Back to Journals » Psoriasis: Targets and Therapy » Volume 12
Reducing the Risk of Developing Psoriatic Arthritis in Patients with Psoriasis
Authors Gisondi P , Bellinato F, Maurelli M , Geat D, Zabotti A , McGonagle D, Girolomoni G
Received 26 April 2022
Accepted for publication 4 August 2022
Published 10 August 2022 Volume 2022:12 Pages 213—220
DOI https://doi.org/10.2147/PTT.S323300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Uwe Wollina
Paolo Gisondi,1 Francesco Bellinato,1 Martina Maurelli,1 Davide Geat,1 Alen Zabotti,2 Dennis McGonagle,3 Giampiero Girolomoni1
1Section of Dermatology and Venereology, Department of Medicine, University of Verona, Verona, Italy; 2Rheumatology Clinic, Department of Medical and Biological Sciences, University Hospital ‘Santa Maria della Misericordia’ c/o University of Udine, Udine, Italy; 3Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK
Correspondence: Paolo Gisondi, Section of Dermatology and Venereology, Department of Medicine, University of Verona, Piazzale A. Stefani 1, Verona, 37126, Italy, Email [email protected]
Abstract: Psoriatic arthritis (PsA) is a heterogeneous chronic inflammatory arthritis associated with psoriasis, which may manifest with different domains such as dactylitis, enthesitis, synovitis and spondylitis. The estimated prevalence of PsA in patients with psoriasis ranges widely between 6% and 42%. In most cases, PsA is preceded by skin involvement by an average time of 7– 8 years. In the complex patho-mechanisms involved in the transition from psoriasis to PsA, the gut and skin have been proposed as the sites of immune activation triggering or contributing to the development of PsA. In such a transition, a subclinical phase has been identified, characterized by enthesopathy where soluble biomarkers and imaging findings but no clinical symptoms are detectable. Recent studies have provided some evidence that timely treated psoriasis may reduce the risk of developing PsA.
Keywords: psoriatic arthritis, psoriasis, therapy, prevention, early intervention, disease modification
Epidemiology of Psoriatic Arthritis
Psoriatic arthritis (PsA) is a heterogeneous chronic inflammatory arthritis associated with cutaneous and/or nail psoriasis. The estimated prevalence of PsA is up to 30% of patients with psoriasis, ranging between 6% and 42% depending on its definition.1,2 Considering only the studies which applied the Classification Criteria for Psoriatic Arthritis (CASPAR criteria), the prevalence is 23.8% (95% CI, 20.1–27.6%).3 In approximately 72% of PsA patients, psoriasis precedes the development of arthritis by an average of 7–8 years.4–6 Hence, the presence of cutaneous or nail psoriasis represents an important risk factor for PsA, and such a latency between the two conditions may have the potential of becoming a precious window of opportunity for preventive interventions. Of note, the annual incidence of PsA among psoriasis patients was found to be constant after the initial diagnosis of psoriasis, varying across the studies from 3.4 to 8 events per 100 000 person-years.7 Furthermore, the risk of developing PsA was shown to increase with psoriasis severity, being highest in patients with the more severe disease.8 Patients presenting to Rheumatologists with PsA without prior extensive psoriasis may not have skin involvement to a degree that warrants systemic therapy, whereas more severe cutaneous disease in psoriasis cases that presents to Dermatology naturally places the Dermatologist in a position whereby psoriasis directed systemic therapy can be strategically implemented, not just to treat skin involvement, but to potentially intercept PsA.
Clinical Manifestations
The initial description of the clinical features of PsA subgroups appeared in 1973.9 PsA was originally defined by Moll and Wright as a seronegative inflammatory arthritis in the presence of psoriasis presenting with five different types, namely: distal interphalangeal joint arthritis, asymmetrical oligoarthritis, polyarthritis, spondylitis and arthritis mutilans. Although these patterns may change and overlap during the clinical course of the disease, asymmetrical oligoarthritis and polyarthritis are generally considered as the most common.10 Furthermore, enthesitis is observed in 30–50% and dactylitis in 40–50% although these were not part of the original classification.10
The diagnosis of PsA is based on clinical and imaging findings in a patient with psoriasis (Figure 1). Among laboratory investigations, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in 40% while anti–cyclic citrullinated peptide antibodies (ACPA) and/or rheumatoid factor (RF) are negative in 95%.11 As there are no specific diagnostic tests, diagnosing PsA can be challenging even for skilled physicians, and even more so in the case of early PsA. Indeed, symptoms and signs of prodromal and early PsA may be subtle and fluctuating, as shown by a recent German retrospective analysis that found that unspecific arthritic symptoms (such as acute rheumatism) precede the development of PsA.12 Similarly, a Bayesian network model found that the musculoskeletal symptoms that predict the development of PsA in psoriatic patients are non-specific arthritis, fatigue, swelling, arthralgia, myalgia and pain in the back, finger, hand and knee.13 In addition, inflammatory markers are commonly normal or only minimally elevated in early PsA.4 Other confounding factors include the fact that RF and/or ACPA may be positive in up to 10% of patients while, conversely, up to 10% of patients with PsA do not have cutaneous or nail psoriasis at the time of PsA onset.4 Ultimately, separating inflammatory from either degenerative symptoms (osteoarthritis) or fibromyalgia can at times be challenging.14,15 Despite these difficulties, a timely diagnosis of PsA is crucial because its treatment can prevent or limit disease progression. Indeed, a delay in the diagnosis of PsA of about six months was found to result in severe inflammation and irreversible joint damage and erosions.16,17 Recognizing the heterogenous presentations and compounding differential diagnoses including biomechanically related tendinopathies in patients that have high BMIs, diabetes that may be linked to musculoskeletal symptoms and the aforementioned degenerative arthritis and fibromyalgia, it is possible that the initiation of systemic therapy for psoriasis with a subsequent resolution of musculoskeletal symptoms could in fact be a useful diagnostic test for suspected early inflammatory arthritis that accompanies PsA. This paradigm is already familiar to rheumatologists who use good clinical responses to corticosteroids in the setting of suspected polymyalgia rheumatic to confirm the clinical diagnosis. In effect, timely therapy may serve as a diagnostic test where diagnosis is difficult in the absence of visible swelling and absence of raised acute phase reactants.
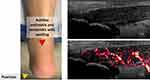 |
Figure 1 Clinical and ultrasonographic findings of PsA showing Achilles tendinitis in a patient with plantar psoriasis. |
Whilst no validated diagnostic criteria for PsA exist, the CASPAR criteria are most commonly used to define PsA in randomized clinical trials and observational studies. These criteria have demonstrated a sensitivity and specificity of 91.4 and 98.7%, respectively.17,18 However, a limitation of CASPAR criteria is that their sensitivity for early PsA is lower than that for established disease. Coates L. et al recruited 111 patients with early PsA (defined as symptom duration of <24 months) and 111 controls with early arthritis from early arthritis clinics. Against a gold standard of PsA diagnosis by a consultant rheumatologist, the sensitivity and specificity of the CASPAR criteria in classifying early PsA were 87.4% and 99.1%.19
Furthermore, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) identified different domains of involvement of psoriatic disease including peripheral arthritis, axial involvement, enthesitis, dactylitis, skin and nail, advocating a tailored treatment approach to achieve the lowest possible level of disease activity in all domains of the disease.20–22 However, when considering these various domains there are two important caveats. Firstly, there are unifying biomechanical features around PsA disease localization to synovio-entheseal complexes and entheses in seemingly disparate pathologies including synovitis and dactylitis.23 Secondly, many of the systemic therapies used to treat psoriasis have an impact on all of the aforementioned domain points towards this unified concept but also augurs well that systemic therapy may prevent the emergence of these previously considered disparate manifestations.
Transition from Psoriasis to PsA
It has been proposed that PsA could be the result of an interplay between environmental factors (musculoskeletal injury, obesity, infection) in patients who are genetically more susceptible. In the transition from psoriasis to PsA, the skin has been considered as a site of onset of immune activation, in some way triggering the inflammatory cascade eventually resulting in PsA. In particular, tissue-resident memory CD8+ T cells derived from the skin are enhanced in the circulation of patients with PsA.24 Also, in an elegant animal model, epidermal alterations were sufficient to initiate both skin lesions and arthritis in psoriasis.25 However, the strong HLA-Cw*06 association with psoriasis is completely absent in PsA that argues against such a simplistic model. Moreover, many PsA patients have mild or limited skin involvement, thus making it difficult to directly link the magnitude of skin disease to joint disease. Furthermore, therapy with topical agents a nb-UVB may beneficially impact on psoriasis but does not seem to prevent PsA development. It has also been recently suggested that there are three clinically quiet stages after psoriasis onset and before clinically detectable PsA.24 First, there is a preclinical phase characterized by aberrant activation of the immune system, which may originate from the skin, intestinal mucosa or the entheses. Secondly, there is a subclinical PsA phase where imaging findings, but no clinical symptoms are detectable. Thirdly, there is a prodromal PsA phase with arthralgia and fatigue but no synovitis and/or enthesitis appreciable by physical exam.24 Regarding the subclinical PsA phase, ultrasonographic enthesopathy was found to be common among patients with psoriasis without clinical signs of arthritis.25 We evaluated through the Glasgow Ultrasound Enthesitis Scoring System (GUESS) entheseal sites commonly involved in PsA and found that the mean GUESS score and tendon thickness were significantly higher in psoriasis patients without musculoskeletal complaints vs controls.26–28 Similarly, Zuliani et al compared the ultrasonographic findings of psoriatic patients without musculoskeletal symptoms and healthy controls and observed active enthesitis and synovitis in 27.5% and 20.0% of psoriatic patients but in none of the controls, with higher PASI score being correlated with active enthesitis (p = 0.034).28 A prospective case control study also confirmed that psoriatic patients showed a significant prevalence of asymptomatic ultrasound synovitis and enthesitis.29 Additionally, the presence of structural entheseal lesions as well as low cortical volumetric bone mineral density at entheseal and intraarticular sites detected by high-resolution peripheral quantitative computed tomography were found to be associated with an increased risk of developing PsA in patients with psoriasis.30 Recently, Wang et al developed a model with a high degree of accuracy to predict the risk of PsA in patients with psoriasis consisting of six ultrasonographic variables, namely hand joint power Doppler (PD) signals, wrist joint synovial thickening, knee joint PD signals, toe joint PD signals, quadriceps tendon and patellar tendon enthesitis, Achilles tendon and plantar aponeurosis enthesitis.31 In regard to prodromal PsA, Zabotti et al found a higher incidence of sonographically detected tenosynovitis in psoriatic patients with arthralgia compared to psoriasis patients without it (29.5% vs 5.3%, p < 0.001), while in a longitudinal analysis of the same study baseline sonographical evidence of enthesitis was able to predict the development of PsA.26,32
Several risk factors for PsA transition have been identified in different systematic reviews, albeit with some controversial results. These include genetics, clinical phenotypes of cutaneous disease, environmental and modifiable risk factor exposure (Table 1).33–35 Genetic markers of PsA comprise specific HLA haplotypes and polymorphisms in IL-12/23 and IL-17/23 axis. Regarding the former ones, HLA-Cw*06, a well-known marker for early onset plaque psoriasis, is only present in a subset of under 30% of PsA patients, and psoriatic patients with HLA-Cw*06 seem to be less likely to also have PsA. Conversely, HLA-B*27 is a genetic biomarker of disease expression in PsA with male predominance and was found to be associated with early onset PsA, axial disease, uveitis and dactylitis.36 Several single nucleotide polymorphisms (SNPs) in the IL-23/IL-17 axis have been associated with PsA, such as the rs33980500 SNP in TRAF 3-interacting protein 2 (TRAF3IP2), which is downstream of IL-17R.37 Furthermore, genome-wide association studies found a stronger association with PsA than with psoriasis and the genes of IL-23 receptor and TNF-induced protein 3 (TNFAIP3), a regulator of NF-κB activity.38,39 With regard to clinical markers, severe psoriasis, defined either by body surface area (BSA) or number of sites involved, is a clinical feature associated with an increased risk of PsA.40–42 The greater extent of cutaneous psoriasis is thought to correlate with a greater burden of systemic inflammation, which, in turn, might trigger joint inflammation.
 |
Table 1 Risk Factors for Psoriatic Arthritis in Patients with Chronic Plaque Psoriasis |
Furthermore, a recent systematic literature review provides a profile of the psoriasis patients at higher risk of developing arthritis, identifying long-term predictors (ie, psoriasis severity, nail involvement, family history of PsA and obesity) and short-term predictors of PsA (ie, arthralgia and subclinical evidence of inflammation detected by imaging).43 The identification of the profile of psoriasis patients at risk for transition represents a benchmark for a preliminary characterization of the psoriasis to PsA march and for the design of PsA interception trial in psoriasis patients.
The proximity between nail matrix and entheses of distal interphalangeal joints may explain the higher risk of enthesopathy in patients with nail psoriasis. Interestingly, such an association was not confirmed for distal onycholysis, crumbling and oil drop discoloration.44–46 Of note, the number of affected nails was not investigated as a continuous variable to be associated with increased risk of PsA. Nail disease in psoriasis is quite common, but the severity nail psoriasis not always reflects the severity of skin disease.45 According to a consensus by Rigopoulos et al, topicals or steroid injections on the nail unit are proposed as the first line of treatment when ≤3 nails are affected. In case of more than 3 nails, systemic treatments including biologics can be used, according to clinical manifestation and quality of life.45 The association between nail psoriasis and increased risk of PsA may support a broader use of biologics in patients with severe nail psoriasis, particularly in the presence of further risk factors of PsA. Psoriasis of the scalp, genital or folds may also predict the onset of PsA.44 Higher BMI and more specific markers of body composure such as abdominal adiposity have been reported as a potential risk factor for PsA, though with conflicting results. This may be due to obesity-associated systemic, chronic low-grade inflammation characterized by an increase in inflammatory cytokines (TNF-alpha, IL-1, IL-6, IL-8, IL-17, IL-23) and in an alteration in adipokines (leptin and adiponectin). On the other hand, obesity increases the risk of biomechanical stress and joint trauma. In turn, microtrauma at entheseal sites represents a critical disease-initiating factor as it can trigger an inflammatory cytokine cascade resulting in monocyte and lymphocyte infiltration, articular inflammatory response, adjacent synovitis, proliferation and fibrous degeneration of the synovia.47 The association between PsA and smoking is also controversial.33 Some studies considered smoking as a protecting factor in psoriatic patients, but a risk factor to develop PsA in the general population. Such a discrepancy seems to be due to a collider bias (ie, when exposure and outcome each influence a common third variable, and that variable or collider is controlled for by design or analysis),48 and it is still unclear whether smoking leads to addition risk for developing PsA. Furthermore, Xie et al reviewed observational studies investigating the association between modifiable lifestyle and environmental factors and risk of PsA.34 No significant associations were observed regarding alcohol consumption (OR, 0.99; 95% CI, 0.88–1.13), female hormonal exposure (OR, 1.45; 95% CI, 0.95–2.20), and psychologically traumatic events.34 Ultimately, the role of the microbiota in driving the development of PsA by promoting the production of pro-inflammatory cytokines such as IL-17, IL-22 and TNF-a is currently under investigation. Dysfunctional intestinal mucosal immunity was reported by different studies; in particular, PsA was associated with a lower overall microbiota intestinal diversity, including reduced proportions of beneficial, commensal microorganisms.49
Prevention of PsA in Patients with Psoriasis
Both conventional Disease Modifying Anti Rheumatic Drug (DMARDs) such as methotrexate and targeted synthetic agents such as PDE4 inhibitors as well as biologics, including TNF alpha, IL-17, IL-12/23 and IL-23 inhibitors, show clear efficacy in reducing the signs and symptoms of both psoriasis and PsA. Assuming shared pathogenetic pathways, it is thus biologically plausible that treatment of moderate to severe psoriasis to be associated with reduced progression to clinically overt PsA.50,51 Studies investigating the hypothesis that treatment of moderate to severe psoriasis prevent psoriatic arthritis are summarized in Table 2. Savage et al found that subclinical enthesopathy in patients with moderate-to-severe psoriasis regressed with the use of ustekinumab.52 In particular, the mean inflammation scores decreased significantly by 42.2% from week 0 to week 24 and by 47.5% by week 42.52 The Interception in Very Early PsA (IVEPSA) study is a single-arm prospective open-label study assessing the effects of secukinumab on the inflammatory and structural changes in the peripheral joints of patients with psoriasis and arthralgia (but not PsA).53 Of the 20 patients included, arthralgia significantly improved after 24 weeks, psoriatic arthritis magnetic resonance imaging scoring system and synovitis sub score significantly improved, while erosions and enthesiophytes did not progress.53 Furthermore, four recent studies found a decreased incidence of PsA in cohorts of patients receiving systemic therapy compared with skin directed therapies (ie, phototherapy and topical therapy).54–57 We found an annual incidence rate of PsA of 1.20 cases (95% CI 0.77 to 1.89) versus 2.17 (95% CI 1.53 to 3.06) per 100 patients/year in patients with psoriasis receiving biologics versus those treated with phototherapy.52 Notably, treatment with biologics was associated with a lower risk of incident PsA (adjusted HR 0.27, 0.11–0.66).54 Felquer et al in a cohort of 1719 patients similarly found that the risk of developing PsA in patients with psoriasis treated with biologics was significantly lower (IRR = 0.26; 95% CI 0.03–0.94; p = 0.0111) compared with those treated only with topicals but not compared with cDMARDs (IRR = 0.35; 95% CI 0.035–1.96; p = 0.1007).55 Adjusted Cox proportional hazards regression analysis showed that biologics use was protective for PsA (HR = 0.19; 95% CI 0.05 to 0.81).55 Furthermore, Solmaz et al reviewed the charts of 203 psoriasis patients referred for musculoskeletal symptoms and found that the onset PsA after initiation of psoriasis treatment was lower in patients treated with systemic therapies (12% for biologics and 9.6% for cDMARDs) compared to those treated with topicals or non-treated cases (37.4%, p < 0.001), suggesting a decreased risk of “de novo” PsA in patients receiving systemic therapies.56 Moreover, among patients diagnosed with PsA, none of the patients on biologics had dactylitis as opposed to 28.6% of those on cDMARDs and 48.6% of those on no or only topical treatment (p = 0.046).56
 |
Table 2 Studies Investigating the Hypothesis That Treatment of Moderate to Severe Psoriasis Prevent Psoriatic Arthritis |
In another cohort study by Rosentahal YS et al, a total of 663 patients who had received biologic treatment for psoriasis and were not diagnosed as having PsA were compared to 663 psoriasis controls patients who had not received biologic treatment. The control group had a significantly higher risk for PsA compared to the biologic treatment group within 10 years of follow-up, aHR 1.39 (95% CI 1.03–1.87).57
Conversely, Meer et al in a retrospective cohort study of 193,709 patients with psoriasis without PsA found that, contrary to the study hypothesis, biologic use was associated with the development of PsA among patients with psoriasis.58 This may be related to confounding by indication and protopathic bias, ie, when a pharmaceutical agent is inadvertently prescribed for an early manifestation of a disease that has not yet been diagnostically detected.58 Indeed, studying the influence of systemic treatment in the transition from psoriasis to PsA is undermined by several issues. Patients need to be followed up longitudinally for a long period because arthritis develops on average of several years after initial diagnosis of psoriasis.24 The accuracy of the diagnosis of PsA is difficult to confirm (ascertainment bias) because of the heterogeneity of disease presentation and the lack of specific biomarkers.24 Also, different domains of PsA could differ in therapeutic response. Confounding by indication (ie, the reason for receiving a treatment, such as severe psoriasis, is also associated with the outcome of interest, such as PsA) and protopathic bias (ie, when a pharmaceutical agent is inadvertently prescribed for an early manifestation of a disease that has not yet been diagnostically detected) may distort the association between therapy and the development of the arthritis.58 Assessing whether treating the skin disease with biologics may prevent development of arthritis, or instead treat early or mild forms of PsA that it is less clinically apparent is problematic. Predictive algorithms that use machine learning and related approaches may contribute to analyze relevant factors that settle disease from background events in the future. Further research with long-term follow-up is needed to assess the impact of psoriasis therapy on the prevention of and/or the delay in development of PsA.
Conclusions
The clinical observation that plaque psoriasis precedes PsA in most patients implies that having psoriasis, particularly moderate-to-severe form, is a key risk factor for PsA. This could have very important implications as biological treatment of moderate-to-severe psoriasis might therefore not only treat psoriasis but also prevent the development of PsA. We acknowledge that there is currently limited evidence to confidently state whether systemic treatment of moderate-to-severe psoriasis reduces the risk of PsA, and that real-life observational studies have major methodological limitations. Planning a randomized trial for addressing this hypothesis is hardly feasible because it would imply enrolling two groups of patients with moderate-to-severe psoriasis and comparing the effect of treatment versus no treatment for very long time periods. However, the consideration of reduction of PsA development as new clinical endpoint in PsO clinical trials may be of great importance, particularly in the subset of PsO patients at high risk for transition. The issue of “Treat the skin To Intercept PsA” will be a fascinating challenge for the next years.59–62
Disclosure
Gisondi P has been a consultant and/or speaker for Abbvie, Almirall, Amgen, Janssen, Leo-pharma, Eli Lilly, Novartis, Pierre Fabre, Sandoz, Sanofi, UCB. Girolomoni G served as consultant and/or speaker for AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli-Lilly, Leo Pharma, Novartis, Pfizer, Regeneron, Sanofi, Samsung and UCB. Zabotti A has been a consultant and/or speaker fo Abbvie, Amgen, Janssen, Eli Lilly, Novartis, UCB.
D McGonagle has been a consultant and/or speaker for Abbvie, Amgen, Janssen, Eli Lilly, Novartis, UCB, Pfizer, BMS. The authors report no other conflicts of interest in this work.
References
1. Dominguez-Rosado I, Moutinho V, DeMatteo RP, Kingham PT, D’Angelica M, Brennan MF. Outcomes of the memorial sloan kettering cancer center international general surgical oncology fellowship. J Am Coll Surg. 2016;222(5):961–966. doi:10.1016/j.jamcollsurg.2016.01.049
2. Scotti L, Franchi M, Marchesoni A, Corrao G. Prevalence and incidence of psoriatic arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2018;48:28–34.
3. Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–265.e19.
4. Pennington SR, FitzGerald O. Early origins of psoriatic arthritis: clinical, genetic and molecular biomarkers of progression from psoriasis to psoriatic arthritis. Front Med. 2021;18(8):723944.
5. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957–970. doi:10.1056/NEJMra1505557
6. Charlton RA, Tillett W, Nightingale AL, et al.; the PROMPT Study Group. Interval between onset of psoriasis and psoriatic arthritis: comparing the United Kingdom Clinical Practice Research Datalink with a hospital-based cohort. Rheumatology. 2017;56:66.
7. Christophers E, Barker JN, Griffiths CE, et al. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. J Eur Acad Dermatol Venereol. 2010;24(5):548–554.
8. Merola JF, Tian H, Patil D, et al. Incidence and prevalence of psoriatic arthritis in patients with psoriasis stratified by psoriasis disease severity: retrospective analysis of an electronic health records database in the United States. J Am Acad Dermatol. 2022;86(4):748–757. doi:10.1016/j.jaad.2021.09.019
9. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55–78.
10. McHugh NJ, Balachrishnan C, Jones SM. Progression of peripheral joint disease in psoriatic arthritis: a 5-yr prospective study. Rheumatology. 2003;42:778–783.
11. Gisondi P, Altomare G, Ayala F, et al. Consensus on the management of patients with psoriatic arthritis in a dermatology setting. J Eur Acad Dermatol Venerol. 2018;32:515–528.
12. Rech J, Sticherling M, Stoessel D, Biermann MHC, Häberle BM, Reinhardt M. Psoriatic arthritis epidemiology, comorbid disease profiles and risk factors: results from a claims database analysis. Rheumatol Adv Pract. 2020;4:rkaa033.
13. Green A, Tillett W, McHugh N, Smith T; PROMPT Study Group. Using Bayesian networks to identify musculoskeletal symptoms influencing the risk of developing psoriatic arthritis in people with psoriasis. Rheumatology. 2022;61(2):581–590.
14. McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29–38.
15. Marchesoni A, De Marco G, Merashli M, et al. The problem in differentiation between psoriatic-related polyenthesitis and fibromyalgia. Rheumatology. 2018;57:32–40.
16. Patrick MT, Stuart PE, Raja K, et al. Genetic signature to provide robust risk assessment of psoriatic arthritis development in psoriasis patients. Nat Commun. 2018;9:4178.
17. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H; CASPAR Study Group. Classification criteria for psoriatic arthritis; development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673.
18. Chandran V, Schentag CT, Gladman DD. Sensitivity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Rheum. 2007;57:1560–1563.
19. Coates LC, Conaghan PG, Emery P, et al. Sensitivity and specificity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Rheum. 2012;64:3150–3155.
20. Kehl AS, Corr M, Weisman MH. Review: enthesitis: new insights into pathogenesis, diagnostic modalities, and treatment. Arthritis Rheumatol. 2016;68:312.
21. Kaeley GS, Eder L, Aydin SZ, et al. Dactylitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum. 2018;48:263–273.
22. Leijten EF, van Kempen TS, Olde Nordkamp MA, et al. Tissue-resident memory CD8+ T cells from skin differentiate psoriatic arthritis from psoriasis. Arthritis Rheumatol. 2021;73:1220–1232.
23. McGonagle D, Tan AL, Watad A, Helliwell P. Pathophysiology, assessment and treatment of psoriatic dactylitis. Nat Rev Rheumatol. 2019;15:113–122.
24. Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. 2019;15:153–166.
25. Zenz R, Eferl R, Kenner L, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–375.
26. Zabotti A, Tinazzi I, Aydin SZ, McGonagle D. From psoriasis to psoriatic arthritis: insights from imaging on the transition to psoriatic arthritis and implications for arthritis prevention. Curr Rheumatol Rep. 2020;22(6):24.
27. Gisondi P, Tinazzi I, El-Dalati G, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67:26–30.
28. Zuliani F, Zabotti A, Errichetti E, et al. Ultrasonographic detection of subclinical enthesitis and synovitis: a possible stratification of psoriatic patients without clinical musculoskeletal involvement. Clin Exp Rheumatol. 2019;37(4):593–599.
29. Naredo E, Möller I, de Miguel E, et al. Ultrasound school of the Spanish society of rheumatology and Spanish eco-aps group. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology. 2011;50:1838–1848.
30. Simon D, Tascilar K, Kleyer A, et al. Association of structural entheseal lesions with an increased risk of progression from psoriasis to psoriatic arthritis. Arthritis Rheumatol. 2022;74:253–262.
31. Wang Y, Zhang L, Yang M, et al. Development of a predictive model for screening patients with psoriasis at increased risk of psoriatic arthritis. Dermatol Ther (Heidelb). 2022;12:419–433.
32. Zabotti A, McGonagle DG, Giovannini I, et al. Transition phase towards psoriatic arthritis: clinical and ultrasonographic characterisation of psoriatic arthralgia. RMD Open. 2019;5(2):e001067.
33. Mulder MLM, van Hal TW, Wenink MH, et al. Clinical, laboratory, and genetic markers for the development or presence of psoriatic arthritis in psoriasis patients: a systematic review. Arthritis Res Ther. 2021;23:168.
34. Xie W, Huang H, Deng X, Gao D, Zhang Z. Modifiable lifestyle and environmental factors associated with onset of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2021;84:701–711.
35. Winchester R, FitzGerald O. The many faces of psoriatic arthritis: their genetic determinism. Rheumatology. 2020;59(Suppl 1):i4–i9.
36. Batalla A, Coto E, González-Lara L, et al. Association between single nucleotide polymorphisms IL17RA rs4819554 and IL17E rs79877597 and psoriasis in a Spanish cohort. J Dermatol Sci. 2015;80:111–115.
37. Vecellio M, Hake VX, Davidson C, Carena MC, Wordsworth BP, Selmi C. The IL-17/IL-23 axis and its genetic contribution to psoriatic arthritis. Front Immunol. 2021;11:596086.
38. Bowes J, Budu-Aggrey A, Huffmeier U, et al. Dense genotyping of immune-related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat Commun. 2015;6:6046.
39. Bowes J, Loehr S, Budu-Aggrey A, et al. PTPN22 is associated with susceptibility to psoriatic arthritis but not psoriasis: evidence for a further PsA-specific risk locus. Ann Rheum Dis. 2015;74:1882–1885.
40. Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology. 2013;52:568–575.
41. Soltani-Arabshahi R, Wong B, Feng BJ, Goldgar DE, Duffin KC, Krueger GG. Obesity in early adulthood as a risk factor for psoriatic arthritis. Arch Dermatol. 2010;146:721–726.
42. Tey HL, Ee HL, Tan AS, Theng TS, Wong SN, Khoo SW. Risk factors associated with having psoriatic arthritis in patients with cutaneous psoriasis. J Dermatol. 2010;37:426–430.
43. Zabotti A, De Lucia O, Sakellariou G, et al. Predictors, risk factors, and incidence rates of psoriatic arthritis development in psoriasis patients: a systematic literature review and meta-analysis. Rheumatol Ther. 2021;8:1519–1534.
44. Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61:233–239.
45. Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915–923.
46. Rigopoulos D, Baran R, Chiheb S, Daniel CR. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228–240.
47. Schett G, Lories RJ, D’Agostino MA, et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol. 2017;13:731–741.
48. Holmberg MJ, Andersen LW. Collider Bias. JAMA. 2022;327:1282–1283.
49. Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128–139.
50. Magina S, Filipe P. Pathophysiology of moderate to severe plaque psoriasis: anti-IL-17 towards disease modification. Drugs Today. 2021;57(5):347–357.
51. Soriano ER. Interventions on modifiable risk factors for the development of psoriatic arthritis. Curr Treat Options in Rheum. 2019;5:313–325.
52. Savage L, Goodfield M, Horton L, et al. Regression of peripheral subclinical enthesopathy in therapy-naive patients treated with Ustekinumab for moderate-to-severe chronic plaque psoriasis: a fifty-two-week, prospective, open-label feasibility study. Arthritis Rheumatol. 2019;71:626–631.
53. Kampylafka E, Simon D. Disease interception with interleukin-17 inhibition in high-risk psoriasis patients with subclinical joint inflammation-data from the prospective IVEPSA study. Arthritis Res Ther. 2019;21:178.
54. Gisondi P, Bellinato F, Targher G, Idolazzi L, Girolomoni G. Biological disease-modifying antirheumatic drugs may mitigate the risk of psoriatic arthritis in patients with chronic plaque psoriasis. Ann Rheum Dis. 2022;81:68–73.
55. Acosta Felquer ML, LoGiudice L, Galimberti ML, Rosa J, Mazzuoccolo L, Soriano ER. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Ann Rheum Dis. 2022;81:74–79.
56. Shalev Rosenthal Y, Schwartz N, Sagy I, et al. Psoriatic arthritis incidence among patients receiving biologic medications for psoriasis: a nested case control study. Arthritis Rheumatol. 2021;1:845.
57. Solmaz D, Ehlebracht A, Karsh J, Bakirci S, McGonagle D, Aydin SZ. Evidence that systemic therapies for psoriasis may reduce psoriatic arthritis occurrence. Clin Exp Rheumatol. 2020;38(2):257–261.
58. Meer E, Merola JF, Fitzsimmons R, et al. Does biologic therapy impact the development of PsA among patients with psoriasis? Ann Rheum Dis. 2022;81:80–86.
59. McGonagle DG, Zabotti A, Watad A, et al. Intercepting psoriatic arthritis in patients with psoriasis: buy one get one free? Ann Rheum Dis. 2022;81:7–10.
60. Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045–1050.
61. Queiro R, Morante I, Cabezas I, Acasuso B. HLA-B27 and psoriatic disease: a modern view of an old relationship. Rheumatology. 2016;55:221–229.
62. Tinazzi I, McGonagle D, Biasi D, et al. Preliminary evidence that subclinical enthesopathy may predict psoriatic arthritis in patients with psoriasis. J Rheumatol. 2011;38:2691–2692.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
