Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Effects of Early TACE Refractoriness on Survival in Patients with Hepatocellular Carcinoma: A Real-World Study
Authors Yang C, Luo YG , Yang HC , Yao ZH , Li X
Received 11 May 2022
Accepted for publication 13 July 2022
Published 21 July 2022 Volume 2022:9 Pages 621—631
DOI https://doi.org/10.2147/JHC.S373112
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Manal Hassan
Chao Yang, Yin-gen Luo, Hong-cai Yang, Zhi-hang Yao, Xiao Li
Department of Interventional Therapy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Xiao Li, Department of Interventional Therapy, National Cancer Center/National Clinical Research, Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 Panjiayuan Nanli, Chaoyang District, Beijing, 100021, People’s Republic of China, Tel/Fax +86 010 8778 8309, Email [email protected]
Purpose: To investigate the effect of early transarterial chemoembolization (TACE) refractoriness on hepatocellular carcinoma (HCC) patient survival and to explore whether viable lesions > 50% after two consecutive TACE treatments negatively affect the prognosis of HCC patients.
Patients and Methods: From January 2014 to August 2017, 323 HCC patients who received TACE as the initial treatment were analyzed. TACE refractoriness was diagnosed according to the Japan Society of Hepatology 2021 version. Propensity score matching (PSM) was used to create a 1:1 matched group (nonrefractoriness vs refractoriness). To determine survival outcomes and prognostic factors, the Kaplan-Meier method and Cox proportional hazards model were used.
Results: In total, 51.1% of patients developed early TACE refractoriness (n = 165). After PSM, 120 patients from each group were matched and analyzed. The median overall survival (OS) time of the early TACE refractoriness group was significantly shorter than that of the nonrefractory group [21 months (95% CI: 15.7– 26.3) vs 34 months (95% CI: 27.5– 40.5), p = 0.002]. Thirty-eight patients with viable lesions > 50% after two consecutive TACE procedures were identified and matched with patients of non-refractoriness. No significant difference in median OS was observed [35 months (95% CI: 21.6– 48.5) vs 31 months (95% CI: 25.4– 36.6), p = 0.611]. Multivariate analysis revealed that the BCLC stage, tumor size, tumor capsule, tumor distribution, α-fetoprotein level (AFP), and early TACE refractoriness were independent risk factors for prognosis in HCC patients.
Conclusion: Early TACE refractoriness may shorten the OS of HCC patients. However, viable lesions > 50% after two consecutive TACE treatments did not impair the survival of patients. It may be inappropriate to consider these patients as having developed TACE refractoriness.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, TACE refractoriness, overall survival, viable lesions
Introduction
For patients with intermediate stage hepatocellular carcinoma (HCC), transarterial chemoembolization (TACE) is the first-line treatment.1 Clinically, some patients with early and advanced HCC are also treated with TACE,1–4 and TACE still plays an important role in the treatment of HCC. Repeated TACE may improve the tumor response and prolong survival.5–8 However, some tumors do not respond to repeated TACE treatments and exhibit progression; thus, patients lose the opportunity to switch to other treatments in a timely manner.
The Japanese Society of Hepatology (JSH) proposed the concept of “TACE refractoriness/failure” (TACE refractoriness) for the first time and revised it in 2014 and 2021.9–11 The Barcelona Clinic Liver Cancer strategy (BCLC) updated in 2022 recommends targeted therapy or immune checkpoint inhibitor therapy for HCC patients when TACE refractoriness develops.12 However, the definition of TACE refractoriness varies across different regions and is controversial.13,14 The revised definition of TACE refractoriness by JSH has been accepted into clinical treatment guidelines in some Asian regions and serves as the key factor for terminating TACE treatment. Patients who develop TACE refractoriness after two consecutive TACE sessions, which is called early TACE refractoriness,15 may have a different survival compared with patients who develop TACE-refractoriness after more TACE treatments. A multicenter retrospective study found that the tumor response rate to a third TACE treatment was 58.3% and 48% in patients with stable tumors after the second TACE session of two cohorts.7 This conflicts with the concept of “viable lesions> 50% after two consecutive TACE treatments have TACE refractoriness” proposed by JSH.
Therefore, it is necessary to clarify whether early TACE refractoriness, especially in patients with viable lesions >50% after two consecutive TACE treatments, affects HCC patient survival.
Materials and Methods
Patients and Design
This retrospective study was conducted in accordance with the Declaration of Helsinki and approved by our institutional review board. The requirement to obtain informed consent was waived because the study provided no more than minimal risk to the patients, the waiver did not adversely affect the rights and welfare of the patients, and the research could not practicably be done without the waiver. Nonidentified information was used to protect patient confidentiality. Patients diagnosed with HCC at our institution were enrolled in this retrospective analysis from January 2014 to August 2017. Inclusion criteria for the study were as follows: (1) diagnosed with HCC by pathological biopsy, cytology, or imaging based on the HCC diagnostic criteria formulated by the American Society for the Study of Liver Diseases (AASLD), (2) age ≥18, (3) Child–Pugh stage A or B, (4) ECOG performance scores ≤1, and (5) life expectancy over 3 months. The exclusion criteria were as follows: (1) incomplete data, (2) initial treatment was not TACE, (3) combined with other antitumor treatments, (4) diagnosis of HCC and other tumors, (5) extrahepatic metastasis, and (6) severe dysfunction of important organs.
Treatment
Conventional TACE (cTACE) was performed by interventional radiologists with over 10 years of experience in the procedure. Enhanced CT or MRI was conducted to evaluate the treatment effect within 2 weeks before each on-demand cTACE. After local anesthesia using 2% lidocaine, the femoral artery of the patients was successfully punctured using the Seldinger technique. Under the guidance of digital subtraction angiography (DSA, Allura Xper FD20, Philips, Netherlands), an RH catheter (TERUMO, Japan) was inserted into the hepatic artery, mesenteric, and diaphragmatic arteries, and then high-pressure injection angiography was performed. If necessary, angiography of the intercostal arteries, internal thoracic arteries, and renal arteries was performed to clarify the distribution of arterial blood supply for HCC. Then, a microcatheter (2.0–2.8 Fr) was inserted into the feeding artery of the tumors to conduct superselective angiography, drugs (anthracycline and platinum) and iodized oil (5–20 mL, GUERBET, France) were infused through the microcatheter, and finally, gelatin sponge particles (350–560 un/560-710 µm, Alicon, China) were injected to embolize the arterial blood supply. The endpoint of embolization was the disappearance of tumor staining and the appearance of lipiodol filling in peripheral portal veins surrounding the tumor.16 In the next TACE treatment, the arteries supplying the same target tumor/tumors were fully evaluated again, and chemotherapy drugs were replaced if necessary. When the patients were unable to continue TACE therapy during follow-up, subsequent treatments such as targeted agents, immune checkpoint inhibitors, hepatic arterial infusion chemotherapy, etc., were selected based on the tumor burden, liver function, and ECOG performance status.
Criteria of TACE Refractoriness
The JSH revised definition of TACE refractoriness in 2021 states the following: (a) progressions in the liver for two or more consecutive times, poor responses of the target tumor (viable lesions >50%), or new tumor lesions appearing even when the chemotherapeutic agent was changed and/or the tumor artery was reconfirmed as observed on response evaluation CT/MRI at 1–3 months after selective TACE procedure, (b) extrahepatic metastasis or vascular invasion, (c) continuously elevated tumor markers after TACE is observed, regardless of a slight transient decrease. TACE refractoriness after the first two consecutive TACE sessions was considered early refractoriness.
Follow-Up
Enhanced CT or MRI was performed 4–6 weeks after each treatment and repeated every 3–4 months until the treatment ended or death. In our study, the endpoints of TACE treatment included complete tumor remission, inability to embolize the tumor artery technically and TACE contraindications (such as C liver function or Child–Pugh class B with decompensated liver cirrhosis, extrahepatic metastasis, ECOG score ≥ 2, and severe coagulation disorder). Vascular invasion was not considered a contraindication to further TACE. When the patient was suspected of having extrahepatic metastases, chest CT, bone scan, or PET-CT examination was performed. Overall survival (OS) was calculated from the first TACE treatment to death or last follow-up.
PSM Analysis
R statistical software (version 4.1.2) was used to conduct the propensity score matching (PSM) analysis. The baseline variables, including sex, age, total bilirubin (TBIL), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), AFP, ascites, cirrhosis, hepatitis B virus (HBV) infection, BCLC stage, Child–Pugh stage, Eastern Cooperative Oncology Group score (ECOG), tumor number, size of the largest tumor, tumor capsule, TACE sessions, and bilobular lesions were matched in the analysis. PSM was carried out at a 1:1 ratio with a caliper of 0.05.
Statistical Analysis
Data are shown as the mean ± standard deviation or median (mean) based on the distribution of the data. Comparisons between the two groups were performed using independent t-tests or Mann–Whitney U-tests, chi-square tests, or Fisher’s exact tests for categorical and consecutive variables. Kaplan-Meier estimates were used to plot the OS curves for each group; the Log rank test was used to compare them, while Cox regression models were used to analyze covariates. Differences were considered statistically significant if the p–value was less than 0.05. Statistical analyses were performed by using R statistical software (version 4.1.2).
Results
Baseline Characteristics
A total of 1731 HCC patients received TACE treatment at the Interventional Department of our hospital from January 2014 to August 2017, and 1408 patients were excluded according to the exclusion criteria (Figure 1). Among them, 664 patients had incomplete data, 612 patients underwent TACE and other antitumor therapy, 96 patients did not receive an initial TACE treatment, 11 patients were diagnosed with HCC and other tumors, and 25 patients had extrahepatic metastasis. Finally, 323 patients with HCC who underwent TACE were enrolled (mean age, 58.3±10.7 years; 281 men). A total of 66 patients were diagnosed based on pathology, and 257 patients were diagnosed depending on noninvasive diagnostic criteria. There were 104, 170, and 49 patients in BCLC stages A, B, and C, respectively. The median number of TACE procedures was 5.3 (range: 1–28). More patients with BCLC stage C, bilobular tumors, larger tumors, more lesions, and high levels of ALT and AST were observed in the early TACE refractoriness group (p < 0.05) (Table 1). In our study, the proportion of early TACE refractoriness was 51.1% (165/323), and the rates of early TACE refractoriness with different BCLC stages were 36.5% (stage A, 38/104), 52.9% (stage B, 90/170), and 75.5% (stage C, 37/49). During treatment, the liver function of three patients in the early TACE refractoriness group deteriorated to Child-Pugh grade C, while no significant deterioration of liver function was observed in patients without early TACE refractoriness.
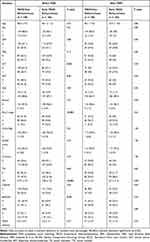 |
Table 1 Patients Characteristics |
 |
Figure 1 Flowchart of patient inclusion and exclusion. Abbreviations: TACE, transarterial chemoembolization; PSM, propensity score matching. |
Survival Analysis Before and After PSM
The median follow-up time of the early TACE-refractory group was 62 months (range, 58.6–65.4 months), while it was also 62 months (range, 56.5–67.5 months) in the group without early TACE-refractoriness (p = 0.399). Before PSM, early TACE refractoriness was observed in 165 patients, and 158 patients did not. The median OS of the early TACE refractoriness group was 21 m (95% CI: 17.3–24.7), and it was 39 m (95% CI: 29.2–48.8) in the group without early TACE refractoriness (p = 0.000) (Figure 2A). After PSM at a 1:1 ratio, 120 pairs of patients were enrolled. Table 1 shows a comparison of the baseline characteristics of the two groups. The median OS of the early TACE refractoriness group was still significantly shorter than that of the group without early TACE refractoriness, and the difference was statistically significant [21 m (95% CI: 15.7–26.3) vs 34 m (95% CI: 27.5–40.5), p = 0.002; HR: 2.02, 95% CI: 1.57–2.61] (Figure 2B). Subgroup analysis showed that stage A and stage B patients with early TACE refractoriness exhibited shorter OS [(Stage A: 28 m, 95% CI: 22.0–34.0; 57 m, 95% CI: 51.3–62.7, p = 0.000; HR: 2.39, 95% CI: 1.49–3.83), (Stage B: 20 m, 95% CI: 16.1–23.9; 36 m, 95% CI: 29.2–42.8, p = 0.002; HR: 1.71, 95% CI: 1.21–2.41)] (Figure 3A and B). However, no significant difference was observed between stage C patients with and without early TACE refractoriness (14 m, 95% CI: 6.1–21.9; 25 m, 95% CI: 19.3–30.7, p = 0.398; HR: 1.34, 95% CI: 0.67–2.70) (Figure 3C).
In the study population, 38 patients (23.03%) were judged to be early TACE refractoriness only because of viable lesions > 50% after two consecutive TACE sessions. PSM at a 1:1 ratio was used to correct the difference between the patients above and patients without early TACE refractoriness, and 33 pairs of patients were matched. Table 2 shows the demographic and baseline characteristics of the two groups above. The 1-, 2-, and 3-year survival rates of the early TACE-refractory group (viable lesions > 50%) were 75.8%, 60.6%, and 42.4%, respectively, and 90.2%, 72.6%, and 47.1%, respectively, for the nonrefractory group. The median OS was 35 m (95% CI: 21.6–48.5) in the early TACE-refractory group (viable lesions > 50%) and 31 m (95% CI: 25.4–36.6) in the nonrefractory group (HR: 1.15, 95% CI: 0.66–2.00). However, no significant difference between the two groups was observed (p = 0.611) (Figure 4).
 |
Table 2 Patients Characteristics After PSM (TACE-Non-Refractoriness and TACE-Refractoriness*) |
Predictors for Survival
In the study population after PSM, univariate analysis revealed that AFP, BCLC stage, tumor size, bilobular tumors, tumor capsule, and early TACE refractoriness were significant predictors for OS (all p < 0.05). Multivariate analysis showed that advanced BCLC stage (HR: 1.82, 95% CI: 1.12–2.96, p = 0.016), larger tumor size (HR: 1.67, 95% CI: 1.14–2.44, p = 0.008), tumors distributed in bilateral hepatic lobes (HR: 1.45, 95% CI: 1.08–1.95, p = 0.015), AFP (HR: 1.66, 95% CI: 1.20–2.31, p = 0.003), and early TACE refractoriness (HR: 1.60, 95% CI: 1.20–2.14, p = 0.001) were significantly associated with poor survival, while the presence of tumor capsule (HR: 0.46, 95% CI: 0.35–0.74, p = 0.001) may lead to a longer survival (Table 3).
 |
Table 3 Predictors of Death for HCC Patients Treated by TACE |
Discussion
The TACE refractoriness concept proposed by JSH has been widely used in Asian regions, which has affected the treatment of HCC to a certain extent; however, the criteria for its definition are still controversial.7,13,14 A survey based on Chinese interventional doctors revealed that 46.1% of doctors chose to continue treat HCC patients with TACE-based therapy after the patients developed TACE refractoriness.17 According to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), the response evaluation of patients with viable lesions >50% after TACE includes stable disease (SD) or partial response (PR), and the treatment can be considered effective in clinical practice.18 In contrast, viable lesions > 50% after two consecutive TACE treatments are identified as TACE refractoriness and the recommendation is to terminate TACE treatment, although there is no evidence to support this definition. Our study shows that early TACE refractoriness can indeed shorten the OS of early- and intermediate-stage HCC patients, but the difference in OS was not statistically significant between patients with viable lesions >50% after two consecutive TACE sessions and patients without early TACE refractoriness.
Similar to the 49.0% TACE refractoriness rate reported in previous studies,19 in our study, it was 51.08%. The rate of early refractoriness was related to the BCLC stage. A total of 36.54% of stage A patients developed TACE refractoriness versus 52.94% and 75.51% of stage B and stage C patients, respectively. Early TACE refractoriness significantly shortened the median OS of HCC patients [21 months (95% CI: 15.7–26.3) vs 34 months (95% CI: 27.5–40.5), p = 0.002]. Chen et al20 studied HCC populations in two cohorts and found that the median OS of patients without TACE refractoriness was 1257 days (95% CI, 338.8–275.2) and 1324 days (95% CI, 183.5–2464.5), respectively. The median OS of patients with TACE refractoriness was 540 days (95% CI, 400.8–679.1) and 568 days (95% CI, 416.3–719.7). Compared with the study above, our study showed a similar OS for patients with TACE refractoriness and a shorter OS for TACE-nonrefractory patients. This might be because our study enrolled stage C patients, and some patients were still alive at the end of follow-up, which may impair the survival of the study population.
Our study compared the survival of patients with early TACE refractoriness identified by viable lesions > 50% after two TACE sessions and without TACE refractoriness. The median OS did not differ significantly between the two groups of patients [35 months (95% CI: 21.6–48.5) vs 31 months (95% CI: 25.4–36.6), p = 0.611]. Therefore, viable lesions > 50% after two TACE treatments did not impair patient survival in our study. In these patients, the tumor had the obvious characteristics of multiple lesions (63.16%, 24/38) and large size (mean size, 8.14±4.07 cm), which resulted in a heavier tumor burden. This may suggest that even two effective TACE treatments in such patients cannot induce complete necrosis of all lesions. Currently, determining the treatment strategy after TACE refractoriness is a research hotspot. Generally, targeted therapy or immune checkpoint inhibitor therapy is recommended.12 Although they are effective, the survival benefit is modest, and the cost is high.21–24 In our study, up to 23.03% of patients with early TACE refractoriness were identified by viable lesions > 50% after two TACE treatments. These patients could continue to receive TACE, and the survival was not shortened. Therefore, the risk of misjudgment of TACE refractoriness would be reduced, and the administration of inappropriate second-line or third-line treatments to patients would be avoided. In addition, multivariate analysis suggested that tumor stage, size, and distribution, AFP level, and tumor capsule are independent risk factors for prognosis, which consistent with the results of other TACE prognostic factor studies.25–28 Considering that the patients enrolled in our study underwent TACE treatment in recent years, more precise TACE technology has been widely used, and there are a decreasing number of cases in which it is impossible to embolize all the lesions for technical reasons. Therefore, the number of tumors and survival in this study did not show a significant correlation.
The present study had certain limitations. First, as a retrospective study, it had inherent defects. However, patients can be matched more accurately, and selection bias was reduced by PSM. Second, most patients in our study had hepatitis B (74.9%), and the conclusions need to be validated in HCC patients with other diseases, such as hepatitis C and alcoholic liver disease. Therefore, further multicenter, prospective, randomized controlled trials are needed to verify our findings.
Conclusion
In conclusion, early TACE refractoriness may impair the survival of HCC patients. However, viable lesions >50% after two consecutive TACE treatments did not shorten the survival of patients, and these patients should not be considered to have TACE refractoriness. Therefore, it may be necessary to reconsider the rationale behind identifying this group as TACE refractory.
Ethics Approval and Informed Consent
Ethics committee of National cancer center/Cancer hospital, Chinese academy of medical sciences and Peking union medical college approved this retrospective study conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki (Ethical review no. 21/433-3104). The ethics committee waived all informed consents because patient medical data was analyzed retrospectively.
Author Contributions
Each author made substantial contributions to the work presented, whether it was through conception, study design, execution, data collection, analysis, and interpretation, or in all of these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
References
1. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
2. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
3. Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258(2):627–634. doi:10.1148/radiol.10101058
4. Silva JP, Berger NG, Tsai S, et al. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB. 2017;19(8):659–666. doi:10.1016/j.hpb.2017.04.016
5. Vogl TJ, Trapp M, Schroeder H, et al. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214(2):349–357. doi:10.1148/radiology.214.2.r00fe06349
6. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
7. Chen S, Peng Z, Zhang Y, et al. Lack of response to transarterial chemoembolization for intermediate-stage hepatocellular carcinoma: abandon or repeat? Radiology. 2021;298(3):680–692. doi:10.1148/radiol.2021202289
8. Zheng L, Fang S, Wu F, et al. Efficacy and safety of TACE combined with sorafenib plus immune checkpoint inhibitors for the Treatment of Intermediate and Advanced TACE-refractory hepatocellular carcinoma: a retrospective study. Front Mol Biosci. 2020;7:609322. doi:10.3389/fmolb.2020.609322
9. Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Digestive Dis. 2011;29(3):339–364. doi:10.1159/000327577
10. Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31. doi:10.1159/000368142
11. Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181–223. doi:10.1159/000514174
12. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
13. Kim HY, Park JW, Joo J, et al. Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27(6):1051–1056. doi:10.1111/j.1440-1746.2011.06963.x
14. Raoul JL, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Cancer. 2014;3(2):119–124. doi:10.1159/000343867
15. Wang TC, An TZ, Li JX, Zhang ZS, Xiao YD. Development and validation of a predictive model for early refractoriness of transarterial chemoembolization in patients with hepatocellular carcinoma. Front Mol Biosci. 2021;8:633590. doi:10.3389/fmolb.2021.633590
16. Lu J, Zhao M, Arai Y, et al. Clinical practice of transarterial chemoembolization for hepatocellular carcinoma: consensus statement from an international expert panel of International Society of Multidisciplinary Interventional Oncology (ISMIO). Hepatobiliary Surg Nutr. 2021;10(5):661–671. doi:10.21037/hbsn-21-260
17. Zhong BY, Wang WS, Zhang S, Zhu HD. Re-evaluating transarterial chemoembolization failure/refractoriness: a survey by Chinese college of interventionalists. J Clin Transl Hepatol. 2021;1:10.
18. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
19. Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3(3–4):458–468. doi:10.1159/000343875
20. Chen L, Yu CX, Zhong BY, et al. Development of TACE refractoriness scores in hepatocellular carcinoma. Front Mol Biosci. 2021;8:615133. doi:10.3389/fmolb.2021.615133
21. Cao Y, Ouyang T, Xiong F, et al. Efficacy of apatinib in patients with sorafenib-transarterial chemoembolization refractory hepatocellular carcinoma: a retrospective study. Hepatol Int. 2021;15(5):1268–1277. doi:10.1007/s12072-021-10198-3
22. Martin SP, Fako V, Dang H, et al. PKM2 inhibition may reverse therapeutic resistance to transarterial chemoembolization in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39(1):99. doi:10.1186/s13046-020-01605-y
23. Arizumi T, Ueshima K, Minami T, et al. Effectiveness of sorafenib in patients with Transcatheter Arterial Chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253–262. doi:10.1159/000367743
24. Kobayashi S, Tajiri K, Murayama A, et al. Drug-eluting bead-transcatheter arterial chemoembolization for advanced hepatocellular carcinoma refractory to conventional lipiodol-based transcatheter arterial chemoembolization. J Hepatocell Carcinoma. 2020;7:181–189. doi:10.2147/JHC.S273929
25. Peng CW, Teng W, Lui KW, et al. Complete response at first transarterial chemoembolization predicts favorable outcome in hepatocellular carcinoma. Am J Cancer Res. 2021;11(10):4956–4965.
26. Han G, Berhane S, Toyoda H, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response-based approach. Hepatology. 2020;72(1):198–212. doi:10.1002/hep.31022
27. Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2261–2273. doi:10.1002/hep.26256
28. Kim BK, Shim JH, Kim SU, et al. Risk prediction for patients with hepatocellular carcinoma undergoing chemoembolization: development of a prediction model. Liver Int. 2016;36(1):92–99. doi:10.1111/liv.12865
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



