mHAP),11 mHAP-II12 and six-and-twelve13 scoring systems. Whether these systems based mostly on the data of treatment-naïve patients are also applicable for patients with recurrent HCC is unclear. Currently, there has been no established prognostic systems for patients who received TACE for recurrent HCC after hepatectomy.
Breakthroughs in systemic therapies have encouraged treatment stage migration (TSM) for patients with middle-stage HCC, who are expected to be refractory to TACE. The 2022 Barcelona Clinic Liver Cancer (BCLC) guidelines suggest systemic therapies rather than TACE as the optimal treatment for middle-stage patients with diffuse, infiltrative, and extensive HCC liver involvement.14 Nevertheless, there is no strict cut-off to define such a subgroup, especially for recurrent HCC. Herein, we established and validated a prognostic nomogram for patients who underwent TACE for post-hepatectomy recurrent HCC to predict individual survival and to select high-risk subgroups as candidates for systemic therapy.
Materials and Methods
Study Population
A continuous series of patients who underwent TACE for post-resection recurrent HCC in the Liver Cancer Institute, Zhongshan Hospital between January 2009 and December 2015 were retrospectively screened from our database. The inclusion criteria were as follows: (1) History of R0 resection for HCC; (2) no residual cancer confirmed by multiphasic computed tomography (CT) or contrast-enhanced magnetic resonance imaging (MRI) 4 to 6 weeks after hepatectomy; (3) recurrent lesions diagnosed based on radiological confirmation according to the American Association for the Study of Liver Diseases (AASLD) criteria or pathologic evaluation; (4) Child-Pugh liver function grade A or B; and (5) performance scoring 0 point. The exclusion criteria included: (1) vascular invasion or extrahepatic spread; (2) loco-regional therapy or systemic therapy during TACE treatment; (3) decompensated cirrhosis; (4) gastrointestinal bleeding, encephalopathy, or other contraindications for embolization; (5) history of other malignancies; and 6) absence of imaging information at baseline or during follow-up. The patients included in the analyses were randomly assigned to the training or validation dataset according to a computer-generated randomized number. Morphological features, including tumor diameter, tumor number, and the absence of vascular invasion, were evaluated using contrast-enhanced MRI or CT. The study was approved by the Institutional Ethics Committee of the Zhongshan Hospital, Fudan University.
Treatment Procedures
Conventional TACE was performed as previously described.15 Briefly, a 4-Fr or 5-Fr RH catheter was inserted through femoral artery to access hepatic artery. Common hepatic artery, superior mesenteric artery, and phrenic artery angiography were performed to present hepatic artery anatomy and identify the tumor-feeding artery. Chemotherapeutic agents, including 5-fluorouracil (0.5–1.0g) and oxaliplatin (100–150 mg) were perfused through the proper hepatic artery. The tumor-feeding arteries were embolized either selectively or superselectively using an emulsion of mixtures of lipiodol (3–20 mL) and chemotherapeutic agents, such as doxorubicin (10–50 mg), epirubicin (10–50 mg), or oxaliplatin (50–100 mg). Thereafter, a gelatin sponge was introduced to reduce the tumor arterial flow.
Contrast-enhanced CT or MRI was performed one month after TACE. In case of remnant viable tumor or intrahepatic recurrence, TACE was repeated on demand at interval of 6 to 12 weeks. Upon radiological confirmation of no viable residual tumor or stable disease, the patients were followed up at an interval of 8 to 12 weeks. During each follow-up period, radiological evaluations and laboratory tests, including liver function and AFP levels, were collected. The study was censored on October 30th, 2019, to avoid the impact of novel systemic therapies particularly immunotherapy on response evaluation.
Statistical Analysis
Statistical analyses were performed using the SPSS software (version 22.0; IBM Inc., Chicago, IL, USA) and R 4.0.2 (http://www.r-project.org). OS was defined as the time interval from the first TACE procedure to all-cause death. Patients who survived at the last follow-up date or were lost to follow-up were censored. Continuous variables were presented as mean ± standard deviation or median (interquartile ranges, IQRs) and compared using Student’s t-test or Mann–Whitney U-test, as appropriate. Categorical variables were shown as counts (percentages) and were compared using the chi-squared test or Fisher’s exact test. The best cutoff value for continuous data was determined by clinical evaluation or using X-tile software (https://medicine.yale.edu/lab/rimm/research/software/). It was difficult to determine the sample size beforehand because of scarce evidence in developing a prognostic model for patients treated with TACE for post-resection recurrent HCC. However, the total number of events (all-cause death) reached 296 and exceeded the ratio of 10 events per variable, suggesting sufficient accuracy of the estimation.16 The prognostic factors identified in the univariate Cox proportional hazards regression analyses (P < 0.1) were included in the multivariate analysis. Based on independent parameters determined via multivariate analysis, a nomogram was established using “rms” and “nomogramFormula” R package. The discrimination of the nomogram was evaluated using the concordance index (C-index) and time-dependent area under the receiver operating characteristic curve (AUROC). Nomogram accuracy was determined using a calibration curve. Comparisons between the nomogram and other prognostic models including up-to-seven,8 HAP,10 mHAP,11 mHAP-II,12 albumin-bilirubin (ALBI)17 and six-and-twelve13 were performed using “rcorrp.cens” package and assessed by the C-index and time-dependent AUROC. Based on the scores calculated via the nomogram, the patients were stratified into three subgroups with the cutoff points determined by the X-tile. P < 0.05 was considered statistically significant.
Results
Clinicopathologic Characteristics
A total of 518 patients were included (Figure 1), with hepatitis B virus (HBV) infection (95.2%) as a major risk factor. Patients with detectable HBV DNA or hepatitis C virus (HCV) RNA received antiviral treatment. Approximately 11.6% of the patients underwent multiple resections before TACE. The median time interval between the last resection and TACE was 11.2 (IQR 6.0–19.2) months. The target population received a median of 3 (IQR, 2–5) TACE sessions during the study period. All the included patients were randomly divided into the training (n = 345) and validation (n = 173) groups at a ratio of 2:1. The baseline characteristics were comparable between the two groups (Table 1).
 |
Table 1 Patient Characteristics at Baseline |
 |
Figure 1 Flowchart of included patients. |
Overall Survival
The median follow-up were 15.1 (IQR 7.0–26.2) months for the entire cohort, and 15.2 (IQR 7.3–27.5) months and 14.3 (IQR 7.2–23.5) months for the training cohort and the validation cohort respectively. The median OS for the entire cohort was 22.0 (95% confidence interval [CI], 21.0–23.4) months, with 1-, 2- and 3-year survival rate of 71.9%, 44.4% and 29.7%, respectively. No significant difference in median OS (23.0, 95% CI 19.7–26.3 months vs 20.0, 95% CI, 16.5–23.5 months, P = 0.307) was observed between the training cohort and the validation group (Figure 2).
 |
Figure 2 Comparison of overall survival between the training cohort and the validation cohort. |
Independent Prognostic Predictors
According to the X-tile analysis, the cut-off value of the time interval between the last resection and TACE for subdivision was 6 months. Likewise the cutoff value of ALBI was −2.6, consistent with the cutoff point in a previous study.17 Based on univariate and multivariate analyses of the training cohort (Table 2), resection-to-TACE time interval ≤6 months (HR = 1.432, 95% CI 1.050–1.955, P = 0.024), recurrent tumor number (HR = 1.143, 95% CI 1.073–1.217, P < 0.001), tumor size (HR = 1.111, 95% CI 1.026–1.204, P = 0.010), AFP > 20ng/mL (HR = 1.464, 95% CI 1.103–1.944, P = 0.017), and GGT >60U/L (HR = 1.429, 95% CI 1.057–1.932, P = 0.020) were independent risk factors for patients undergoing TACE for recurrent HCC.
 |
Table 2 Univariate and Multivariate Analyses of Factors Predicting Overall Survival in the Training Cohort |
Prognostic Nomogram for OS
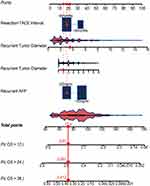
A novel nomogram was established for patients who underwent TACE for recurrent HCC, based on independent risk factors for OS in the training cohort. The C-index of the nomogram that integrated all five parameters was 0.67 (95% CI, 0.63–0.71), the same to that of the nomogram established based on four parameters without GGT. To develop an easy-to-use stratification tool, a final nomogram was developed based on four predictors including resection-to-TACE time interval, AFP value, recurrent tumor number, and tumor size (Figure 3). The 1-year, 2-year and 3-year survival probabilities were estimated based on the nomogram. An example of using the nomogram to predict survival probability of a given patient was also displayed in Figure 3.
In the validation set, the C-index value of the nomogram was 0.71 (95% CI, 0.68–0.74). The 1-year, 2-year, 3-year AUROC for the training cohort and the validation cohort were 0.72 (95% CI, 0.65–0.78), 0.70 (95% CI, 0.63–0.76), 0.70 (95% CI, 0.62–0.79) and 0.73 (95% CI, 0.66–0.81), 0.74 (95% CI, 0.67–0.82), 0.80 (95% CI, 0.73–0.89) respectively. The calibration curve for 3-year survival showed a high congruence between the predicted and actual survival probabilities (Figure 4).
 |
Figure 4 Calibration curve for predicting 3-year survival in the training cohort (upper figure) and the validation cohort (lower figure). |
Comparison Between Current Model and Other Prognostic Systems
The performance of the established model was compared with other models including up-to-seven, six-and-twelve, HAP, mHAP, m-HAP-II and ALBI scoring systems (Table 3). Based on the time-dependent AUROC curves (Figure 5) and C-indices, the current model displayed favorable performance and discrimination compared to the previous models.
 |
Table 3 Comparisons of AUROC and C-Index Among Different Prognostic Models |
Survival Stratification Based on the Nomogram
Based on the score calculated using the nomogram for each patient, the entire cohort was subdivided into three groups: high-risk (scoring ≥53 points), middle-risk (scoring <53 and ≥26 points), and low-risk (scoring <26 points) group. The median OS for the high-risk, middle-risk and low-risk groups were 10.1 (95% CI, 6.1–13.9) months, 20.3 (95% CI, 17.5–22.5) months and 47.0 (95% CI, 34.2–59.8) months respectively (Figure 6).
 |
Figure 6 Different risk stratification for overall survival based on the nomogram. |
Discussion
For patients who underwent TACE for post-resection recurrent HCC, the current nomogram could predict individual survival with favorable performance and discrimination. According to the 2022 BCLC proposal, the expected median OS for newly-diagnosed patients who received TACE for intermediate HCC was over 30 months.14 The median OS in our cohort was only 20.1 months, similar to the result of a previous systemic review.18 In addition to increased aggressiveness of recurrent HCC, the availability of effective systemic therapies after TACE progression in recent years has contributed to the improved expected OS in recent guidelines. In our study, patients who received TACE for recurrent HCC were divided into three prognostic strata with a median OS of 47.0, 20.3 and 10.1 months respectively. The median OS was 49.1, 32.0 and 15.8 months for treatment-naive TACE candidates using the six-and-twelve scoring system, the heterogeneity of which was similar to our nomogram.13
Consistent with previous prognostic models for treatment-naïve patients receiving TACE,10–13 tumor burden, including tumor size and tumor number, was also a major risk factor for OS in our model for recurrent HCC. Although albumin and total bilirubin were established as independent risk factors for OS in the HAP10 and mHAP models,11 the ALBI score integrating albumin and total bilirubin showed an insignificant association with OS in the current nomogram and the six-and-twelve scoring systems. One reason for this discrepancy might be the different background liver diseases. The current nomogram and the six-and-twelve model were established based on Chinese cohorts mainly consisting of HBV-related HCC, whereas other models, such as HAP, were developed from Japanese cohorts with HCV-related HCC. HBV-related HCC, which belongs to the proliferation molecular subclass, is characterized by poor differentiation and higher aggressiveness, whereas HCV-associated HCC, which belongs to the non-proliferation class, is characterized by well or moderate differentiation and less invasiveness.4 Considering liver function reserve, patients with HCV infection are more likely to develop cirrhosis with impaired liver function preserve compared to those with HBV infection.19,20 As a result, the heterogeneity of the tumor burden and liver function among different cohorts contributes to a variety of independent parameters for prognosis prediction.
The time interval between radical resection and recurrence is an independent factor for OS in patients after resection.21–25 Early recurrence is usually derived from intrahepatic metastasis of remnant viable tumor cells, whereas late recurrence is regarded as de novo HCC.26,27 Notably, although intrahepatic lesions occurring > two years after hepatectomy were usually referred to as late recurrence,28 evolutionary tree analysis via sequencing showed the possibility of intrahepatic metastasis in recurrent nodules 28 months after resection.29 The cutoff value of the resection-to-recurrence time interval to discriminate early recurrence from late recurrence varied among different studies.23–25,28 Consistent with the study conducted by Liu et al23 patients with recurrence within 6 months had significantly inferior OS than those with recurrence beyond 6 months in our study. As the C-index and AUROC revealed good performance of the current model, other features, such as the clinicopathological characteristics of resected tumor nodules, were not included in this study for easy-to-use purposes.
The median OS for the high-risk group according to the current nomogram was 10.1 months, whereas the median OS for patients who received sorafenib for early and intermediate HCC ineligible for locoregional therapy was 14.5 months.30 In addition to sorafenib, systemic therapies for HCC have significantly improved since 2017. The median OS for patients who received lenvatinib as first-line therapy for unresectable HCC was 13.6 months.31 According to a proof-of-concept study, lenvatinib as an initial treatment resulted in a more favorable OS than TACE in patients with intermediate-stage HCC beyond up-to-seven.32 The synergistic efficacy of immunotherapy plus target agents and dual immunotherapy has also been demonstrated in the first-line setting. Atezolizumab plus bevacizumab for advanced HCC have resulted in median OS of 19.2 months for overall cohort33 and 24.0 months for the Chinese cohort,34 significantly superior to sorafenib. Tremelimumab plus durvalumab also yielded superior OS compared to sorafenib (16.4 vs 13.7 months, P=0.0035) for unresectable HCC.35 For TACE-unsuitable patients, defined as those within the intermediate stage but with inferior survival after TACE, systemic treatments with prolonged OS of more than 20 months are recommended over TACE according to recent guidelines.14 For patients with recurrent intermediate HCC, our nomogram can serve as an effective stratification tool to select high-risk patients who may benefit more from systemic therapies rather than TACE. Prospective, controlled, and randomized studies are needed to demonstrate the efficacy of our nomogram as a tool to guide treatment allocation.
Our study had some limitations. First, although we included a large cohort of 518 consecutive patients, selection bias was unavoidable in observational studies. Second, the nomogram was based on data from a single center without external validation, which weakened its reliability. Moreover, the majority of the patients included in our study had HBV-related HCC. Therefore, exploration of our nomogram for HCC patients with other etiologies, such as non-alcoholic steatohepatitis (NASH), is unclear.
Conclusion
In conclusion, we established a prognostic nomogram involving four clinical parameters to stratify patients who underwent TACE for post-resection recurrent HCC. The model could predict individualized prognosis with adequate performance and stratify patients into three subgroups with significantly different OS, facilitating treatment allocation. Further validation in patients with different etiologies and its efficacy in risk stratification for clinical use are warranted.
Data Sharing Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethical Statement
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Medical Ethics Committee of Fudan University Zhongshan Hospital (NO.B2016-086). Since the study was retrospective, informed consent was waived in accordance with national legislation and institutional requirements. The data of the participants have been anonymized.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. doi:10.1016/j.jhep.2022.08.021
3. de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62(4):1190–1200. doi:10.1002/hep.27969
4. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
5. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
6. Park JW. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022;28(4):583–705. doi:10.3350/cmh.2022.0294
7. Sangro B, Salem R. Transarterial chemoembolization and radioembolization. Semin Liver Disease. 2014;34(4):435–443. doi:10.1055/s-0034-1394142
8. Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi:10.1016/S1470-2045(08)70284-5
9. Yamakado K, Miyayama S, Hirota S, et al. Subgrouping of intermediate-stage (BCLC stage B) hepatocellular carcinoma based on tumor number and size and Child-Pugh grade correlated with prognosis after transarterial chemoembolization. Jpn J Radiol. 2014;32(5):260–265. doi:10.1007/s11604-014-0298-9
10. Kadalayil L, Benini R, Pallan L, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24(10):2565–2570. doi:10.1093/annonc/mdt247
11. Pinato DJ, Arizumi T, Allara E, et al. Validation of the hepatoma arterial embolization prognostic score in European and Asian populations and proposed modification. Clin Gastroenterol Hepatol. 2015;13(6):1204–8 e2. doi:10.1016/j.cgh.2014.11.037
12. Park Y, Kim SU, Kim BK, et al. Addition of tumor multiplicity improves the prognostic performance of the hepatoma arterial-embolization prognostic score. Liver Int. 2016;36(1):100–107. doi:10.1111/liv.12878
13. Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol. 2019;70(5):893–903. doi:10.1016/j.jhep.2019.01.013
14. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
15. J-H L, Xie X-Y, Zhang L, et al. Oxaliplatin and 5-fluorouracil hepatic infusion with lipiodolized chemoembolization in large hepatocellular carcinoma. World J Gastroenterol. 2015;21(13):3970–3977. doi:10.3748/wjg.v21.i13.3970
16. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503–1510. doi:10.1016/0895-4356(95)00048-8
17. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
18. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind J-FH. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
19. Sasaki Y, Yamada T, Tanaka H, et al. Risk of recurrence in a long-term follow-up after surgery in 417 patients with hepatitis B- or hepatitis C-related hepatocellular carcinoma. Ann Surg. 2006;244(5):771–780. doi:10.1097/01.sla.0000225126.56483.b3
20. Nomoto S, Hishida M, Inokawa Y, Sugimoto H, Kodera Y. Management of hepatocellular carcinoma should consider both tumor factors and background liver factors. Hepatobil Surg Nutr. 2014;3(2):82–85. doi:10.3978/j.issn.2304-3881.2014.02.13
21. Zou Q, Li J, Wu D, et al. Nomograms for pre-operative and post-operative prediction of long-term survival of patients who underwent repeat hepatectomy for recurrent Hepatocellular carcinoma. Ann Surg Oncol. 2016;23(8):2618–2626. doi:10.1245/s10434-016-5136-0
22. Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol. 2015;41(2):236–242. doi:10.1016/j.ejso.2014.11.002
23. Liu Y-W, Yong C-C, Lin -C-C, et al. Six months as a cutoff time point to define early recurrence after liver resection of hepatocellular carcinoma based on post-recurrence survival. Updates Surg. 2021;73(2):399–409. doi:10.1007/s13304-020-00931-2
24. Wei T, Zhang X-F, Bagante F, et al. Early versus late recurrence of hepatocellular carcinoma after surgical resection based on post-recurrence survival: an international multi-institutional analysis. J Gastrointest Surg. 2021;25(1):125–133. doi:10.1007/s11605-020-04553-2
25. Kuo M-J, L-R M, Chen C-L. Factors predicting long-term outcomes of early-stage hepatocellular carcinoma after primary curative treatment: the role of surgical or nonsurgical methods. BMC Cancer. 2021;21(1):250. doi:10.1186/s12885-021-07948-9
26. Ding X, He M, Chan AWH, et al. Genomic and epigenomic features of primary and recurrent hepatocellular carcinomas. Gastroenterology. 2020. doi:10.1053/j.gastro.2019.09.056
27. Xie D-Y, Fan H-K, Ren Z-G, Fan J, Gao Q. Identifying clonal origin of multifocal Hepatocellular carcinoma and its clinical implications. Clin Transl Gastroenterol. 2019;10(2):e00006. doi:10.14309/ctg.0000000000000006
28. Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207. doi:10.1016/S0168-8278(02)00360-4
29. Furuta M, Ueno M, Fujimoto A, et al. Whole genome sequencing discriminates hepatocellular carcinoma with intrahepatic metastasis from multi-centric tumors. J Hepatol. 2017;66(2):363–373. doi:10.1016/j.jhep.2016.09.021
30. Bruix J, Raoul J-L, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a Phase III trial. J Hepatol. 2012;57(4):821–829. doi:10.1016/j.jhep.2012.06.014
31. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
32. Kudo M, Ueshima K, Chan S, et al. Lenvatinib as an initial treatment in patients with intermediate-stage Hepatocellular Carcinoma beyond up-to-seven criteria and Child-Pugh a liver function: a Proof-of-Concept Study. Cancers. 2019;11(8):1084. doi:10.3390/cancers11081084
33. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable Hepatocellular carcinoma. New Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
34. Qin S, Ouyang X, Bai Y. Subgroup analysis of Chinese patients in a phase 3 study of lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma. Hepatol Internat. 2019;2019(13):1. doi:10.1097/MD.0000000000000388
35. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(4_suppl):379. doi:10.1200/JCO.2022.40.4_suppl.379
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Effects of Early TACE Refractoriness on Survival in Patients with Hepatocellular Carcinoma: A Real-World Study
Yang C, Luo YG, Yang HC, Yao ZH, Li X
Journal of Hepatocellular Carcinoma 2022, 9:621-631
Published Date: 21 July 2022
Histological Severity of Cirrhosis Influences Surgical Outcomes of Hepatocellular Carcinoma After Curative Hepatectomy
Liang BY, Gu J, Xiong M, Zhang EL, Zhang ZY, Lau WY, Wang SF, Guan Y, Chen XP, Huang ZY
Journal of Hepatocellular Carcinoma 2022, 9:633-647
Published Date: 23 July 2022
Comparison of the Efficacy and Safety of Transarterial Chemoembolization with or without Lenvatinib for Unresectable Hepatocellular Carcinoma: A Retrospective Propensity Score–Matched Analysis
Chen YX, Zhang JX, Zhou CG, Liu J, Liu S, Shi HB, Zu QQ
Journal of Hepatocellular Carcinoma 2022, 9:685-694
Published Date: 1 August 2022
Nomogram for the Preoperative Prediction of the Macrotrabecular-Massive Subtype of Hepatocellular Carcinoma
Shan Y, Yu X, Yang Y, Sun J, Wu S, Mao S, Lu C
Journal of Hepatocellular Carcinoma 2022, 9:717-728
Published Date: 10 August 2022
Development and Validation of a Prediction Model for Hepatitis B Virus-Related Hepatocellular Carcinoma Patients Receiving Postoperative Adjuvant Transarterial Chemoembolization
Tu X, Zhang J, Li M, Lu F, Wang T, Gong W, Xiang B
Journal of Hepatocellular Carcinoma 2023, 10:1881-1895
Published Date: 24 October 2023