Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Histological Severity of Cirrhosis Influences Surgical Outcomes of Hepatocellular Carcinoma After Curative Hepatectomy
Authors Liang BY, Gu J, Xiong M, Zhang EL, Zhang ZY, Lau WY, Wang SF, Guan Y, Chen XP, Huang ZY
Received 28 March 2022
Accepted for publication 7 July 2022
Published 23 July 2022 Volume 2022:9 Pages 633—647
DOI https://doi.org/10.2147/JHC.S368302
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ahmed Kaseb
Bin-Yong Liang,1,* Jin Gu,2,* Min Xiong,3 Er-Lei Zhang,1 Zun-Yi Zhang,1 Wan-Yee Lau,4 Shao-Fa Wang,5 Yan Guan,1 Xiao-Ping Chen,1 Zhi-Yong Huang1
1Hepatic Surgery Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Department of Hepatobiliary and Pancreatic Surgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, People’s Republic of China; 3Department of Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 4Faculty of Medicine, the Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, New Territories, Hong Kong SAR, People’s Republic of China; 5Institute of Organ Transplantation, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhi-Yong Huang, Hepatic Surgery Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095 Jie Fang Da Dao, Wuhan, People’s Republic of China, Tel +86-27-83665392, Fax +86-27-83663432, Email [email protected]
Background: Hepatocellular carcinoma (HCC) is frequently associated with cirrhosis. The present study investigated the impact of histological severity of cirrhosis on surgical outcomes for HCC and further developed novel nomograms to predict postoperative recurrence and survival.
Methods: A total of 1524 consecutive patients undergoing curative hepatectomy for HCC between 1999 and 2015 were retrospectively studied. Cirrhotic severity was histologically staged according to the Laennec staging system. Short- and long-term outcomes were investigated. Recurrence-free survival (RFS) and overall survival (OS) predictive nomograms were constructed based on the results of multivariate analysis. The predictive accuracy of the nomograms was measured by the concordance index (C-index) and calibration.
Results: Patients in the severe cirrhosis group had significantly higher morbidity and mortality rates than patients in the no, mild, and moderate cirrhosis groups. The 5-year RFS and OS rates were 36.8% and 64.5%, respectively, in the no cirrhosis group, compared to 34.8% and 60.4% in the mild cirrhosis group, 17.3% and 43.4% in the moderate cirrhosis group, and 6.1% and 20.1% in the severe cirrhosis group. Long-term survival outcomes were significantly worse as cirrhotic severity was increased. The C-index was 0.727 for the RFS nomogram and 0.746 for the OS nomogram. Calibration curves showed good agreement between actual observations and nomogram predictions. The 2 nomograms had a superior discriminatory ability to predict RFS and OS compared to other staging systems.
Conclusion: Histological severity of cirrhosis significantly affected surgical outcomes in HCC patients undergoing curative hepatectomy. The novel nomograms, including histological severity of cirrhosis, showed an accurate prediction of postoperative recurrence and survival.
Keywords: hepatocellular carcinoma, Laennec staging system, prognosis, nomogram, hepatectomy
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy worldwide and is a leading cause of cancer-related death in cirrhotic patients.1 Liver resection (LR) is the primary curative treatment for HCC. Improvements in surgical techniques, preoperative evaluation, and perioperative management have significantly improved the perioperative outcomes of patients after LR. Unfortunately, the HCC recurrence rate remains high.2,3
Greater than 90% of HCC cases occur in the background of chronic liver disease.4 Cirrhosis from any etiology, including infection by hepatitis B virus (HBV) or hepatitis C virus (HCV), chronic alcohol consumption, non-alcoholic steatohepatitis, and aflatoxin, is the strongest risk factor for HCC.5,6 HCC and cirrhosis affect the prognosis of these patients.7,8 The cirrhotic liver remnant may develop multicentric HCC recurrences after LR, and the cirrhotic background may progress to liver-related deaths due to liver failure or complications of portal hypertension. Previous studies indicated that cirrhosis adversely affected the long-term outcomes after LR.9 However, these studies simply regarded cirrhosis as a one-stage condition and ignored differences in its histological severity. Histologically, cirrhosis is defined as a diffuse state in which the normal lobular architecture is replaced by abnormal nodules and fibrous septa.10 The Laennec staging system divides cirrhosis into 3 stages (F4A, F4B, and F4C) based on the fibrous septal thickness and nodule size.11 Histological subclassification of cirrhosis using the Laennec staging system correlates with the grade of portal hypertension12 and the development of liver-related events, which include HCC and hepatic decompensation.13 However, liver surgeons do not pay much attention to the histological severity of cirrhosis except to evaluate liver functional reserve. Several recent studies demonstrated that the histological severity of cirrhosis was very useful in predicting prognosis in HCC patients with cirrhosis after LR.14,15 However, these studies did not draw a firm conclusion due to small sample sizes and short follow-up periods.
Nomograms derived from regression coefficients of all independent variables have been used as a straightforward tool to predict the survival of patients with various types of malignancies.16–18 However, no predictive nomogram including the histological severity of cirrhosis was constructed in HCC patients.
The present study emphasized the correlation between the histological severity of cirrhosis and surgical outcomes in HCC patients after LR. We also constructed novel prognostic nomograms that integrated the histological severity of cirrhosis and other independent risk factors to predict the possibility of tumor recurrence and survival.
Materials and Methods
Patients
The present study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the medical ethics committee of Tongji Hospital, Huazhong University of Science and Technology, China. Written informed consent was obtained from each patient in the study for the use of his or her data in clinical research. The clinicopathological data of consecutive patients with HCC who underwent curative LR between 1999 and 2015 were obtained from the computerized database maintained by Tongji Hospital. Portal hypertension, as defined by the presence of esophageal varices on endoscopy or splenomegaly with a platelet count <100×109/L, was not a contraindication to LR.19 Hospital mortality was defined as death during the postoperative hospital stay. Postoperative complications were defined using the Dindo-Clavien classification, and major complications were defined as grade 3 and above.20 Major hepatectomy was defined as resection of ≥3 Couinaud liver segments.21
Operative Technique
The surgeries were performed as described previously.22,23 Briefly, LR was performed via a right subcostal incision with an upward midline extension and a left subcostal extension in some patients. Operative ultrasonography was used routinely to locate the tumor and mark the transection plane. The liver was initially mobilized before hepatic parenchymal transection in most patients. The anterior approach with or without the hanging technique was used in the remaining patients. Liver transection was performed using a Cavitron ultrasonic surgical aspirator (CUSA), Biclamp, Harmonic Scalpel, or TissueLink, in addition to finger fracturing and clamp crushing. Pringle’s maneuver was only used when there was excessive bleeding during liver transection.
Histological Subclassification of Cirrhosis
Cirrhosis was staged using the Laennec staging system.11,12 Specimens were collected from the patients, and histopathological slides were prepared. Two liver pathologists who were blinded to the clinical data independently re-evaluated the slides from each patient for this study. Any disagreement on the cirrhosis staging between the 2 pathologists was discussed between them until a consensus was reached. The livers were histologically diagnosed as cirrhotic (F4) or non-cirrhotic (F0-F3). The cirrhotic livers (F4) were staged by the thickness of the fibrous septa as follows: F4A, mild cirrhosis, definite or probable; F4B, moderate cirrhosis (at least 2 broad septa); and F4C, severe cirrhosis (at least 1 very broad septum or many minute nodules).
Follow-Up
All patients were evaluated using serum alpha-fetoprotein (AFP), ultrasonography or computed tomography, and chest X-ray 1 month after surgery. Patients were followed up once every 2 months for the first 2 years and once every 3 months thereafter. Further magnetic resonance imaging, bone scans, or positron emission tomography were performed when tumor recurrence was suspected. Patients with tumor recurrence were actively treated with repeat resection, microwave or radiofrequency ablation, ethanol injection, transarterial chemoembolization (TACE), radiotherapy, or oral sorafenib depending on the general condition of the patients, HCC recurrence pattern, and liver functional status. Overall survival (OS) was calculated from the date of LR to the date of death or last follow-up. Recurrence-free survival (RFS) was calculated from the date of LR to the date of recurrence, death, or last follow-up.
Statistical Analysis
Statistical analyses were performed using SPSS 20.0 statistical software (SPSS, Inc., Chicago, IL, USA) and R version 4.0.0 for Windows (http://www.r-project.org). Categorical variables are reported as numbers (n) and proportions (%) and were compared using the chi-squared test with Yates’ correction or Fisher’s exact test, as appropriate. Continuous variables are reported as medians and ranges and were compared using the Kruskal-Wallis H-test or the Mann-Whitney U-test, as appropriate. The RFS and OS were calculated using the Kaplan-Meier method and compared using the Log rank test. The Cox proportional hazards model was used to identify risk factors associated with RFS and OS using univariate and multivariate analyses.
The nomograms for RFS and OS were formulated based on the results of multivariate analysis using the rms package in R version 4.0.0. To use the nomogram, the value of an individual patient is located on each variable axis, and a line is drawn upward to determine the number of points received for each variable value. The sum of these numbers is located on the total points axis, and a line is drawn downward to the survival axes to determine the likelihoods of 3- and 5-year RFS and 3- and 5-year OS. The predictive performance of the nomograms was measured using the concordance index (C-index) and Kaplan-Meier curves of the tertiles of predictions. Performance was further evaluated using a calibration curve that compared the nomogram-predicted survival probability with the observed Kaplan–Meier estimates of survival probability. Internal validation was performed using bootstraps with 1000 resamples. The discriminatory powers of the nomograms were also compared with 2 current staging systems, the American Joint Committee on Cancer (TNM) staging system24 and the Barcelona Clinic Liver Cancer (BCLC) staging system,19 using the C-index. Based on the constructed nomogram, each patient obtained a nomogram score for risk stratification of recurrence or postoperative mortality. Patients were divided into 3 different risk groups using the tertiles of the nomogram scores as the cut-off points. P <0.05 was considered statistically significant for all analyses.
Results
Baseline Characteristics
There were 1524 patients in this study. According to the Laennec staging system, 220 patients were without cirrhosis, 575 patients were diagnosed with mild cirrhosis, 597 patients had moderate cirrhosis, and the remaining 132 patients had severe cirrhosis. The clinicopathological data of each group (no, mild, moderate, and severe cirrhosis) are shown in Table 1. There were no significant differences in the year of hepatectomy, age, sex, serum AFP level, vascular invasion, tumor differentiation, or proportion of patients within Milan criteria between the 4 groups (P >0.05). The no cirrhosis group had significantly larger tumor sizes (P <0.001), a greater proportion of patients with a single tumor (P = 0.006), and a smaller proportion of patients with positive surface antigen of HBV (HBsAg) (P <0.001) than the other 3 groups. Compared to patients in the other 3 groups, patients in the no cirrhosis group had lower levels of alanine aminotransferase (ALT) (P = 0.004), total bilirubin (TBIL) (P < 0.001), prothrombin time (PT) (P < 0.001), and platelets (PLT) (P <0.001), and higher levels of albumin (ALB) (P < 0.001) and a lower proportion of patients with Child-Pugh class A liver function (P < 0.001). The frequencies of patients with portal hypertension in the mild, moderate, and severe cirrhosis groups were 11.8%, 34.2%, and 77.3%, respectively. An increased frequency of portal hypertension was observed as the histological severity of cirrhosis was increased (P < 0.001).
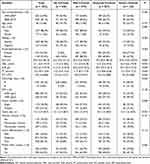 |
Table 1 Baseline Characteristics |
Perioperative Outcomes
The detailed perioperative data are summarized in Table 2. The proportion of major hepatectomy in the no cirrhosis group was higher than the other 3 groups (P = 0.011). In contrast, patients in the severe cirrhosis group had significantly higher transfusion rates (P <0.001), higher major complication rates (P <0.001), and higher postoperative mortality rates (P <0.001) than patients in the other 3 groups. Compared with the other 3 groups, the proportion of patients with intraoperative blood loss >1000 mL was also higher in the severe group, but significant differences were not demonstrated (P = 0.361).
 |
Table 2 Correlation Between Histological Severity of Cirrhosis and Perioperative Outcomes |
Long-Term Outcomes
For all patients, the 1-, 3-, 5-, 7-, and 10-year RFS rates were 53.6%, 36.3%, 26.6%, 22.1%, and 16.0%, respectively. The corresponding OS rates were 80.7%, 62.5%, 51.8%, 40.5%, and 31.6%, respectively (Figure 1A and B). After patients were stratified by the histological severity of cirrhosis, the 5-year RFS and OS rates were 36.8% and 64.5%, respectively, in the no cirrhosis group, 34.8% and 60.4% in the mild cirrhosis group, 17.3% and 43.4% in the moderate cirrhosis group, and 6.1% and 20.1% in the severe cirrhosis group, respectively (Figure 1C and D). The RFS and OS rates did not differ significantly between the no and mild cirrhosis groups (P = 0.447 and 0.355, respectively), but patients in the mild cirrhosis group had significantly better RFS and OS rates than patients in the moderate and severe cirrhosis groups (P <0.001). Patients in the moderate cirrhosis group had significantly better RFS and OS rates than patients in the severe cirrhosis group (P <0.001).
The 5-year RFS rates in patients without portal hypertension were 36.8%, 38.5%, 21.9%, and 8.7% in the no, mild, moderate, and severe cirrhosis groups, respectively (Figure 2A). The 5-year OS rates were 64.5%, 64.7%, 42.9%, and 23.1% in the no, mild, moderate, and severe cirrhosis groups, respectively (Figure 2B). Patients in the no and mild cirrhosis groups had significantly better RFS and OS rates than patients in the moderate and severe cirrhosis groups (P ≤0.01). However, no significant differences were observed in the RFS and OS rates between the moderate and severe cirrhosis groups (P = 0.484 and 0.179, respectively) or between the no and mild cirrhosis groups (P = 0.974 and 0.911, respectively). The 5-year RFS rates in patients with portal hypertension were 10.4%, 10.1%, and 5.0% in the mild, moderate, and severe cirrhosis groups, respectively (Figure 2C). The 5-year OS rates were 31.0%, 44.0%, and 17.6% in the mild, moderate, and severe cirrhosis groups, respectively (Figure 2D). Patients in the severe cirrhosis group had significantly worse RFS and OS rates than patients in the mild and moderate cirrhosis groups (P ≤0.01). However, no significant differences were observed in the RFS and OS rates between the mild and moderate cirrhosis groups (P = 0.232 and 0.480, respectively).
The 5-year RFS rates in patients without portal hypertension and with tumors within the Milan criteria were 63.7%, 58.9%, 36.5%, and 16.7% in the no, mild, moderate, and severe cirrhosis groups, respectively (Figure 3A). The 5-year OS rates were 79.1%, 83.3%, 52.6%, and 33.3% in the no, mild, moderate, and severe cirrhosis groups, respectively (Figure 3B). Patients in the no and mild cirrhosis groups had significantly better RFS and OS rates than patients in the moderate and severe cirrhosis groups (P <0.05). However, no significant differences were observed in the RFS and OS rates between the no and mild cirrhosis groups (Ps = 0.651 and 0.749, respectively) or between the moderate and severe cirrhosis groups (Ps = 0.829 and 0.311, respectively). The 5-year RFS rates in patients with portal hypertension and with tumors within the Milan criteria were 17.0%, 16.5%, and 8.0% in the mild, moderate, and severe cirrhosis groups, respectively (Figure 3C). The 5-year OS rates were 44.4%, 48.9%, and 25.1% in the mild, moderate, and severe cirrhosis groups, respectively (Figure 3D). Patients in the mild and moderate cirrhosis groups had significantly better RFS and OS rates than patients in the severe cirrhosis group (P <0.05). However, no significant differences were observed in the RFS and OS rates between the mild and moderate cirrhosis groups (Ps = 0.619 and 0.796, respectively).
Prognostic Factors for RFS and OS
To identify independent prognostic factors for RFS and OS, Cox univariate and multivariate regression analyses were performed. The variables selected included the year of hepatectomy, age, sex, HBsAg, portal hypertension, Child-Pugh class, histological severity of cirrhosis, ALT level, ALB level, TBIL level, PT, AFP level, tumor number, tumor size, vascular invasion, tumor differentiation, extent of hepatectomy, and intraoperative blood transfusion (Table 3). Significant risk factors (P <0.05) identified by univariate analysis were entered into the Cox multivariate analysis. The results showed that HBsAg, portal hypertension, histological severity of cirrhosis, AFP level, tumor number, tumor size, vascular invasion, tumor differentiation, and intraoperative blood transfusion were independent risk factors associated with RFS and OS (Table 4).
 |
Table 3 Univariate Analysis of Risk Factors for Recurrence-Free and Overall Survival |
 |
Table 4 Independent Prognostic Factors for Recurrence-Free and Overall Survival Using Multivariate Analysis |
Construction and Validation of the Histological Severity of Cirrhosis-Based Nomograms for RFS and OS
We used these 9 independent prognostic factors to construct predictive RFS (Figure 4A) and OS nomograms (Figure 4B). The C-indexes of the RFS and OS nomograms were 0.727 (95% CI, 0.711–0.743) and 0.746 (95% CI, 0.726–0.766), respectively, with 1000 cycles of bootstrapping. Calibration curves based on the independent prognostic factors are shown in Figure 5. There was good agreement between the actual observations and nomogram-predicted probabilities for 3- and 5-year RFS and 3- and 5-year OS.
Comparison of Predictive Powers of Nomograms with Conventional Staging Systems
The discriminatory powers of the RFS- and OS-nomograms were compared with TNM stage and BCLC stage. Our nomograms showed better discriminatory powers in predicting postoperative RFS and OS than the competing models. The C-index of the RFS nomogram (0.727) was significantly higher than TNM and BCLC staging (0.643 for TNM stage and 0.633 for BCLC stage, P <0.001 for all). The C-index of the OS nomogram (0.746) was significantly higher than the TNM stage and BCLC stage (0.66 for TNM stage and 0.648 for BCLC stage, P <0.001 for all).
Performance of Nomograms in Stratifying Patient Risk
Based on the tertiles of RFS nomogram scores, patients were classified into low-, intermediate-, and high-risk groups of recurrence. The 5-year RFS rates of these 3 groups were 48.1%, 21.7%, and 8.4%, respectively (P <0.001; Figure 6A). According to the tertiles of OS nomogram scores, patients were also stratified into low-, intermediate-, and high-risk postoperative mortality groups. The 5-year OS rates of these 3 groups were 74.2%, 48.9%, and 24.8%, respectively (P <0.001; Figure 6B).
Discussion
Our study analyzed the long-term outcomes of 1524 patients with HCC who underwent curative LR in a single center over 17 years. The key findings were that the RFS and OS rates decreased significantly with increasing histological severity of cirrhosis, and multivariate analysis identified histological severity of cirrhosis as an independent adverse prognostic factor of RFS and OS. The underlying mechanisms of tumor recurrence in patients with a cirrhotic liver are not clear. Previous studies suggested that the enhanced aberrant microenvironment in cirrhotic livers, which is characterized by abundant stroma, carcinoma-associated fibroblasts, and altered expression of tumor suppressor genes, played key roles in liver carcinogenesis.25,26 Hoshida et al27 reported that gene expression profiles in the surrounding liver tissues, rather than the tumor itself, correlated with the risk of late HCC recurrence after LR, which emphasizes the significance of evaluating the characteristics of the remnant liver.
Our study also showed that patients who had no portal hypertension, tumors within the Milan criteria and who did not have cirrhosis had a 5-year OS rate of 79.1%. The corresponding figures for patients who had mild cirrhosis were 83.3%, moderate cirrhosis 52.6%, and severe cirrhosis 33.3%. The long-term survival outcomes of patients without cirrhosis and with mild cirrhosis after LR were comparable to patients after liver transplantation (LT) for HCC within the Milan criteria, as reported in other studies.28,29 However, the long-term survival outcomes of patients with moderate and severe cirrhosis after LR were worse than patients after LT. This result reflects the fact that underlying liver disease is an important factor affecting long-term survival after LR, but LT can overcome it and achieve much better long-term outcomes.
Our study showed that the histological severity of cirrhosis significantly correlated with portal hypertension. Portal hypertension was more common in patients with severe cirrhosis. However, the signs of portal hypertension did not accurately reflect cirrhotic severity because uncertain collateral circulation developed in some patients with severe cirrhosis. A total of 65.8% of patients with moderate cirrhosis and 22.7% with severe cirrhosis did not present any clinical signs of portal hypertension in our study. The 5-year OS was 57.2% in patients without portal hypertension in our study, and it decreased to 35.0% in patients with portal hypertension. For patients without portal hypertension, patients with mild cirrhosis had significantly better RFS and OS rates than patients with moderate or severe cirrhosis. Therefore, the histological severity of cirrhosis was more reliable than portal hypertension in predicting prognosis after LR for HCC.
We further constructed RFS and OS nomograms to predict RFS and OS in HCC patients who underwent curative LR. The nomograms were used as a straightforward tool to provide individualized risk evaluation for each patient. The advantage of using nomograms is that all the variables are ordinary clinical characteristics, and no advanced mathematical calculation is needed. The C-indexes were 0.727 and 0.746 for the nomograms to predict RFS and OS, respectively, which were significantly higher than the TNM stage and BCLC stage. Notably, the calibration plots exhibited good agreement with actual observations and nomogram-predicted probabilities for RFS and OS, which confirmed the predictive accuracy of these nomograms. The high predictive accuracy of the nomograms stems from the use of comprehensive and relatively sufficient prognostic factors associated with tumor stage, tumor invasiveness, surgical procedure, prognostic tumor biomarkers, and underlying liver diseases. Notably, the nomogram demonstrated that vascular invasion was the largest contributor to prognostic prediction. Vascular invasion is an expression of aggressive biological behavior by the tumor, and it is a harbinger of systemic metastatic spread.30 The presence of vascular invasion is a critical determinant of HCC recurrence and prognosis according to the results of multiple retrospective studies,30–32 and it is one of the most important parts of an established staging system.33 Past studies suggested that HCC recurrence after curative hepatectomy primarily correlated with 2 leading causes, multicentric occurrence (MO) and intrahepatic metastasis (IM). MO is primarily associated with hepatitis virus-related cirrhosis, and IM is primarily a tumor that spreads via vascular invasion.34–36 Therefore, the nomograms constructed in the present study had unique features that included tumor-related characteristics and the histological severity of cirrhosis. The proposed nomograms clearly stratified patients into 3 distinct prognostic groups with significant differences in recurrence and mortality. Therefore, these nomograms may help surgeons create closer surveillance plans and design therapeutic clinical trials for HCC patients with a high risk of recurrence.
The present study has several limitations. First, this study was a retrospective study from a single center, and selection biases were unavoidable. Second, our results showed that the histological severity of cirrhosis was important for surgical decision-making, but a preoperative liver biopsy is invasive and not accepted as a routine clinical practice. Third, most patients in our study had HBV-related hepatitis, and the results may not be applicable to other etiologies of HCC. Fourth, antiviral treatments for HBV after LR may decrease the risk of HCC recurrence and cirrhosis progression that could not be histologically evaluated. Fifth, we only used our population to construct 2 nomograms, and our findings were not verified in a validation cohort. Therefore, the reliability of the 2 nomograms must be further confirmed using multicenter and prospective validation in patients with different etiologies of HCC.
Conclusions
The histological severity of cirrhosis significantly influenced postoperative complications, tumor recurrence, and OS in HCC patients who underwent curative LR. We developed 2 nomograms that accurately and objectively predicted RFS and OS in patients with HCC after curative hepatectomy. The combined application of these predictive models provided an accurate individualized estimation of tumor recurrence and postoperative survival and will help surgeons adopt a close surveillance plan and postoperative adjuvant therapy for patients with a high risk of tumor recurrence.
Abbreviations
HCC, hepatocellular carcinoma; LR, liver resection; HBV, hepatitis B virus; HCV, hepatitis C virus; POD, post-operative day; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization; OS, overall survival; RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; C-index, concordance index; BCLC, Barcelona Clinic Liver Cancer; ALT, alanine transaminase; ALB, albumin; TBIL, total bilirubin; PT, prothrombin time; PLT, platelet count; LT, liver transplantation.
Data Sharing Statement
The data supporting the conclusions of this article will be made available upon reasonable request to the corresponding author.
Ethics Statement
This study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the ethics committee of Tongji Hospital, Huazhong University of Science and Technology, China. Written informed consent was obtained from each patient for the use of his or her data in clinical research.
Acknowledgments
The authors thank Dr. Guo-ping Wang and Dr. Yao-bing Chen, Department of Pathology, Tongji Hospital, Huazhong University of Science and Technology, China, for their assistance in the histopathological study. Bin-Yong Liang and Jin Gu are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by funding from the National Science and Technology Major Project of China (No.2017ZX10203207-002-005) to Prof. Zhi-yong Huang and funding from the Natural Science Foundation of Hubei Province of China (No. 2017CFB258) to Dr. Bin-yong Liang.
Disclosure
The authors have no conflict of interests to declare.
References
1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi:10.1016/S0140-6736(18)30010-2
2. Huang G, Li PP, Lau WY, et al. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels: a randomized controlled trial. Ann Surg. 2018;268:943–954. doi:10.1097/SLA.0000000000002727
3. Yang T, Tabrizian P, Zhang H, et al. Comparison of patterns and outcomes of liver resection for hepatocellular carcinoma: east vs west. Clin Gastroenterol Hepatol. 2017;15:1972–1974. doi:10.1016/j.cgh.2017.06.025
4. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi:10.1038/s41572-020-00240-3
5. European Association For The Study Of The Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi:10.1016/j.jhep.2018.03.019
6. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association For The Study Of Liver Diseases. Hepatology. 2018;68:723–750. doi:10.1002/hep.29913
7. Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology. 2017;65:1196–1205. doi:10.1002/hep.28895
8. Pinter M, Trauner M, Peck-Radosavljevic M, et al. Cancer and liver cirrhosis: implications on prognosis and management. ESMO Open. 2016;1:e42. doi:10.1136/esmoopen-2016-000042
9. Taura K, Ikai I, Hatano E, et al. Influence of coexisting cirrhosis on outcomes after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery. 2007;142:685–694. doi:10.1016/j.surg.2007.05.009
10. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi:10.1016/S0140-6736(14)60121-5
11. Kutami R, Girgrah N, Wanless IR, et al. The Laennec grading system for assessment of hepatic fibrosis: validation by correlation with wedged hepatic vein pressure and clinical features. Hepatology. 2000;32:407A.
12. Kim MY, Cho MY, Baik SK. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol. 2011;55:1004–1009. doi:10.1016/j.jhep.2011.02.012
13. Kim SU, Oh HJ, Wanless IR, et al. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol. 2012;57:556–563. doi:10.1016/j.jhep.2012.04.029
14. Kim SU, Jung KS, Lee S, et al. Histological subclassification of cirrhosis can predict recurrence after curative resection of hepatocellular carcinoma. Liver Int. 2014;34:1008–1017. doi:10.1111/liv.12475
15. Huang ZY, Chen G, Hao XY, et al. Outcomes of non-anatomic liver resection for hepatocellular carcinoma in the patients with liver cirrhosis and analysis of prognostic factors. Langenbecks Arch Surg. 2011;396:193–199. doi:10.1007/s00423-010-0700-8
16. Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33:861–869. doi:10.1200/JCO.2014.56.6661
17. Huang YQ, Liang CH, He L, et al. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol. 2016;34:2157–2164. doi:10.1200/JCO.2015.65.9128
18. Kattan MW, Sternberg CN, Mehmud F, et al. Development and validation of a prognostic nomogram for progression-free survival in patients with advanced renal cell carcinoma treated with pazopanib. Oncology. 2015;89:235–241. doi:10.1159/000430989
19. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi:10.1055/s-2007-1007122
20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi:10.1097/01.sla.0000133083.54934.ae
21. Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257:377–382. doi:10.1097/SLA.0b013e31825a01f6
22. Zhu P, Lau WY, Chen YF, et al. Randomized clinical trial comparing infrahepatic inferior vena cava clamping with low central venous pressure in complex liver resections involving the Pringle manoeuvre. Br J Surg. 2012;99:781–788. doi:10.1002/bjs.8714
23. Huang ZY, Liang BY, Xiong M, et al. Severity of cirrhosis should determine the operative modality for patients with early hepatocellular carcinoma and compensated liver function. Surgery. 2016;159:621–631. doi:10.1016/j.surg.2015.09.002
24. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi:10.1245/s10434-010-0985-4
25. Revill K, Wang T, Lachenmayer A, et al. Genome-wide methylation analysis and epigenetic unmasking identify tumor suppressor genes in hepatocellular carcinoma. Gastroenterology. 2013;145:1424–1435. doi:10.1053/j.gastro.2013.08.055
26. Wu SD, Ma YS, Fang Y, et al. Role of the microenvironment in hepatocellular carcinoma development and progression. Cancer Treat Rev. 2012;38:218–225. doi:10.1016/j.ctrv.2011.06.010
27. Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi:10.1056/NEJMoa0804525
28. Agopian VG, Harlander-Locke MP, Ruiz RM, et al. Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within Milan criteria undergoing liver transplantation: analysis of 3601 patients from the US multicenter HCC transplant consortium. Ann Surg. 2017;266:525–535. doi:10.1097/SLA.0000000000002381
29. Proneth A, Zeman F, Schlitt HJ, et al. Is resection or transplantation the ideal treatment in patients with hepatocellular carcinoma in cirrhosis if both are possible? A systematic review and metaanalysis. Ann Surg Oncol. 2014;21:3096–3107. doi:10.1245/s10434-014-3808-1
30. Rodriguez-Peralvarez M, Luong TV, Andreana L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325–339. doi:10.1245/s10434-012-2513-1
31. Yang L, Xu J, Ou D, et al. Hepatectomy for huge hepatocellular carcinoma: single institute’s experience. World J Surg. 2013;37:2189–2196. doi:10.1007/s00268-013-2095-5
32. Wang CC, Iyer SG, Low JK, et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2009;16:1832–1842. doi:10.1245/s10434-009-0448-y
33. Chun YS, Pawlik TM, Vauthey JN. 8th edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25:845–847. doi:10.1245/s10434-017-6025-x
34. Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi:10.1016/S0168-8278(02)00360-4
35. Cucchetti A, Piscaglia F, Caturelli E, et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009;16:413–422. doi:10.1245/s10434-008-0232-4
36. Cheng Z, Yang P, Qu S, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB. 2015;17:422–427. doi:10.1111/hpb.12367
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






