Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Development and Validation of a Prediction Model for Hepatitis B Virus-Related Hepatocellular Carcinoma Patients Receiving Postoperative Adjuvant Transarterial Chemoembolization
Authors Tu X , Zhang J , Li M, Lu F, Wang T, Gong W, Xiang B
Received 1 August 2023
Accepted for publication 5 October 2023
Published 24 October 2023 Volume 2023:10 Pages 1881—1895
DOI https://doi.org/10.2147/JHC.S422565
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Imam Waked
Xinyue Tu,1,* Jie Zhang,2,* Minjun Li,1 Fei Lu,3 Ting Wang,3 Wenfeng Gong,2 Bangde Xiang2
1Guangxi Medical University Cancer Hospital, Nanning, 530021, People’s Republic of China; 2Department of Hepatobiliary Surgery, Guangxi Medical University Cancer Hospital, Nanning, 530021, People’s Republic of China; 3Department of Radiotherapy, Guangxi Medical University Cancer Hospital, Nanning, 530021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bangde Xiang; Wenfeng Gong, Department of Hepatobiliary Surgery, Guangxi Medical University Cancer Hospital, No. 71 Hedi Road, Nanning, 530021, People’s Republic of China, Email [email protected]; [email protected]
Background: Hepatocellular carcinoma (HCC) patients who are at significant risk of tumor recurrence and mortality can benefit from postoperative adjuvant transarterial chemoembolization (PA-TACE). However, the benefits of PA-TACE remain unclear. Herein, we aimed to develop a model for predicting the prognosis of HBV-related patients who undergo PA-TACE and endeavored to guide individualized clinical treatment.
Methods: We included 432 HBV-related patients who underwent PA-TACE after curative resection were included. The dataset was divided into a training set (n=216) and an internal validation set (n=216). For identifying independent risk factors, the least absolute shrinkage and selection operator and univariate and multivariate Cox analyses were performed. We derived a prognostic model from the training set that was internally validated. The concordance index (C-index), receiver operating characteristic (ROC) curve, calibration curve, and risk stratification were used to evaluate the performance of the nomogram.
Results: Patients undergoing PA-TACE had significantly longer overall survival (OS) than those who did not undergo PA-TACE. Age, albumin levels, macrovascular invasion, tumor size, and, stages of Barcelona Clinic Liver Cancer were identified as independent risk variables and concluded into the nomogram to predict the OS of HBV-related patients who received PA-TACE. The nomogram’s C-index values OS were 0.710 and 0.652 in the training and internal validation sets, respectively. Both time-dependent AUC and the calibration curve showed good discrimination and model fitness. The risk score − 0.12 was kept as the cut-off value that would accurately divide patients into high-risk and low-risk groups; furthermore, the Kaplan–Meier curve showed a high discriminative ability of the model.
Conclusion: We developed a predictive model. comprising a formula and nomogram to predict the OS and provide risk stratification for HBV-related patients undergoing PA-TACE, which could contribute to suitable treatment options for this patient population.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, hepatitis B virus, nomogram, prognostic model
Introduction
Hepatocellular carcinoma (HCC), the most prevalent primary liver malignancy, accounts for 75–85% of all liver cancers. The International Agency for Research on Cancer (IARC) has estimated that the morbidity and mortality due to liver cancer ranks sixth and third among all tumors, respectively, thus significantly threatening people’s health.1,2 Infection with the hepatitis B virus (HBV) is a significant risk factor for HCC. HBV activates multiple signals, promotes viral replication and inflammatory progression, accelerates liver cancer development, and is correlated with a high mortality rate, especially in Asian China.3–5 Patients with HBV-associated HCC who have undergone liver resection have a poorer prognosis than those with metabolic HCC.6 Moreover, a nationwide survey found that HCC patients without HBV infection had a noticeably decreased probability of recurrence than those with HBV infection.7
With advancements in HCC management, significant emphasis is placed on customized therapy to improve the patient’s prognosis. The most effective treatment for HCC is thought to be curative therapy. Patients with early-stage HCC are more likely to benefit from hepatectomy and transplantation, possibly resulting in a higher survival rate.3,8 Nevertheless, researches have shown a 60–100% recurrence risk for HCC after hepatectomy. Furthermore, there is a considerable risk of complications with HCC liver resection, thereby resulting in a poor prognosis.9,10
The most effective palliative care method for patients with intermediate-stage liver cancer (Barcelona Clinic Liver Cancer [BCLC] grade B) is transarterial chemoembolization (TACE).8 TACE causes tumor necrosis by the combined effect of ischemia, hypoxia, and toxicity of chemotherapy drugs when vascular embolic agents and high-concentration chemotherapy drugs are injected into the blood vessels feeding the tumors. Moreover, it is widely used as an adjuvant therapy in combination with targeted drugs, ablation, transplantation, and surgery.11 It can significantly reduce the potential complications and recurrence rate.12,13 For HCC patients with bile duct tumor thrombus, PA-TACE had superior survival outcomes than hepatectomy alone.14 One retrospective research revealed that postoperative adjuvant treatment delayed intrahepatic recurrence and prolonged overall survival (OS) in patients with resectable, multiple HCCs larger than those specified in Milan criteria.15 Moreover, PA-TACE is an independent protective factor for large HCC patients after liver resection.16 Hence, we should take PA-TACE into consideration as a conventional therapy for HCC patients. Additionally, PA-TACE eliminates HBV in cancer cells and reduces viral burden, thereby marginally enhancing the OS of hepatitis B virus-associated HCC (HBV-HCC) patients.17 Nevertheless, in some patients, such as those with tumors that are less than 5 cm in diameter, single tumors, or MVI-negative tumors, PA-TACE does not confer better survival outcomes and may even encourage postoperative recurrence.18,19 Moreover, HCC patients with microscopic portal vein invasion do not significantly benefit from PA-TACE.20 Presently, no consensus or guidelines exist on the indications for PA-TACE, and the prognostic indicators for outcomes in patients undergoing PA-TACE remain unclear.21,22
According to a novel online calculator that predicts HCC patients’ overall survival after PA-TACE and some other research, several factors, including BCLC stages, tumor diameter, multinodular tumors, alpha-fetoprotein (AFP), and microvascular invasion (MVI), have been proven to be associated with the prognoses of these patients.18,23,24 Moreover, some models predicting recurrence after PA-TACE for HBV-HCC patients have been developed.25,26 Yet, there is no commendable evaluation system to predict the OS of HBV-HCC who have had PA-TACE directly; therefore, in certain situations, it is difficult for clinicians to decide on initiating PA-TACE, especially for individuals without any significant recurrence risk factors.
Thus, a reliable prediction system that aids clinicians in patient selection, thereby achieving better OS with PA-TACE is urgently needed. Herein, the aim of our project was to create and verify an original prediction model applying a nomogram and formula, which could identify high-risk and low-risk HBV-HCC patients treated with PA-TACE and predict their OS.
Methods
Patients
This retrospective analysis comprised 1820 consecutive patients at the Cancer Hospital Affiliated with Guangxi Medical University who received a diagnosis of HCC between 2012 and 2017. The inclusion criteria are as follows: (1) Patients diagnosed with HCC according to the principles of the authoritative guidelines through histological examination or clinicoradiological criteria; (2) patients infected with HBV; and (3) patients treated with curative liver resection as their initial therapy and receiving PA-TACE within 4 to 8 weeks. The exclusion criteria were: (1) Patients without curative resection confirmed by image; (2) Patients died during the perioperative period (within 90 days after operation); (3) Treated with other anti-tumor treatments after operation: radiotherapy, systemic therapy (TKI therapy, PD-1 immunotherapy.); (4) Incomplete critical baseline data; (5) Follow-up time less than 2 months. After the application of the abovementioned criteria, the final analysis covered 432 patients. These participants were split into the internal validation and derivation groups in a 1:1 ratio based on random numbers using the SPSS random function. The study was approved by the Ethical Review Committee of the Guangxi Medical University Cancer Hospital (LW2023094). It was conducted according to the ethical guidelines of the World Medical Association Declaration of Helsinki, and informed patient consent was obtained from all participants.
Treatment
All patients underwent curative hepatectomy after the completion of the preoperative examination. Each patient was reexamined 4 weeks postoperatively, and once the liver function recovered, after the surgeon’s evaluation, TACE was carried out on the residual liver. All patients were followed up regularly after treatment and various indexes were monitored, including physical examination, serum AFP level, ultrasound or enhanced CT scan or MRI of the chest and abdomen. If the patient tends to relapse, all imaging examinations should be carried out in time for early diagnosis. According to the recurrence of the tumor, the residual liver function reserve, and the general situation of patients, the treatment surgeon decides the subsequent management, such as re-resection, TACE, local ablation, radiotherapy, systemic treatment with oral sorafenib or the best supportive treatment.
Data Collection and Outcomes
Preoperatively, 28 baseline patient characteristics were examined, including AFP levels, serum biochemistry, liver function index, and BCLC and Edmondson stages. Preoperative imaging was performed routinely. Most factors were divided into two groups based on a recognized cut-off value. All parameter values were the first recorded data. Every patient was followed up regularly, and their health conditions were documented. The primary outcome was OS estimated from the date of HCC diagnosis to the date of death from any cause.
Statistical Analysis
Normally distributed data are displayed as mean±standard deviation, and skewed data are displayed as interquartile ranges. Categorical variables are presented as percentiles. For missing data, the numerical and categorical variables were imputed with an average value and mode, respectively. Mann–Whitney U-test or Student’s t-test to compare numerical variables between the derivation and internal validation sets was carried out. Furthermore, the Fisher’s exact test or the chi-squared test was used to evaluate categorical variables. Correlations between variables were assessed using Spearman correlation analysis and if the absolute value of the correlation coefficient between two variables is greater than 0.8, they are considered to be highly correlated. Lasso regression can considerably reduce multicollinearity among variables and reduce data dimension. Therefore, we used it to preliminarily screen variables associated with OS. The potential prognostic variables with a significant difference (P 0.05) were chosen using a univariate Cox proportional-hazards regression analysis. Next, the chosen covariates were included in the multivariate Cox regression. The final independent factors were screened out with backward stepwise selection based on the same standard of P value. Incorporating the relevant parameters, a nomogram was constructed to predict patients’ survival outcomes. Each patient could be scored using this nomogram model. Furthermore, X-tile software (Yale School of Medicine, USA)27 generated a cut-off value for risk categorization utilizing which the patients were separated into groups with high and low risk. The Kaplan–Meier (KM) analysis with Log rank test was used to compare OS differences between the two groups. The internal validation group was used to verify and test the model. The area under the curve (AUC) of the time-dependent receiver operating characteristic (ROC) was calculated to evaluate the model’s accuracy. The C-index was applied to estimate the model’s discrimination ability. Moreover, to assess the consistency between the predictions and observations, the calibration curve was employed, which is a visual representation of the Hosmer–Lemeshow goodness-of-fit test.
Data processing, including statistics of baseline information and univariate and multivariate Cox regression analyses, was performed using SPSS 25.0 (IBM Corporation, 2020, USA). Lasso regression analyses, nomogram construction, survival and calibration curve constructions, and time-dependent ROC calculations were performed using R version 3.6.2 (http://www.r-project.org/), with package dependencies: “rms”, “glmnet”, “survival”, “survminer”, and “timeROC”. A C-index and AUC >0.7 indicated a better predictive ability of the model. A two-tailed P value <0.05 was considered statistically significant.
Result
Patient Baseline Characteristic
The final analysis included 432 HBV-HCC patients who complied with the selection criteria. These participants were split in a 1:1 ratio between the derivation and validation groups (n=216, each group) (Figure 1). The baseline data comprised 28 preoperative indicators. The distribution of each character in the two groups did not significantly differ from one another (Table 1). The median OS of patients from the training and validation groups was 57 and 40 months, respectively, with no significant between-group difference seen on the KM curve (P=0.62, Figure 2A).
 |
Table 1 Baseline Characteristics of Study Patients |
 |
Figure 1 Flowchart of patient selection. |
 |
Figure 2 The difference in OS between the training and validation sets examined by Kaplan-Meier survival curves (A). Kaplan–Meier survival curves for PA-TACE’s impact on OS of the patients (B). |
Survival Benefits for Patients Undergoing PA-TACE
Patients who underwent PA-TACE are designated as the PA-TACE group, whereas patients who did not have PA-TACE are designated as the non-PA-TACE group. The KM curve evidently shows that patients who underwent PA-TACE had longer OS than those who did not undergo PA-TACE . Additionally, there was a tremendous prognostic difference between patients who received adjuvant therapy and those who did not (P<0.05). The median survival times of patients who underwent and did not undergo PA-TACE were 46 and 37 months, respectively. Meanwhile, the 1-, 3-, and 5-year OS rates were all significantly higher for patients in the PA-TACE group than for those in the non-PA-TACE group (Figure 2B).
Independent Prognostic Factors Selection
In the training set, the correlation between variables was analyzed and the heat map was depicted (Figure 3). Expect the WBC and neutrophils (r>0.8, p<0.05), there were no highly correlated variables. Then LASSO regression analysis was carried out, and LASSO coefficient profiles of the feature were shown. By applying the minimal criteria, the LASSO model’s optimum parameter (lambda) selection was determined. The curve of the partial likelihood deviance (binomial deviance) versus the log (lambda) is presented. By performing lambda.min and lambda.1se, dotted vertical lines are displayed at the ideal values. Lastly, sex, age, BCLC, prothrombin time (PT), albumin (ALB), AFP, size, capsule, macrovascular invasion, and tumor rupture were selected as the initial prognostic factors based on the optimal value that corresponds to the lowest value of lambda (Figure 4). And the corresponding coefficients derived from Lasso regression were as follows: 0.011, −0.283, 0.342, 0.074, −0.412, 0.069, 0.334, −0.011, 0.591, −0.018, which can reflect the influence of related variables on prognosis to some extent.
 |
Figure 3 The value of the correlation coefficient between variables is displayed in the form of a heat map, and the value is mainly expressed by color depth. |
 |
Figure 4 28 prognostic factors’ expression patterns (A). The LASSO model’s 10-fold cross-validation for selecting variables (B). |
The univariate Cox analysis included the abovementioned 10 prognostic factors that were identified by LASSO regression. Age, BCLC stage, ALB, AFP, macrovascular invasion, size, and capsule were statistically significant with minimal P values. Next, for the final variables, the multivariate Cox analysis was conducted, and age, BCLC stage, ALB, size, and macrovascular invasion were discovered to be independent prognostic factors of OS (Table 2).
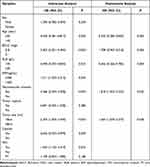 |
Table 2 Uni- and Multivariate Analysis for Overall Survival in the Derivation Cohort |
Development of the Model
A formula and a nomogram were created to more accurately predict the patients’ survival results after incorporating the abovementioned independent factors (Figure 5). The formula used to figure out each patient’s corresponding score is as follows: risk score = 0.535 × BCLC stage (0, 0-B; 1, C) + 0.598 × macrovascular invasion (0, negative; 1, positive) + 0.447 × maximal tumor diameter (MTD) (0, <8cm; 1, ≥8cm) – 0.562 × age (0, ≤45; 1, >45) – 0.786 × ALB (0, <35g/L; 1, ≥35g/L)
 |
Figure 5 Nomogram for predicting OS in HBV-HCC (hepatitis B virus-associated hepatocellular carcinoma) patients with PA-TACE. |
Risk Stratification Construction
According to the patient points from the training set, risk stratification was constructed. The ideal cut-off value −0.12, based on which patients can be divided into high-risk and low-risk categories, was determined using the X-tile program. Moreover, the KM curves of the OS of high-risk and low-risk groups for the training (Figure 6A) and the validation sets (Figure 6B) were plotted, respectively. In both sets, the low-risk group had a superior OS than the high-risk group, thereby demonstrating the excellent discriminative ability of the scoring system (P<0.05). Meanwhile, the patients without PA-TACE were also divided into high-risk and low-risk groups according to the same criteria, and the KM analysis was conducted between patients with and without PA-TACE. In the high-risk group, the OS of patients with PA-TACE is obviously better than that of patients without PA-TACE (P < 0.05). As depicted by the KM curve, patients with PA-TACE showed significant survival benefits, especially before 30 months. (Figure 6C) In the low-risk group, although the P value is less than 0.05, the curve shows that the survival difference between patients with and without PA-TACE is mild, and it becomes obvious only after 45 months. (Figure 6D)
Evaluation and Validation of the Model
The calibration curves show good agreement of OS rate between the model’s prediction and actual observation. The standard lines in the training set nearly overlap with the calibration curves of the 3 – (Figure 7A) and 5- (Figure 7B) year OS rates. Additionally, an accurate C-index of risk score was estimated through 500 bootstraps resampling, and the C-indices of the risk scores were 0.710 [95% CI, 0.6555–0.755] and 0.652 [95% CI, 0.613–0.691] for the training and the validation sets, respectively. Furthermore, time-dependent ROC curves of the risk score system were plotted to verify the model’s prediction ability. The AUCs for the 1-, 3-, and 5-year survival rates were 0.74, 0.79, and 0.84, respectively, in the training set (Figure 7C). Similarly, the AUCs for the 1-, 3-, and 5-year survival rates were 0.74, 0.68, and 0.66, respectively (Figure 7D).
Discussion
The decision of whether a patient should undergo PA-TACE remains controversial. Although many scoring systems and models have been developed, presently there is no consensus on the indications for PA-TACE. Liang et al created a cutting-edge online tool to assess the survival advantage of HCC patients with PA-TACE by constructing two nomograms to estimate the survival of patients who undergo and those who do not undergo PA-TACE, respectively.24 Additionally, many studies have established relevant models to predict tumor recurrence in HBV-HCC patients after PA-TACE,25,26 A prognostic model that can predict early recurrence in HCC patients after PA-TACE has also been constructed.28 Nevertheless, there is no model for predicting the OS and survival outcomes of HBV-HCC patients. Herein, to accurately predict HBV-HCC patients’ OS after PA-TACE, a model was constructed. Compared with previous models, our model is more targeted, mainly focuses on HBV-positive patients, and provides more meaningful information on their survival. The model was also exceptionally capable of distinguishing high-risk groups from low-risk groups. The corresponding formula for risk stratification can directly calculate a patient’s score, and the nomogram can be utilized for predicting patients’ 1-, 3-, and 5-year survival rates, which can aid clinicians in better evaluating whether patients need PA-TACE.
In this study, the cut-off value of age was 45 years, and this was incorporated into the model. Patients aged >45 years are more likely to benefit from PA-TACE and may show better OS after PA-TACE. This is the initial study to demonstrate that age may be a critical variable in assessing survival after PA-TACE in HCC-HBV patients, although, one study has demonstrated the association of age with OS after liver resection of HCC in octogenarian patients.29 From a molecular perspective, age-dependent NFR2 expression levels also affect the prognosis of HCC patients.30 Zeng et al’s study found that in 699 young patients (age 40 years) with HBV-HCC who had radical resection, tumor size and macrovascular invasion were shown to be linked with poor OS. Another study showed that younger patients had better liver function but worse prognoses than older patients.31 Furthermore, In elderly individuals diagnosed with HCC, drug-eluting bead transarterial chemoembolization (DEB-TACE) is well tolerated.32 However, further studies have yet to explore how different age groups affect the outcome of PA-TACE in HBV-HCC patients.
ALB level is a crucial indicator for assessing liver function and is a component of the Child–Pugh score. ALB level when combined with other indicators is an essential and stable prognostic marker for HCC.33 In the random survival forest model developed by Lin et al, ALB level was a reliable indicator of survival in BCLC-B HCC patients.34 Moreover, postoperative ALB levels are utilized as well to evaluate a patient’s prognosis for HCC. The survival rate of HCC patients after hepatectomy is mainly related to tumor characteristics and ALB levels.35 For TACE therapy, abnormal ALB level is a potential prognostic factor for HCC patients after conventional TACE.36 Li et al found that HCC patients who had ALB levels ≤ 35 g/L were at risk for extrahepatic progression following TACE, and early combination treatment was strongly advised for them.35 Besides, In Chinese HCC patients, CalliSpheres DEB-TACE has been proven effective and well-tolerated, and ALB level abnormality is independently associated with poor prognosis.37,38
A critical tumor characteristic is macrovascular invasion, which includes invasion of the portal vein, hepatic vein, and inferior vena cava. Previous studies have suggested that macrovascular invasion can be used as an independent predictor of OS after PA-TACE, which can be used to individually estimate the net survival benefit of PA-TACE.24 Ji et al found that combination therapy may prolong the survival of HCC patients with portal vein tumor thrombus.39 We found that macrovascular invasion is also an independent OS prediction in PA-TACE, which agrees with the findings of earlier researches.
In our study, 8 cm was kept as the cut-off value for the MTD. As expected, MTD was found to be a critical variable in our prognostic model. The correlation between tumor size and prognosis has been verified in multiple studies and centers, and our results are consistent with those of most studies. Tumor size is essential in predicting HBV-HCC recurrence after PA-TACE.25 A previous study showed that multiple diameter thresholds, such as 2 cm, 3 cm, 4 cm, 5 cm, 8 cm, and 10 cm, showed strong prognostic differentiators for HCC.40,41 Carr et al discovered that HCC characteristics might transform into a more aggressive phenotype as the tumor grows larger. With higher MTD categories, there was a rise in the proportion of patients with multifocal tumors, portal vein tumor thrombus, and median serum AFP levels. Patients were grouped according to MTDs of 2.1–5.0 cm, 5.1–8.0 cm, and 8.1–11 cm. Compared with patients of different groups, with an increase in the MTD, the OS of the three-parameter group decreased significantly.42,43
The most used staging and therapy methodology for HCC is the BCLC system, which is also regarded as an essential prognostic predictor for OS.17 Hu et al indicated that HCC patients diagnosed with BCLC-B stage might benefit from PA-TACE after liver resection.44 Therefore, when performing the multivariate Cox regression analysis, the P value conditions were relaxed. BCLC stage was also incorporated into the model, with a P value of 0.065. We found that PA-TACE may be beneficial for BCLC stage 0-B patients, and this outcome is in accordance with previous research.
In this research, we were committed to screening out patients who can benefit from PA-TACE. However, it’s critical to take other treatments into consideration combined with TACE as well.45 Zhong et al have found that TACE combined with immune checkpoint inhibitors plus tyrosine kinase inhibitors significantly improved OS, over immune checkpoint inhibitors plus tyrosine kinase inhibitors for advanced HCC.46 Furthermore, lenvatinib combined with anti-PD-1 antibodies plus TACE for neoadjuvant treatment of resectable hepatocellular carcinoma provided better survival outcomes than surgery alone.47 Thus, in clinical practice, in addition to PA-TACE, perhaps we can also give some other adjuvant therapies to provide more comprehensive treatment to improve the prognosis of patients, which need further exploration.
Our study has some limitations. The model was only internally validated and lacks external validation. To refine the model and make it more convincing, further external validation and multi-center, prospective cohort, clinical studies are needed. Furthermore, the patient’s postoperative clinical data and HBV-DNA load can be taken into consideration.
Conclusion
In summary, A clinical prognostic model based on age, tumor size, macrovascular invasion, ALB, and BCLC stage was created and validated by our team. The formula and nomogram provided an understandable clinical tool for clinicians to create reasonable and personalized postoperative treatment plans.
Abbreviations
PA-TACE, Postoperative adjuvant transarterial chemoembolization; HCC, hepatocellular carcinoma; C-index, concordance index; ROC, receiver operating characteristic; OS, overall survival; HBV, Hepatitis B virus; TACE, transarterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer; HBV-HCC, hepatitis B virus-associated HCC; AFP, alpha-fetoprotein; MVI, microvascular invasion; KM, Kaplan–Meier; AUC, area under the curve; DEB-TACE, transarterial chemoembolization; PT, prothrombin time; ALB, albumin; MTD, maximal tumor diameter.
Ethics Approval and Informed Consent
The Guangxi Medical University Cancer Hospital’s Ethical Review Committee gave the study its blessing (LW2023094). All subjects gave their informed permission and the ethical guidelines outlined in the Declaration of Helsinki were followed during the study’s execution.
Acknowledgments
We appreciate Bullet Edits Limited for the linguistic editing and proofreading of the original manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by Youth Science Foundation of Guangxi Medical University (GXMUYSF202346), Youth Program of Scientific Research Foundation of Guangxi Medical University Cancer Hospital (2021-08), National Natural Science Foundation of China (81960450) and Medical Science Research Fund of Beijing Medical And Health Foundation (YWJKJJHKYJJ-1322016).
Disclosure
The authors report no conflicting interests in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
3. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):7. doi:10.1038/s41572-021-00245-6
4. Papatheodoridi M, Tampaki M, Lok AS, Papatheodoridis GV. Risk of HBV reactivation during therapies for HCC: a systematic review. Hepatology. 2022;75(5):1257–1274. doi:10.1002/hep.32241
5. Jiang Y, Han QJ, Zhang J. Hepatocellular carcinoma: mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25(25):3151–3167. doi:10.3748/wjg.v25.i25.3151
6. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi:10.1002/hep.31288
7. Utsunomiya T, Shimada M, Kudo M, et al. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg. 2015;261(3):513–520. doi:10.1097/SLA.0000000000000821
8. Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13(2):125–137. doi:10.1007/s12072-018-9919-1
9. Katz SC, Shia J, Liau KH, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249(4):617–623. doi:10.1097/SLA.0b013e31819ed22f
10. Roayaie S, Obeidat K, Sposito C, et al. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57(4):1426–1435. doi:10.1002/hep.25832
11. Zhong BY, Jin ZC, Chen JJ, Zhu HD, Zhu XL. Role of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Clin Transl Hepatol. 2023;11(2):480–489. doi:10.14218/JCTH.2022.00293
12. Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi:10.1016/j.ctrv.2018.11.002
13. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
14. Chen ZH, Feng JK, Sun JX, et al. Postoperative adjuvant transarterial chemoembolization improves outcomes of hepatocellular carcinoma associated with bile duct tumor thrombus: a propensity score matching analysis. HPB. 2022;24(4):547–557. doi:10.1016/j.hpb.2021.09.005
15. Dong ZR, Zhang PF, Wang CH, et al. Postoperative adjuvant transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond the Milan criteria: a retrospective analysis. Am J Cancer Res. 2015;5(1):450–457.
16. Wang H, Yu H, Qian YW, Cao ZY, Wu MC, Cong WM. Postoperative adjuvant transcatheter arterial chemoembolization improves the prognosis of patients with huge hepatocellular carcinoma. Hepatobil Pancreat Dis Int. 2021;20(3):232–239. doi:10.1016/j.hbpd.2020.12.018
17. Chen MY, Juengpanich S, Hu JH, et al. Prognostic factors and predictors of postoperative adjuvant transcatheter arterial chemoembolization benefit in patients with resected hepatocellular carcinoma. World J Gastroenterol. 2020;26(10):1042–1055. doi:10.3748/wjg.v26.i10.1042
18. Chen W, Ma T, Zhang J, et al. A systematic review and meta-analysis of adjuvant transarterial chemoembolization after curative resection for patients with hepatocellular carcinoma. HPB. 2020;22(6):795–808. doi:10.1016/j.hpb.2019.12.013
19. Wang H, Du PC, Wu MC, Cong WM. Postoperative adjuvant transarterial chemoembolization for multinodular hepatocellular carcinoma within the Barcelona Clinic Liver Cancer early stage and microvascular invasion. Hepatobiliary Surg Nutr. 2018;7(6):418–428. doi:10.21037/hbsn.2018.09.05
20. Qiu Y, Yang Y, Wang T, Shen S, Wang W. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microscopic portal vein invasion. Front Oncol. 2022;12:831614. doi:10.3389/fonc.2022.831614
21. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
22. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
23. Sun JJ, Wang K, Zhang CZ, et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol. 2016;23(4):1344–1351. doi:10.1245/s10434-015-5008-z
24. Liang L, Li C, Wang MD, et al. Development and validation of a novel online calculator for estimating survival benefit of adjuvant transcatheter arterial chemoembolization in patients undergoing surgery for hepatocellular carcinoma. J Hematol Oncol. 2021;14(1):165. doi:10.1186/s13045-021-01180-5
25. Zou Y, Chen Z, Lou Q, et al. A novel blood index-based model to predict hepatitis B virus-associated hepatocellular carcinoma recurrence after curative hepatectomy: guidance on adjuvant transcatheter arterial chemoembolization choice. Front Oncol. 2021;11:755235. doi:10.3389/fonc.2021.755235
26. Huang J, Liu FC, Li L, et al. Prognostic nomogram for hepatitis B virus-related hepatocellular carcinoma with adjuvant transarterial chemoembolization after radical resection. Am J Clin Oncol. 2020;43(1):20–27. doi:10.1097/COC.0000000000000619
27. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
28. Mao S, Shan Y, Yu X, et al. A new prognostic model predicting hepatocellular carcinoma early recurrence in patients with microvascular invasion who received postoperative adjuvant transcatheter arterial chemoembolization. Eur J Surg Oncol. 2022;49(1):129–136. doi:10.1016/j.ejso.2022.08.013
29. Tan LLY, Chew VTW, Syn N, et al. Effect of age on the short- and long-term outcomes of patients undergoing curative liver resection for HCC. Eur J Surg Oncol. 2022;48(6):1339–1347. doi:10.1016/j.ejso.2021.12.027
30. Atyah M, Zhou C, Zhou Q, et al. The age-specific features and clinical significance of NRF2 and MAPK10 expression in HCC patients. Int J Gen Med. 2022;15:737–748. doi:10.2147/IJGM.S351263
31. Zeng J, Lin K, Liu H, et al. Prognosis factors of young patients undergoing curative resection for hepatitis B virus-related hepatocellular carcinoma: a multicenter study. Cancer Manag Res. 2020;12:6597–6606. doi:10.2147/CMAR.S261368
32. Yang Q, Jin X, Ye F, et al. Safety and efficacy analysis of DEB-TACE treatment in elderly patients with hepatocellular carcinoma: a comparative cohort study. Oncol Res. 2018. doi:10.3727/096504018X15223171140640
33. Pang S, Zhou Z, Yu X, et al. The predictive value of integrated inflammation scores in the survival of patients with resected hepatocellular carcinoma: a Retrospective Cohort Study. Int J Surg. 2017;42:170–177. doi:10.1016/j.ijsu.2017.04.018
34. Lin H, Zeng L, Yang J, Hu W, Zhu Y. A machine learning-based model to predict survival after transarterial chemoembolization for BCLC stage B hepatocellular carcinoma. Front Oncol. 2021;11:608260. doi:10.3389/fonc.2021.608260
35. Li S, Wang Q, Mei J, et al. Risk factors of extra-hepatic progression after transarterial chemoembolization for hepatocellular carcinoma patients: a retrospective study in 654 cases. J Cancer. 2019;10(20):5007–5014. doi:10.7150/jca.35355
36. Chen C, Qiu H, Yao Y, et al. Comprehensive predictive factors for CalliSpheres® microspheres (CSM) drug-eluting bead-transarterial chemoembolization and conventional transarterial chemoembolization on treatment response and survival in hepatocellular carcinoma patients. Clin Res Hepatol Gastroenterol. 2021;45(2):101460. doi:10.1016/j.clinre.2020.05.008
37. Peng Z, Cao G, Hou Q, et al. The comprehensive analysis of efficacy and safety of callispheres(®) drug-eluting beads transarterial chemoembolization in 367 liver cancer patients: a multiple-center, cohort study. Oncol Res. 2020;28(3):249–271. doi:10.3727/096504019X15766663541105
38. Sun J, Zhou G, Xie X, et al. Efficacy and safety of drug-eluting beads transarterial chemoembolization by CalliSpheres(®) in 275 hepatocellular carcinoma patients: results from the Chinese CalliSpheres(®) Transarterial Chemoembolization in Liver Cancer (CTILC) study. Oncol Res. 2020;28(1):75–94. doi:10.3727/096504019X15662966719585
39. Ji M, Zou H, Shu B, et al. Prognostic analysis of hepatocellular carcinoma with macrovascular invasion after liver resection and a successful case of conversion therapy. Front Surg. 2022;9:1042431. doi:10.3389/fsurg.2022.1042431
40. Liu H, Yang Y, Chen C, et al. Reclassification of tumor size for solitary HBV-related hepatocellular carcinoma by minimum p value method: a large retrospective study. World J Surg Oncol. 2020;18(1):185. doi:10.1186/s12957-020-01963-z
41. Zhang H, Yuan SX, Dai SY, et al. Tumor size does not independently affect long-term survival after curative resection of solitary hepatocellular carcinoma without macroscopic vascular invasion. World J Surg. 2014;38(4):947–957. doi:10.1007/s00268-013-2365-2
42. Carr BI, Guerra V, Donghia R, et al. Changes in hepatocellular carcinoma aggressiveness characteristics with an increase in tumor diameter. Int J Biol Markers. 2021;36(1):54–61. doi:10.1177/1724600821996372
43. Carr BI, Guerra V, Donghia R, et al. Identification of clinical phenotypes and related survival in patients with large HCCs. Cancers. 2021;13(4):592. doi:10.3390/cancers13040592
44. Hu S, Gan W, Qiao L, et al. a new prognostic algorithm predicting HCC recurrence in patients with Barcelona clinic liver cancer stage B who received PA-TACE. Front Oncol. 2021;11:742630. doi:10.3389/fonc.2021.742630
45. Singal AG, Kudo M, Bruix J. Breakthroughs in hepatocellular carcinoma therapies. Clin Gastroenterol Hepatol. 2023;21(8):2135–2149. doi:10.1016/j.cgh.2023.01.039
46. Huang JT, Zhong BY, Jiang N, et al. Transarterial chemoembolization combined with immune checkpoint inhibitors plus tyrosine kinase inhibitors versus immune checkpoint inhibitors plus tyrosine kinase inhibitors for advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:1217–1228. doi:10.2147/JHC.S386672
47. Wu JY, Wu JY, Li YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for neoadjuvant treatment of resectable hepatocellular carcinoma with high risk of recurrence: a multicenter retrospective study. Front Oncol. 2022;12:985380. doi:10.3389/fonc.2022.985380
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


