Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Cost-Effectiveness Analysis of the Oncotype DX Breast Recurrence Score® Test in Node-Negative Early Breast Cancer
Authors Berdunov V , Millen S, Paramore A , Griffin J, Reynia S, Fryer N, Brown R, Longworth L
Received 26 May 2022
Accepted for publication 13 August 2022
Published 19 September 2022 Volume 2022:14 Pages 619—633
DOI https://doi.org/10.2147/CEOR.S360049
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Vladislav Berdunov,1 Steve Millen,2 Andrew Paramore,2 Jane Griffin,3 Sarah Reynia,2 Nina Fryer,2 Rebecca Brown,1 Louise Longworth1
1PHMR Ltd, London, UK; 2Exact Sciences, London, UK; 3Jane Griffin Associates, East Molesey, UK
Correspondence: Vladislav Berdunov, PHMR Ltd, Berkeley Works, Berkley Grove, London, NW1 8XY, UK, Tel +44 20 3432 7450, Email [email protected]
Background: The 21-gene assay (the Oncotype DX Breast Recurrence Score® test) is a validated multigene assay which produces the Recurrence Score® result (RS) to inform decisions on the use of adjuvant chemotherapy in human epidermal growth factor receptor 2-negative (HER2-), hormone receptor positive (HR+) early invasive breast cancer. A model-based economic evaluation estimated the cost-effectiveness of the 21-gene assay against the use of clinical risk tools alone based on the latest evidence from prospective studies.
Methods: The proportion of patients assigned to chemotherapy conditional on their RS result was obtained from retrospective data from the Clalit registry. The probability of distant recurrence with endocrine and chemo-endocrine therapy conditional on RS result was obtained from TAILORx and NSABP B-20 trials. The cost-effectiveness of the 21-gene assay compared to using clinical risk tools alone was estimated in terms of cost per quality-adjusted life-year (QALY) over a lifetime horizon.
Results: The 21-gene assay was more effective (0.17 more quality-adjusted life years) at a lower cost (-£ 519) over a lifetime compared to clinical risk alone. The model results were sensitive to assumptions around the magnitude of benefit of chemotherapy in the high RS result subgroup. Other assumptions underpinning the model, such as the proportion of patients assigned to chemotherapy in the low and mid-range RS result subgroups and long-term distant recurrence probabilities, had a smaller impact on the results.
Conclusion: The analysis showed that the cost-effectiveness of the 21-gene assay is sensitive to assumptions for chemotherapy sparing for patients with RS 0– 25 whose outcomes with endocrine therapy are no worse compared to chemotherapy-assigned patients, and a chemotherapy benefit in the RS 26– 100 group. Future studies need to incorporate a wider set of tumour profiling tests other than the 21-gene assay to allow a direct comparison of their cost-effectiveness.
Keywords: cost-effectiveness, multigene assay, breast cancer, chemotherapy, the Oncotype DX test, 21-gene assay
Background
Breast cancer accounts for up to 25% of all malignancies in the world and represents the most common cause of cancer-related mortality in women.1,2 Over 55,000 cases of breast cancer are diagnosed in the UK annually.3 Breast cancer is often described by stage of development linked to invasive tumours of increasing severity. Early-stage invasive breast cancer refers to tumours that are either stage 1, 2 or 3A.
The standard of care for early invasive breast cancer is breast-conserving surgery or mastectomy followed by adjuvant treatment for the prevention of metastases.4,5 Over 70% of all breast cancer tumours are human epidermal growth factor receptor 2-negative (HER2-) and hormone receptor positive (HR+),6 and this subset of patients, when treated appropriately, has the highest survival rate amongst the different molecular subtypes.7 Targeted endocrine treatment significantly reduces the risk of distant recurrence of this type of breast cancer.8
The addition of adjuvant chemotherapy can further reduce the risk of distant recurrence in a minority of HR+/HER2- patients.9–12 However, the risk of chemotherapy-related toxicity needs to be weighed against the likelihood of long-term benefit of treatment.13,14 Gene expression profiling of tumours using multigene assays (MGAs) can estimate the risk of distant recurrence and in some cases predict chemotherapy benefit, thus supporting decisions on the use of chemotherapy. The 21-gene assay (the Oncotype DX Breast Recurrence Score® test, Exact Sciences, Madison, WI, USA) assesses the expression of 21 genes in tumor tissue and reports a Recurrence Score® (RS®) result along with an estimate of the risk of distant recurrence and likely chemotherapy benefit.15,16 The prognostic and predictive ability of the 21-gene assay has been explored in several large-scale prospective randomised studies,17–20 and this level of evidence has led to its inclusion in clinical guidelines and adoption in clinical practice.21 Recently, an alternative RS result cut-point of 25 has been applied for node-negative patients in the prospective randomised controlled trial TAILORx, which demonstrated no statistically significant difference in distant recurrence-free interval between patients with an RS result between 11–25 randomised to chemo-endocrine therapy or endocrine therapy alone.15
The use of the 21-gene assay is recommended by NICE in the UK as an option for guiding adjuvant chemotherapy decisions in node-negative early breast cancer with intermediate clinical risk.22 This recommendation was on the basis of an economic model informed by a bespoke analysis of TransATAC dataset and estimated reduction in the use of chemotherapy based on routinely collected data from the UK. The analysis presented in this manuscript aimed to build upon the methods and findings of the NICE model to estimate the cost-effectiveness of the 21-gene assay compared to decisions based on clinical risk estimating tools alone using new RS cut-points which define the threshold for chemotherapy benefit for node-negative early breast cancer reported in the TAILORx trial.
Methods
Systematic Literature Review Update
The model was informed by an update of the systematic literature reviews (SLRs) reported in the UK National Institute for Health and Care Excellence (NICE) diagnostic guidance on tumour profiling tests in early breast cancer (DG34).22 An update of the NICE SLRs was performed to identify clinical and economic peer-reviewed evidence (from March 2017 to April 2020) for the 21-gene assay in HR+/HER2- early breast cancer. A detailed description of the search and data extraction methodology is described in the SLR update protocol available from the authors of the article upon request.
Model Structure
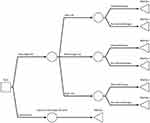
The cost-effectiveness of the 21-gene assay was assessed using a decision-analytic model developed in Microsoft Excel® and Visual Basic for Applications®. The model consisted of a decision-tree which stratified the model population according to genomic risk and assigned chemo-endocrine therapy or endocrine therapy alone (Figure 1). A Markov model simulated the lifetime treatment pathway in early breast cancer using 6-month cycles (Figure 2). The analysis was conducted from the perspective of the UK National Health Service (NHS) and personal social services according to the reference case set by the National Institute for Health and Care Excellence.23 The model population consisted of people with newly diagnosed early invasive HR+/HER2-, node-negative or micrometastatic breast cancer.
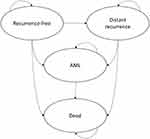 |
Figure 2 Markov model diagram representing the four health states. |
The Markov model consisted of four health states: “distant recurrence-free”, “distant recurrence”, “acute myeloid leukemia (AML)” and “death”. All patients were assumed to start in the recurrence-free health state and could experience disease recurrence depending on their genomic and clinical risk, as well as the assigned adjuvant treatment. The model included a separate health state to represent AML, which can be a long-term adverse event (AE) of chemotherapy.24,25 The key assumptions used in the model are summarised in Table 1.
 |
Table 1 Key Assumptions in the Cost-Effectiveness Model |
The base case analysis followed a lifetime horizon with all future costs and outcomes discounted at a rate of 3.5% per year. Costs were presented in 2020 Pound Sterling, with unit costs published in previous years uplifted using the Hospital and Community Health Services (HCHS) index.26
Clinical Inputs
The distribution of RS results and distant recurrence-free intervals (DRFI) with endocrine therapy conditional on the RS result were obtained from TAILORx and NSABP B-20 RCTs15,16 and converted to 6-month transition probabilities using standard formulas assuming a constant hazard over time. The hazard rate for distant recurrence was assumed to be time-independent in line with assumptions used in models reported NICE DG3422 and Ward et al,24 which were used in previous appraisals of the 21-gene assay in the UK. Probability of distant recurrence with chemotherapy in the RS 11–25 group was estimated by applying the hazard ratio (HR) reported in TAILORx to the hazard rate with endocrine therapy. Given that patients in the low and high RS groups were not randomised in TAILORx, the HR reported in a re-analysis of NSABP B-20 informed the probability of distant recurrence with chemotherapy for RS 0–10 and endocrine therapy for RS 26–100 groups. Due to the retrospective nature of the re-analysis of NSABP B-20, the assumed chemotherapy benefit in the high RS group was subject to uncertainty. The sensitivity of the model results was tested using the upper limit of the range for this value. Chemotherapy assignment conditional on RS group (using RS cut-offs which are consistent with the TAILORx study) was based on a population of LN0 patients enlisted in the Clalit registry, which was assumed to be representative of the UK population.27 The proportion of patients who received chemotherapy based on assessment of clinical risk alone was obtained from NICE DG34 based on the National Cancer Registration and Analysis Service (NCRAS).22 The baseline probability of distant recurrence without genomic risk information was based on the distant recurrence-free intervals from TAILORx with adjustment using the HR from NSABP B-20 reflecting the underlying distribution of RS results that these patients would have had if the 21-gene assay had been used. The probability of developing AML was applied to chemotherapy-treated patients only based on a meta-analysis by Petrelli et al.28 Short-term AEs of chemotherapy were informed by the TACT trial (Table S1).29 No excess breast cancer-related mortality was assumed in the recurrence-free health state and all-cause probability of death was derived from UK life tables for females in 2016–18 (Table S2).30 Probability of death after distant recurrence of breast cancer was derived from the abemaciclib and fulvestrant arm of the MONARCH 2 trial.31 The probability of death in the AML health state was based on the CPX-351 treatment group of Study 301 reported in the NICE technology appraisal of liposomal cytarabine-daunorubicin for AML in the UK.32 Full details of clinical inputs and sources can be found in Table S3.
Subgroup Analysis Inputs
Inputs for the node-negative clinical low and intermediate-risk subgroups were obtained from the bespoke analysis of TransATAC in DG34, which reported genomic risk distributions and 10-year distant recurrence-free interval (DRFI) for low-risk patients (Nottingham Prognostic Index (NPI) ≤ 3.4) and intermediate-risk patients (NPI>3.4). RS result distributions and probability of chemotherapy in patients with micrometastatic tumour node involvement were obtained from the SEER database.33 Where specific inputs for patients with micrometastatic nodal involvement were not available, node-negative population inputs were used due to similarities in clinical characteristics and treatment modality. This assumption was validated with clinical experts.
Health-Related Quality of Life and Cost Inputs
Health-related quality of life (HRQoL) was applied in two ways in the model: (i) health state utility values multiplied by the number of cycles spent in each health state; (ii) one-off decrements to account for loss in HRQoL due to administration of chemotherapy in early breast cancer, chemotherapy-related AEs and local recurrence. The main source of utilities was a study of 361 breast cancer patients in Sweden which reported utility values in recurrence-free and distant recurrence states.34 It was assumed that local recurrence was associated with a one-off utility loss of 0.108 based on Campbell et al.35 The utility level in AML was obtained from the NICE appraisal of liposomal cytarabine-daunorubicin for untreated acute myeloid leukemia.32 Health state utilities were adjusted for background morbidity using age-specific general population utilities in the UK.36 A utility decrement of 0.038 based on Campbell et al was applied to all patients receiving chemotherapy to reflect the loss in utility associated with treatment administration and AEs.35
Cost of the 21-gene assay was based on the list price reported in NICE DG34.22 The true opportunity cost of MGA testing for the NHS includes a confidential discount negotiated with NICE in UK, therefore a 20% discount for the list price was explored in a scenario analysis. No specific unit cost was sought for clinical risk alone as it was assumed that the cost of using clinical risk tools such as NHS PREDICT apply to both in the MGA and non-MGA strategy in the model. Acquisition cost of chemotherapy was obtained from eMIT37 and BNF Online.38 The distribution of chemotherapy regimens used in node-negative HR+/HER2- early breast cancer in the UK was obtained from clinical expert opinion in the absence of published data. Although this distribution was assumed to reflect UK clinical practice, in reality the choice of chemotherapy regimen may vary substantially. For example, the use of anthracycline may be avoided for patients at lower risk of breast cancer recurrence due to the associated risk of AML.39 A scenario analysis examine the effect of a decrease in the use of anthracycle-based regimen by 30%, and assignment of this patient group to a taxane-only regimen (TC), with a corresponding adjustment of the probability of AML by 30%, assuming no excess risk of AML associated with a taxane-only regimen based on the findings from the Petrelli et al meta-analysis.28 Other costs of chemotherapy (administration, follow-up visits and monitoring) were estimated using the method in Ward et al.24 The dosage and frequency of chemotherapy regimens were obtained from published UK guidelines and combined with unit costs.40–43 The method for costing supportive medications was based on Hall et al.25 The investigator of this study (Dr. Peter Hall) was consulted to identify any changes to the use of aprepitant and filgrastim in current practice in the UK since the publication of the Hall et al study. Based on advice from Dr. Hall, it was assumed that aprepitant was used in 20% of all anthracycline or taxane chemotherapy cycles. Filgrastim 5 units per chemotherapy cycle was assumed in 20% of anthracycline chemotherapy cycles, and in every cycle with taxane chemotherapy or accelerated epirubicin, cyclophosphamide and paclitaxel (EC90/P).
Due to the short duration of treatment (less than 6 months), the cost of chemotherapy was applied in the first cycle of the Markov model. All patients were assumed to receive ongoing adjuvant endocrine therapy. The method of estimating the cost of endocrine therapy is consistent with the approach used in Ward et al.24 A summary of chemotherapy and endocrine therapy cost calculations is reported in Tables S4 and S5. Drug unit costs are reported in Table S6.
Non-drug costs of managing metastatic breast cancer were obtained from NICE TA563.44 Subsequent chemotherapy treatments were informed by UK guidelines for the management of metastatic breast cancer,45 published UK real-world data on distribution of treatments46 and length of treatment informed by TA563.44 The use of CDK4/6 inhibitors was informed by clinical expert opinion as few published studies on their use in metastatic breast cancer in the UK exist. The cost of treatments for distant recurrence is reported in Table S7. The cost of AML was split into the initial cost of treatment applied in the first 6 months and maintenance treatment cost applied in every cycle thereafter until death, based on cost estimates from Zeidan et al.47
Analytical Approach
The modelling analysis estimated absolute costs, life-years (LY) and quality-adjusted life-years (QALYs) gained, and incremental cost-effectiveness ratios (ICER) for the 21-gene assay compared to clinical risk tools alone. Subgroup analyses included patients with low and intermediate clinical risk of recurrence and patients with micrometastatic tumours. Uncertainty analyses were carried out in the form of one-way sensitivity analyses, a probabilistic sensitivity analysis and scenario analyses. One-way sensitivity analyses for individual parameter values were represented in tornado diagrams. For ease of interpretation, one-way analyses were carried out on net monetary benefit (NMB), which is defined as: NMB = incremental QALYs x willingness-to-pay threshold (WTP) - incremental cost, assuming a WTP of £20,000 per QALY. Probabilistic analyses were interpreted using a scatter plot on a cost-effectiveness plane and a cost-effectiveness acceptability frontier. Acceptability curves demonstrated the percentage of simulations with an ICER below £20,000 per QALY, which corresponds to a cost-effective use of health and social care resources according to NICE. Plausible ranges and distributions for all parameters included in uncertainty analyses are reported in Table S3.
Results
SLR Update
The results of the SLR update are reported in the Online Supplement.
Base Case Cost-Effectiveness Results
The 21-gene assay was found to be dominant compared to clinical risk alone, meaning that it generated more QALYs at a lower total cost over a lifetime (Table 2). The use of the 21-gene assay was associated with a reduction in the use of chemotherapy from 27% to 16% (Figure 3), which translated into a reduction in the mean cost of chemo-endocrine therapy (including short and long-term chemotherapy AEs) of £791. Effective targeted treatment of patients with a high risk of recurrence was expected to generate more life-years and QALYs over a lifetime and reduce the cost of treating local and distant recurrence by £2279 compared to clinical risk alone. The breakdown of cost by category and QALYs by health state are reported in Table 3.
 |
Table 2 Incremental Cost-Effectiveness of the 21-Gene Assay and Other MGAs Compared to Clinical Risk Alone |
 |
Table 3 Breakdown of Cost and QALYs Compared to Clinical Risk Alone |
 |
Figure 3 Proportion of patients assigned to chemo-endocrine therapy after using the 21-gene assay and clinical risk alone. |
Subgroup Analyses
In subgroup analyses, the 21-gene assay was dominant compared to clinical risk alone in the intermediate clinical risk subgroup and was expected to be cost-effective in the micrometastatic subgroup. The ICER in the low clinical risk group was £63,922 (Table 4).
 |
Table 4 Cost-Effectiveness of the 21-Gene Assay Vs Clinical Risk Alone, Model Subgroups |
Scenario Analyses
Several additional analyses were conducted to test the model results with more conservative assumptions. The use of TransATAC DRFI data for patients treated with endocrine therapy alone instead of TAILORx/NSABP B-20 produced an ICER of £1578 if the 21-gene assay is assumed to identify a large group of patients who do not benefit from the addition of chemotherapy based on the conclusions from TAILORx, and £209,867 if the model assumes a chemotherapy benefit regardless of underlying clinical or genomic risk, which was assumed in the NICE DAR (which did not include the findings from the TAILORx study in their analysis). A scenario was conducted to test the assumed magnitude of chemotherapy benefit predicted by the 21-gene assay. Assuming a more conservative treatment effect of chemotherapy based on the upper range for the hazard ratio for distant recurrence in the RS 26–100 group from NSABP B-20 (changing HR from 0.27 to 0.62) resulted in an ICER of £21,694 per QALY compared to clinical risk alone. Increasing the proportion of patients with low or mid-range RS results assigned to chemotherapy by 20% led to an increase in the ICER, although it remained below the NICE threshold. An assumption of no excess risk of distant recurrence after 15 years (consistent with NICE DAR) reduced the incremental cost savings from £519 to £290. A full summary of scenario analysis results is included in Table 5.
 |
Table 5 Results of Scenario Analyses, 21-Gene Assay vs Clinical Risk Alone |
Uncertainty Analyses
Results of one-way sensitivity analyses are presented in Figure 4. The hazard ratio for chemotherapy vs endocrine therapy in the high RS result subgroup was the largest driver of uncertainty in the model. Other parameters which had an impact on the model results were the discount rate applied to outcomes, distant recurrence probability with endocrine therapy in the high RS result subgroup and the hazard ratio in the low RS result subgroup. Other parameters had a minor impact on the results.
Probabilistic sensitivity analysis results are reported in Figure 5. The cloud of points representing the combined parametric uncertainty in the model was distributed across the north-east quadrant (the 21-gene assay is more effective but also more costly than clinical risk alone) and the south-east quadrant (the 21-gene assay is both more effective and less costly). The 21-gene assay was dominant in 72% of the simulations. Based on acceptability curves (Figure 6), the 21-gene assay had a probability of being cost-saving or cost-effective of 97% at the WTP of £20,000 per QALY.
Discussion
Interpretation of Results
This cost-effectiveness analysis combined the most up-to-date evidence for the impact of MGA use on chemotherapy decisions and long-term outcomes in HR+/HER2-, node-negative or micrometastatic early breast cancer. This was the first cost-effectiveness analysis which incorporated the treatment effect of chemotherapy according to RS result cut-offs reported in the TAILORx and NSABP B-20 prospective trials.15,16
The 21-gene assay was found to be more effective and less costly compared to clinical risk alone in the HR+/HER2- node-negative population. It was also expected to be cost-effective in the intermediate clinical risk subgroup and the micrometastatic subgroup according to the accepted willingness-to-pay per QALY in the UK. The 21-gene assay was unlikely to be deemed cost-effective when used in the low clinical risk LN0 subgroup (defined as NPI≤3.4). Chemotherapy assignment in the subgroup analyses was informed by data from the Clalit registry, which was not stratified by clinical risk and thus included a mix of LN0 patients with low and intermediate clinical risk. It is likely that the 21-gene assay may have a different impact on treatment decisions for patients with varying clinical risk and with micrometastatic tumours. Therefore, the true estimate of cost-effectiveness in these subgroups is highly uncertain and requires additional empirical data. The use of MGAs to support chemotherapy decisions in this subgroup could be informed by additional analyses of existing datasets, such as TAILORx or Clalit.
Substantial uncertainty around clinical input values was observed in the sensitivity analyses. The probability of distant recurrence in the RS 26–100 group was informed by a re-analysis of the NSABP B-20 trial data using the updated cut-points based on the TAILORx study, in the absence of evidence from a randomised study. Although this retrospective analysis was less methodologically robust than evidence from an RCT, it represents additional evidence of the significant benefit of chemotherapy in this group previously demonstrated by Paik et al.48 The upper bound for the hazard ratio in Geyer et al (0.62) corresponds to an ICER of £21,694 per QALY, which can be considered to be cost-effective by NICE. An RCT with an endocrine therapy arm for patients with RS>25 is unlikely to be conducted on safety and ethical grounds, leaving retrospective or prospective re-analyses or registry studies as the best source of primary data in this subgroup. Chemotherapy assignment in the RS 0–25 group was also highly uncertain, although increasing the assumed proportion assigned to chemotherapy to 20% from the base case (0% for low RS and 9% for mid-range RS) did not change the study conclusions. Chemotherapy assignment is unlikely to be higher than this upper bound given that endocrine therapy offers the same outcomes in terms distant recurrence risk as chemotherapy in this patient group.
The incremental lifetime cost savings associated with the genomic test strategy were significantly reduced if the probability of distant recurrence is set to zero after 15 years. In the absence of long-term follow-up studies, the model relied on the opinion of clinical experts to support the assumption that distant recurrence risk persists over a patient’s lifetime, albeit at a reduced rate after 10 years. Further consensus is required to address this source of uncertainty.
The uncertainty associated with cost and utility assumptions were tested in scenario analyses and did not change the study conclusions. The probabilistic analysis showed that the 21-gene assay was highly likely to be cost-effective compared to clinical risk alone based on the accepted willingness-to-pay threshold set by NICE.
Comparison to Published Evidence
The cost-effectiveness of the 21-gene assay in HR+/HER2- early breast cancer in the UK was assessed by NICE in DG34 which was informed by a bespoke analysis of TransATAC.22 The NICE base case analysis concluded that the 21-gene assay was not cost-effective compared to using clinical risk alone if the assumption was made that the test was prognostic only, and applied a constant treatment effect of chemotherapy across all clinical and genomic risk groups. A scenario analysis with prediction of chemotherapy benefit based on Paik et al48 showed that the 21-gene assay is dominant vs clinical risk alone. Differences in the approach and sources made it difficult to compare the results of the current analysis to the NICE model. In addition, the current analysis used updated estimates of chemotherapy and distant recurrence cost based on clinical expert opinion and published studies to reflect current clinical practice, which further differentiated this model from the NICE DAR. For validation purposes, the clinical inputs used in the NICE analysis (TransATAC and chemotherapy assignment probabilities reported in the NICE DAR) were implemented in the current model and the results were compared, which showed broad alignment in conclusions between the current model and the NICE analysis.
A recent systematic review of economic analyses of the 21-gene assay found that assumptions regarding the treatment effect of chemotherapy on distant recurrence conditional on RS result had the largest impact on the ICER, alongside assumptions for reduction in chemotherapy use.49 Studies which assumed a differential hazard ratio for distant recurrence between RS result subgroups concluded that the 21-gene assay was either dominant or considered to be cost-effective compared to using clinical-pathologic factors alone.25,50–53 Studies which assumed a constant reduction in the rate of distant recurrence with chemotherapy irrespective of RS result were less likely to conclude that the 21-gene assay is cost-effective.54,55 This is consistent with the findings from the prognostic-only scenario in the current analysis which reported an ICER considerably higher than the NICE WTP per QALY threshold, and the prognostic-only base case assumed in the NICE DAR prior to the publication of results from the TAILORx study, which demonstrated that chemotherapy does not provide additional benefit to patients with RS results 11–25. This suggests that assuming a constant benefit of chemotherapy across all RS subgroups is not supported by the latest evidence. The assumed predictive ability of the 21-gene assay in the RS 26–100 subgroup relies on re-analyses of the NSABP B-20 study,16,48 and the HR in this subgroup remains a substantial source of uncertainty, as demonstrated in the scenario analyses reported earlier.
Study Limitations
The cost-effectiveness analysis was informed by a synthesis of different clinical data sources for chemotherapy assignment, DRFI and chemotherapy benefit, which inherently contributed to overall model uncertainty. Analyses of the low and intermediate-risk subgroups defined by NPI were informed by TransATAC as TAILORx did not report DRFI or chemotherapy benefit by clinical risk subgroup, which limited the comparison between clinical risk subgroup analyses and the main analysis of the full LN0 population.
The NICE analysis in DG34 included parallel cost-effectiveness analyses of other MGAs used in clinical practice in the UK, including MammaPrint®, EPClin® and Prosigna®. Since the publication of DG34, no further studies have been conducted which compared multiple MGAs using the same patient cohort. The retrospective re-analysis of the TransATAC study data conducted by NICE remains the most relevant study of the clinical and cost-effectiveness of EPClin and Prosigna. A new cut-point from the MINDACT study reported 8-year DRFS for MammaPrint, which is likely to have a small impact on its cost-effectiveness as assessed by NICE using the 5-year cut-point from the same study. Due to differences in the design and characteristics of the populations across pivotal studies (TAILORx, MINDACT, TransATAC), a direct or indirect comparison of the cost-effectiveness of the 21-gene assay against other MGAs was considered to be methodologically flawed and was not attempted in analysis reported here. Adjustment based on patient characteristics using a validated indirect treatment comparison method using inverse probability weights or matching could be used to minimise bias but requires access to individual patient-level data.56,57 The feasibility of a network meta-analysis is constrained by differences in inclusion and exclusion criteria, cut points used to categorise patients according to genomic risk, randomisation methods used across the studies involved.
Recommendations for Policy and Future Research
The analysis presented here synthesised the best available evidence for the prognostic and predictive capabilities of the 21-gene assay to guide chemotherapy in node-negative early breast cancer in the UK and contributed towards the evidence base for future appraisals by NICE in the UK and HTA bodies in other countries. The analysis results were sensitive to certain input parameters, particularly rates of distant recurrence with endocrine therapy and estimates of chemotherapy benefit. Further evidence for this subgroup from prospective or retrospective registry studies could help to reduce the plausible range around the ICER. Future studies are needed to examine the cost-effectiveness of the 21-gene assay in the node-positive HR+/HER2- population by incorporating evidence the RxPONDER Phase III trial which demonstrated that adjuvant chemotherapy had no significant benefit for distant recurrence compared to endocrine therapy in postmenopausal patients with 1–3 positive nodes and RS result 0–25.58
Data Sharing Statement
The parameter inputs used in the model were identified from sources in the open domain or peer-reviewed journal articles. All parameter values and their sources are reported in the article and Online Supplement. The Excel data traces used to generate the results of the cost-effectiveness model can be obtained upon written request to the corresponding author.
Ethics Approval and Informed Consent
The authors have attached a statement from the NHS Health Research Authority which confirms that review from NHS Research Ethics Committee was not required for this study. The ethics waiver reasons are fully explained in the attached document.
Acknowledgments
We would like to acknowledge the contribution of Dr. Peter Hall to the design of the study, validation of inputs for the cost-effectiveness model and review of the draft manuscript. We would like to acknowledge the expert input of Dr. Mark Verrill, Prof. Robert Coleman, Prof. Carlo Palmieri and Dr. Richard Simcock to validate model assumptions and identify model inputs.
Author Contributions
All authors have been credited with authorship based on the conditions listed in the IMCJE authorship guidelines.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Exact Sciences, who are the manufacturers of the 21-gene assay. Exact Sciences contributed to the design and reporting of the analysis, and the development of this manuscript.
Disclosure
Steve Millen, Andrew Paramore, Sarah Reynia and Nina Fryer are employees and stockholders of Exact Sciences. The authors report no other conflicts of interest in this work.
References
1. Winters S, Martin C, Murphy D, Shokar NK. Breast cancer epidemiology, prevention, and screening. In: Progress in Molecular Biology and Translational Science. Vol. 151. Elsevier B.V.; 2017:1–32. doi:10.1016/bs.pmbts.2017.07.002
2. Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pacific J Cancer Prev. 2016;17(S3):43–46. doi:10.7314/APJCP.2016.17.S3.43
3. Cancer Research UK. Breast cancer incidence (invasive) statistics. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/incidence-invasive#heading-Zero.
4. Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):e148–e160. doi:10.1016/S1470-2045(11)70383-7
5. National Institute for Health and Care Excellence. Early and locally advanced breast cancer: diagnosis and management; 2018.
6. Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5):e1000279. doi:10.1371/journal.pmed.1000279
7. Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(6):619–626. doi:10.1158/1055-9965.EPI-17-0627
8. Dubsky PC, Jakesz R, Fitzal F, et al. Tamoxifen and anastrozole as a sequencing strategy: a randomized controlled trial in postmenopausal patients with endocrine-responsive early breast cancer from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2012;30(7):722–728. doi:10.1200/JCO.2011.36.8993
9. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
10. Martín M, Ruiz A, Borrego MR, et al. Fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus FAC followed by weekly paclitaxel as adjuvant therapy for high-risk, node-negative breast cancer: results from the GEICAM/2003-02 study. J Clin Oncol. 2013;31:2593–2599. doi:10.1200/JCO.2012.46.9841
11. Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352(22):2302–2313. doi:10.1056/NEJMoa043681
12. Roché H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 trial. J Clin Oncol. 2006;24(36):5664–5671. doi:10.1200/JCO.2006.07.3916
13. Caparica R, Bruzzone M, Poggio F, Ceppi M, de Azambuja E, Lambertini M. Anthracycline and taxane-based chemotherapy versus docetaxel and cyclophosphamide in the adjuvant treatment of HER2-negative breast cancer patients: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res Treat. 2019;174(1):27–37. doi:10.1007/s10549-018-5055-9
14. Friese CR, Harrison JM, Janz NK, et al. Treatment-associated toxicities reported by patients with early-stage invasive breast cancer. Cancer. 2017;123(11):1925–1934. doi:10.1002/cncr.30547
15. Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi:10.1056/NEJMoa1804710
16. Geyer CE, Tang G, Mamounas EP, et al. 21-Gene assay as predictor of chemotherapy benefit in HER2-negative breast cancer. Npj Breast Cancer. 2018;4(1). doi:10.1038/s41523-018-0090-6
17. Sestak I, Buus R, Cuzick J, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor–positive breast cancer a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):545–553. doi:10.1001/jamaoncol.2017.5524
18. Buus R, Sestak I, Kronenwett R, et al. Comparison of endopredict and epclin with oncotype dx recurrence score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst. 2016;108:djw149. doi:10.1093/jnci/djw149
19. Loncaster J, Armstrong A, Howell S, et al. Impact of Oncotype DX breast Recurrence Score testing on adjuvant chemotherapy use in early breast cancer: real world experience in Greater Manchester, UK. Eur J Surg Oncol. 2017;43(5):931–937. doi:10.1016/j.ejso.2016.12.010
20. Curtit E, Vannetzel JM, Darmon JC, et al. Results of PONDx, a prospective multicenter study of the Oncotype DX® breast cancer assay: real-life utilization and decision impact in French clinical practice. Breast. 2019;44:39–45. doi:10.1016/j.breast.2018.12.015
21. National Comprehensive Cancer Network. NCCN Guidelines: breast Cancer, Version 2.2021; 2021.
22. National Institute for Health and Care Excellence. Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer; 2018.
23. National Insitute for Health and Care Excellence. Guide to the methods of technology appraisal; 2013.
24. Ward S, Scope A, Rafia R, et al. Gene expression profiling and expanded immunohistochemistry tests to guide the use of adjuvant chemotherapy in breast cancer management: a systematic review and cost-effectiveness analysis. Health Technol Assess (Rockv). 2013;17(44). doi:10.3310/hta17440
25. Hall PS, Smith A, Hulme C, et al. Value of information analysis of multiparameter tests for chemotherapy in early breast cancer: the OPTIMA Prelim Trial. Value Heal. 2017;20(10):1311–1318. doi:10.1016/j.jval.2017.04.021
26. Curtis LA, Burns A. Unit costs of health and social care 2020; 2021. Available from: https://kar.kent.ac.uk/84818/.
27. Stemmer SM, Steiner M, Rizel S, et al. Ten-year clinical outcomes in N0 ER+ breast cancer patients with Recurrence Score-guided therapy. Npj Breast Cancer. 2019;5(1). doi:10.1038/s41523-019-0137-3
28. Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Mortality, leukemic risk, and cardiovascular toxicity of adjuvant anthracycline and taxane chemotherapy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;135(2):335–346. doi:10.1007/S10549-012-2121-6
29. Ellis P, Barrett-Lee P, Johnson L, et al. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, Phase III, randomised controlled trial. Lancet. 2009;373(9676):1681–1692. doi:10.1016/S0140-6736(09)60740-6
30. Office for National Statistics. National life tables 2016–2018, United Kingdom; 2019. Available from: https://www.ons.gov.uk/releases/nationallifetablesuk2016to2018.
31. Sledge GW, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a Randomized Clinical Trial. JAMA Oncol. 2020;6(1):116–124. doi:10.1001/JAMAONCOL.2019.4782
32. National Institute for Health and Care Excellence. Liposomal Cytarabine–Daunorubicin for Untreated Acute Myeloid Leukaemia; 2018.
33. Roberts MC, Miller DP, Shak S, Petkov VI. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and Oncotype DX Recurrence Score results in the SEER database. Breast Cancer Res Treat. 2017;163(2):303–310. doi:10.1007/s10549-017-4162-3
34. Lidgren M, Wilking N, Jönsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16(6):1073–1081. doi:10.1007/s11136-007-9202-8
35. Campbell HE, Epstein D, Bloomfield D, et al. The cost-effectiveness of adjuvant chemotherapy for early breast cancer: a comparison of no chemotherapy and first, second, and third generation regimens for patients with differing prognoses. Eur J Cancer. 2011;47(17):2517–2530. doi:10.1016/j.ejca.2011.06.019
36. Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (Where Patient Level Data Are Not Available). Value Heal. 2008;11(7):1131–1143. doi:10.1111/j.1524-4733.2008.00352.x
37. Department of Health and Social Care. Drugs and pharmaceutical electronic market information tool (eMIT); 2021. Aailable from: https://www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit.
38. National Institute for Health and Care Excellence. British national formulary; 2020. Available from: https://bnf.nice.org.uk/.
39. Jasra S, Anampa J. Anthracycline use for early stage breast cancer in the modern era: a review. Curr Treat Options Oncol. 2018;19(6):1–17. doi:10.1007/S11864-018-0547-8
40. London Cancer Alliance West and South. LCA breast cancer clinical guidelines; 2016. Available from: http://rmpartners.cancervanguard.nhs.uk/wp-content/uploads/2017/03/lca-breast-cancer-clinical-guidelines-october-2013-updated-march-2016-.pdf.
41. London Cancer Alliance West and South. Breast pathway group FEC75 in early breast cancer; 2016. Available from: https://rmpartners.nhs.uk/wp-content/uploads/2017/03/LCA-Breast-FEC75-EBC-November-2014.pdf.
42. London Cancer Alliance West and South. Breast pathway group - EC x 4 - Docetaxel x 4: epirubicin & cyclophosphamide followed by docetaxel in early breast cancer; 2016. Available from: https://rmpartners.nhs.uk/wp-content/uploads/2017/03/LCA-Breast-EC-Docetaxel-EBC-November-2014.pdf.
43. NHS Thames Valley. Chemotherapy regimens beast cancer. Available from: http://tvscn.nhs.uk/wp-content/uploads/2018/12/Breast-4.2-November-2018.pdf.
44. National Institute for Health and Care Excellence. Abemaciclib with an aromatose inhibitor for previously untreated, hormone receptor-positive, HER2-negative, locally advanced or metastatic breast cancer; 2019.
45. National Institute for Health and Care Excellence. Advanced breast cancer: diagnosis and treatment; 2017. Available from: https://www.nice.org.uk/guidance/cg81.
46. Kurosky SK, Mitra D, Zanotti G, Kaye JA. Treatment patterns and outcomes of patients with metastatic ER + /HER-2 − breast cancer: a multicountry retrospective medical record review. Clin Breast Cancer. 2018;18(4):e529–e538. doi:10.1016/j.clbc.2017.10.008
47. Zeidan AM, Mahmoud D, Kucmin-Bemelmans IT, et al. Economic burden associated with acute myeloid leukemia treatment. Exp Rev Hematol. 2015;9(1):79–89. doi:10.1586/17474086.2016.1112735
48. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. doi:10.1200/JCO.2005.04.7985
49. Wang SY, Dang W, Richman I, Mougalian SS, Evans SB, Gross CP. Cost-Effectiveness analyses of the 21-Gene assay in breast cancer: systematic review and critical appraisal. J Clin Oncol. 2018;36(16):1619–1627. doi:10.1200/JCO.2017.76.5941
50. Blohmer JU, Rezai M, Kümmel S, et al. Using the 21-gene assay to guide adjuvant chemotherapy decision-making in early-stage breast cancer: a cost-effectiveness evaluation in the German setting. J Med Econ. 2012;16(1):30–40. doi:10.3111/13696998.2012.722572
51. Masucci L, Torres MS, Eisen A, et al. Cost-utility analysis of 21-gene assay for node-positive early breast cancer. Curr Oncol. 2019;26(5):307–318. doi:10.3747/co.26.4769
52. Kip M, Monteban H, Steuten L. Long-term cost-effectiveness of Oncotype DX® versus current clinical practice from a Dutch cost perspective. J Comp Eff Res. 2015;4(5):433–445. doi:10.2217/CER.15.18
53. Holt S, Bertelli G, Humphreys I, et al. A decision impact, decision conflict and economic assessment of routine Oncotype DX testing of 146 women with node-negative or pNImi, ER-positive breast cancer in the U.K. Br J Cancer. 2013;108(11):2250–2258. doi:10.1038/bjc.2013.207
54. Hannouf MB, Zaric GS, Blanchette P, et al. Cost-effectiveness analysis of multigene expression profiling assays to guide adjuvant therapy decisions in women with invasive early-stage breast cancer. Pharmacogenomics J. 2020;20(1):27–46. doi:10.1038/s41397-019-0089-x
55. Tsoi DT, Inoue M, Kelly CM, Verma S, Pritchard KI. Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist. 2010;15(5):457–465. doi:10.1634/THEONCOLOGIST.2009-0275
56. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41. doi:10.2307/2335942
57. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399. doi:10.1080/00273171.2011.568786
58. Kalinsky K, Barlow WE, Gralow JR, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385(25):2336–2347. doi:10.1056/NEJMOA2108873/SUPPL_FILE/NEJMOA2108873_DATA-SHARING.PDF
59. De Bock GH, Putter H, Bonnema J, Van Der Hage JA, Bartelink H. The impact of loco-regional recurrences on metastatic progression in early-stage breast cancer: a multistate model. Breast Cancer Res Treat. 2009;117(2):401–408. doi:10.1007/s10549-008-0300-2
60. Färkkilä N, Roine R, Jahkola T, et al. PCN137 Health state utilities in breast cancer. Value Heal. 2011;14(7):A459. doi:10.1016/j.jval.2011.08.1238
61. Yousefi M, Najafi S, Ghaffari S, Mahboub-Ahari A, Ghaderi H. Comparison of SF-6D and EQ-5D scores in patients with breast cancer. Iran Red Crescent Med J. 2016;18(5). doi:10.5812/ircmj.23556
62. Ibarrondo O, Álvarez-López I, Freundlich F, et al. Probabilistic cost-utility analysis and expected value of perfect information for the Oncotype multigenic test: a discrete event simulation model. Gac Sanit. 2020;34(1):61–68. doi:10.1016/j.gaceta.2018.07.012
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.