Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
The Combination of IL-6, PLR and Nail Psoriasis: Screen for the Early Diagnosis of Psoriatic Arthritis
Authors Liu X, Zhao Y , Mu Z, Jia Y, Liu C, Zhang J, Cai L
Received 23 March 2023
Accepted for publication 16 June 2023
Published 28 June 2023 Volume 2023:16 Pages 1703—1713
DOI https://doi.org/10.2147/CCID.S413853
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Xiaoyang Liu,1 Yan Zhao,1 Zhanglei Mu,1 Yuan Jia,2 Chen Liu,3 Jianzhong Zhang,1 Lin Cai1
1Department of Dermatology, Peking University People’s Hospital, Beijing, People’s Republic of China; 2Department of Rheumatology and Immunology, Peking University People’s Hospital, Beijing, People’s Republic of China; 3Department of Clinical Laboratory, Peking University People’s Hospital, Beijing, People’s Republic of China
Correspondence: Lin Cai, Department of Dermatology, Peking University People’s Hospital, # 11. Xizhimen South St, Beijing, 100044, People’s Republic of China, Tel +8610 88325472, Fax +8610 88325474, Email [email protected]
Background: Early screening or timely prediction of psoriatic arthritis (PsA) are crucial. The study aimed to compare the clinical characteristics, cytokines and inflammation index between plaque psoriasis and PsA to explore their values in the early diagnosis of PsA.
Methods: This was a case-control study in a single center from January 2021 to February 2023. The differences in clinical characteristics and laboratory examinations between PsA and plaque psoriasis were conducted. Patients with rheumatoid arthritis (RA) were used as a positive control. The correlation between variables were analyzed and multivariable logistic regression were performed by using the 10-fold cross-validation to find independently risk factors of plaque psoriasis that are developing PsA.
Results: A total of 109 patients with plaque psoriasis (without joint damage), 47 patients with PsA and 41 patients with RA were enrolled in this study. The study found that the proportion of patients with elevated serum IL-6 levels, as well as the value of platelet to lymphocyte ratio (PLR) and systemic immune-inflammation index (SII), were significantly higher in patients with PsA and early PsA (PsA course ≤ 2 years) compared to those with plaque psoriasis (p< 0.05). After adjusting for age, gender, severity of skin lesions, and comorbidities (diabetes, hypertension, hyperlipidemia, hyperuricemia, and overweight/obesity), the study identified nail psoriasis (OR=4.35, 95% CI 1.67– 11.29, p< 0.002), elevated serum IL-6 (OR=6.78, 95% CI 2.34– 19.67, p< 0.001), and PLR (OR=8.37, 95% CI 2.97– 23.61, p< 0.001) as independent risk factors for PsA. A multivariable logistic regression analysis employing 10-fold cross-validation assessing the predictive association between the diagnosis of early PsA and the combination of IL-6, PLR, and nail psoriasis demonstrated that the area under the curve (AUC) was 0.84 (95% CI 0.77– 0.90) and the F1-score was 0.67 (95% CI 0.54– 0.80).
Conclusion: The combination of elevated serum IL-6, PLR, and nail psoriasis can help to predict and screen the early stage of PsA.
Keywords: biomarkers, IL-6, PLR, psoriasis, psoriatic arthritis
Introduction
Psoriatic arthritis (PsA) is an immune-mediated inflammatory disease affecting joints and entheses, and is strongly associated with psoriasis. The majority of patients, approximately 85% to 91.67%, experience psoriasis prior to the onset of arthritis by a span of 7 to 10 years.1–3 This lag time creates a unique opportunity, particularly for dermatologists, to identify patients with an increased risk for developing PsA. Unfortunately, a delay in diagnosing PsA by 6 months resulted in a higher chance of peripheral erosive disease and poorer physical function.4 Consequently, early screening or timely prediction of PsA are crucial. Non-rheumatologists may encounter challenges in accurately identifying PsA or inflammatory joint disease at the early stage. Screening for PsA markers among patients with psoriasis may aid in identifying the onset of PsA as early as possible. However, the specific early biomarkers for PsA remain unclear.
Proinflammatory and proliferation mediators, such as interleukin (IL)-6, IL-8, IL-17, IL-22, IL-23, tumor necrosis factor (TNF)-α, interferon (IFN)-α, and IFN-γ have been proved to be involved in the pathogenesis of psoriasis, and some of these mediators have been targeted for drug development.5,6 However, inhibitors targeting the IL-23/IL-17 axis significantly improve psoriasis outcomes, but their effects on PsA skeletal symptoms are not superior to TNF inhibitors.1 Similarly, compared with placebo, IL-6 monoclonal antibody treatment significantly improved musculoskeletal manifestations (joint signs and symptoms, enthesitis, and dactylitis), but with minimal improvements in skin.7 This disparity may be attributed to differential cytokine or chemokine expression between skin lesions and joint lesions. Pro-inflammatory cytokines such as IL-1, TNF-a, IL-6 and IL-17 induce the production of matrix metalloproteinases by chondrocytes and neutrophils, which enzymatically degrade the extra-cellular matrix of bone and cartilage, thereby contributing to erosions and joint space narrowing.8 It was reported that IL-1, IL-6 and IL-8 seemed to be more closely associated with PsA,9–11 and cutaneous expression of CXCL12 and IL-23R has been observed higher in PsA patients compared to psoriasis vulgaris patients.12,13 Bos et al14 also found that PsA patients had a marked increase in IL-2 production of T cells in the circulation compared with cutaneous psoriasis. Szentpetery et al15 reported that reduction in Treg expression in the synovium of PsA but not in the psoriatic lesion, so IL-10 which was representative Treg cell may also be different in serum between PsA and cutaneous psoriasis. Furthermore, neutrophil and platelet activation play a role in psoriasis pathogenesis by producing various inflammatory cytokines,16,17 which are increased in both the blood and active lesions of psoriatic patients. But the counts of neutrophils and platelet are easily fluctuated due to dehydration or overhydration, dilution and processing of blood specimens. The neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR) and systemic immune-inflammation index (SII) are relatively stable and have emerged as reliable indicators of sub-clinical systemic inflammation and poor prognosis in various diseases, including PsA.18
To the best of our knowledge, no specific biomarkers for PsA have been identified, and only a limited number of studies have investigated the combination of different markers to aid in the diagnosis of PsA. Hence, this study aimed to analyze the differences in serum cytokines (IL-1β, IL-2, IL-6, IL-8, IL-10, IL-17A, IL-17F, IL-22, IFN-γ, TNF-α), NLR, PLR and SII among patients with PsA, plaque psoriasis and rheumatoid arthritis (RA) individually. Furthermore, these laboratory markers were combined with clinical markers to explore their potential in facilitating the early diagnosis of PsA.
Materials and Methods
Study Population
The study population comprised adult patients who visited Peking University People’s Hospital between January 2021 and February 2023. The criteria for enrollment of patients were set as follows: (a) Plaque psoriasis patients were diagnosed by dermatologists according to clinical or histopathological manifestations, without any signs of arthritis or symptoms of joint involvement (including swelling, pain, morning stiffness >30 minutes in any joint, and deformity). (b) PsA patients were diagnosed by rheumatologist according to Classification Criteria for Psoriatic Arthritis (CASPAR criteria), without any other forms of arthritis including RA, osteoarthritis, gouty arthritis, ankylosing spondylitis, and reactive arthritis. (c) RA patients were diagnosed by rheumatologist. (d) Key exclusion criteria included types of psoriasis other than chronic plaque-type psoriasis, including guttate psoriasis, pustular psoriasis, erythrodermic psoriasis, drug-induced psoriasis. (e) In all psoriasis patients, there were no use of biologics in previous 3 months, systemic immune-modulating agents in 1 month, and phototherapy or topical treatment in 2 weeks before collecting of clinical information and laboratory examinations. (f) Patients without any liver and kidney dysfunction, hematological diseases, severe infections, immunosuppression, skeletal muscle injury. (g) Patients with incomplete important data or failed follow-up were excluded.
Study Design
The clinical characteristics of patients with psoriasis were collected from electronic medical records, which included demographical characteristics, duration of psoriasis, nail psoriasis, location of psoriasis, severity of psoriasis measured by body surface area (BSA) and Psoriasis Area and Severity Index (PASI), number of tender and swollen joints, history of psoriasis, comorbidities and treatment history. Patients who were diagnosed with PsA or developed joint symptoms of PsA within 2 years were classified as the early PsA group,19 while those with a duration of PsA exceeding 2 years were categorized as the non-early PsA group.
The laboratory examinations of patients with psoriasis and RA included serum cytokines (IL-1β, IL-2, IL-6, IL-8, IL-10, IL-17A, IL-17F, IL-22, IFN-γ, TNF-α), neutrophils, lymphocytes, platelets, mean platelet volume (MPV), NLR (neutrophil to lymphocyte ratio), PLR (platelet to lymphocyte ratio), systemic immune-inflammation index (SII, platelet × neutrophil / lymphocyte ratio), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). The same time period records were collected for each patient to ensure consistency.
Sample Size Calculation
The sample size was calculated using PASS software (version 15.0.5, NCSS, Kaysville, UT, USA). Based on previous studies, the prevalence of PsA in patients with psoriasis was generally in the range of 14%-30%,20–22 and the incidence of nail involvement was 46.4% in patients with PsA, and 21.0% in patients with psoriasis without joint damage.23 In this study, the ratio of patients with PsA to patients with psoriasis without joint damage was estimated to be 1:2.3, and the sample size was increased by 10% to account for sample quality. The two-sided significance level (alpha) was 0.05, a total of 156 participants (47 for patients with PsA and 109 for patients with psoriasis without joint damage) were calculated to require and obtain 85% power to reject the null hypothesis using a two-sample unequal-proportion test.
Statistical Analysis
All statistical analyses were performed with SPSS version 24.0 software (SPSS, Chicago, IL, USA), GraphPad Prism version 5.0 software (GraphPad, San Diego, CA, USA) and R4.1.2 software rms package (version 6.2–0). Continuous variables were expressed as the mean ± standard deviation or median (interquartile range, IQR). Categorical data were expressed as a frequency (percentage). Quantitative indicators were compared using the Student’s t-test, One-Way ANOVA, or Mann–Whitney U-test according to the data distribution. Categorical indicators were compared by the chi-square test and Fisher exact test. Correlation analysis was performed by calculating the Spearman correlation. Multiple imputations were used for missing PASI score, height and weight, and sensitivity analysis was conducted. Receiver operating characteristic curve (ROC) was used to set out the optimal cut-off values of NLR, PLR and SII. Multivariable logistic regression analysis was performed to find independently related variables for PsA among psoriasis patients, and the 10-fold cross-validation method was used to prevent over-fitting. Furthermore, the area under the curve (AUC), the specificity, the sensitivity, and F1-score were calculated to evaluate the fit of the model. In all analyses, statistical significance was defined as a p value < 0.05 or Bonferroni-corrected p-value.
Results
Patient Characteristics
A total of 156 adult patients with psoriasis were enrolled in the study, including 47 patients with PsA and 109 patients with plaque psoriasis without joint involvement. The mean age of patients with PsA was 47.15±12.97 years, which was older than that of plaque psoriasis group (42.15 ± 12.58 years, p<0.05). The age at onset of psoriasis in the PsA group was later than that in the plaque psoriasis group (p<0.05). There was a significant difference in male to female ratio between the PsA group and the plaque psoriasis group (1:1.14 vs 2.21:1, p<0.05). The proportions of skin lesion involvement in trunk and limbs were significantly higher in patients with plaque psoriasis compared to those with PsA (p<0.05). The proportion of nail damage in patients with PsA was significantly higher than that in plaque psoriasis (68.1% vs 41.3%, p<0.05). The BSA and PASI scores were higher in the plaque psoriasis group than in the PsA group (p<0.05). Among the PsA group, 20 patients (42.6%) had early PsA, and the median duration of arthritis in PsA patients was 2 years (IQR 1.00–6.00). In the PsA group, 80.9% (38/47) of patients had skin lesions prior to joint involvement, and the median duration of joint involvement diagnosed after skin lesions was 8 years (IQR 2.69–21.50). The results after interpolation were consistent with the results before interpolation by sensitivity analysis. A total of 41 patients with RA were included in the study, and their laboratory results were used as a positive control. The demographic and disease characteristics of patients at baseline were shown in Table 1.
 |
Table 1 Clinical Characteristics Plaque Psoriasis, PsA and RA |
Comparison of Laboratory Results Among PsA, Plaque Psoriasis and RA
The proportion of IL-6 and IL-8 elevation in the RA group were significantly higher than those in the PsA group and plaque psoriasis group (p<0.0167). Additionally, the levels of serum IL-6 (29.67 [12.70–83.00] vs 7.49 [2.58–28.32]) and IL-8 (35.21 [15.67–56.72] vs 0 [0–0]) in RA group were significantly higher than those in the PsA group (p<0.01). Furthermore, the proportion of IL-6 elevation in the PsA group was significantly higher than that in the plaque psoriasis group (p<0.0167). Conversely, the proportion of TNF-α elevation in the plaque psoriasis group was significantly higher than that in the PsA group and RA group (p<0.0167). There were no significant differences in IL-1β, IL-2, IL-10, IL-17A, IL-17F and IFN-γ among the three groups (p>0.05). Further details can be found in Table 2 and Figure 1A and B.
 |
Table 2 Comparison of Serum Interleukins Among PsA, Plaque Psoriasis and RA Group |
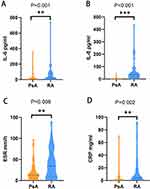 |
Figure 1 Comparison of laboratory results between PsA and RA. The level of IL-6 (A), IL-8 (B), ESR (C) and CRP (D) in RA group were significantly higher than those in PsA group. **p<0.01, ***p<0.001. |
The value of MPV in the PsA group was significantly higher than that in the RA group (adjusted p<0.05). The values of PLR and SII in the plaque psoriasis group were significantly lower than those in the RA group and PsA group (adjusted p<0.05) (Table 3). Within the PsA group, ESR (13.00 [7.00–23.00] vs 34.00 [8.50–63.50]) and CRP (0.67 [0.00–5.80] vs 4.40 [1.05–19.25]) levels were significantly lower than those in the RA group (p<0.01) (Figure 1C and D).
 |
Table 3 Comparison of Blood Routine Among PsA, Plaque Psoriasis and RA Group |
The differences in IL-6, IL-8, NLR, PLR, and SII were separately analyzed among patients with early PsA, non-early PsA and plaque psoriasis. The results revealed that the proportion of serum IL-6, PLR and SII elevation in patients with early PsA was significantly higher than those in patients with plaque psoriasis (p <0.0167), and the proportion of PLR elevation in patients with non-early PsA was significantly higher than that in patients with plaque psoriasis (51.9% vs 25.7%, p<0.0167) (Table 4).
 |
Table 4 Comparison of Biomarkers Among Early PsA, Non-Early PsA and Plaque Psoriasis |
Correlation Analyses of Risk Factors in Patients with PsA
IL-6 exhibited a positive correlation with IL-8 (rs=0.571, p<0.001), ESR (rs=0.347, p<0.05) and CRP (rs=0.311, p<0.05); NLR showed a positive correlation with PLR (rs=0.711, p<0.001), SII (rs=0.906, p<0.001), ESR (rs=0.545, p<0.001) and CRP (rs=0.389, p<0.01); PLR had a positive correlation with SII (rs=0.791, p<0.001), ESR (rs=0.413, p<0.01) and CRP (rs=0.323, p<0.05); SII had a positive correlation with ESR (rs=0.504, p<0.001) and CRP (rs=0.400, p<0.01); ESR was positively correlated with CRP (r=0.617, p<0.001).
Furthermore, the correlations between the number of joints for swelling and tenderness and IL-6, IL-8, NLR, PLR, SII, CRP and ESR were studied separately. The results showed that the level of IL-6 (rs=0.521, p<0.001), IL-8 (rs=0356, p<0.05), ESR (rs=0.517, p<0.001) and CRP (r=0.421, p<0.01) were positively correlated with the number of joints for swelling and tenderness, respectively. However, there were no significant correlations observed between the number of affected joints and NLR, PLR, or SII. Further details were showed in Figure 2.
The Prediction of Risk Factors for PsA
We conducted multivariable logistic regression analyses to assess the association between risk factors, including serum IL-6, serum IL-8, NLR, PLR, SII, and nail psoriasis, and the presence of PsA. The results revealed a significant association between PsA and nail psoriasis, as well as IL-6 and PLR. After adjusting for age, gender, comorbidities related to psoriasis, and the severity of psoriasis lesions, the risk factors for PsA remained consistent, including nail psoriasis, elevated serum IL-6, and PLR (Table 5).
 |
Table 5 Multivariable Logistic Regression |
To mitigate the potential over-fitting issue caused by a small sample size, we employed 10-fold cross-validation. The predictive power was estimated by the area under curve (AUC) with an AUC of 0.84 (95% CI 0.77–0.90), and with a specificity of 0.88 (95% CI 0.82–0.94), a sensitivity of 0.62 (95% CI 0.46–0.77), and F1-score of 0.67 (95% CI 0.54–0.80), these results showed that the combination of nail psoriasis, serum IL-6 and PLR had a good fitting degree and generalization ability for the early diagnosis of PsA. Figure 3 illustrated that the calibration curve fluctuated around the ideal curve, indicating that the adjusted-model had a good prediction performance and repeatability.
 |
Figure 3 Calibration curve for the Model-2 internal verification set. |
Discussion
Mulder et al24 conducted a systematic analysis of 51 relevant clinical markers to evaluate the progression of psoriasis to PsA or the presence of PsA. The results showed that nail pitting, intergluteal skin lesion, uveitis, joint symptoms, the use of corticosteroid and retinoid, and lifting heavy loads were identified as positive predictors of moderate evidence for PsA progression in psoriasis patients. However, most of the other markers showed conflicting results or demonstrated no significant association.24 Nail psoriasis is observed in approximately 15% to 50% of patients with psoriasis, but in about 41% to 93% of patients with PsA.1 In this study, the proportion of nail psoriasis was significantly higher in patients with PsA than plaque psoriasis (68.1% vs 41.3%, p<0.05). Therefore, nail psoriasis, especially pitting lesions, is considered a risk factor for the development of PsA in patients with psoriasis.6 Castellanos-Gonzalez et al25 found that 82.6% of psoriasis with entheseal abnormalities had nail involvement, as the nails were directly anchored to the underlying bone through structures such as the extensor tendons. In addition, on physical examination, nail lesions were often easily detected and diagnosed by dermatologists and rheumatologists, while other manifestations related to PsA were uncommon or difficult to detect. Therefore, nail lesions serve as sensitive clinical markers for the diagnosis of PsA.
Immune disorder plays a pivotal role in the pathogenesis of psoriasis. Upon activation, dendritic cells secrete IL-23, which subsequently stimulates Th17 cells to release proinflammatory cytokines like IL-17 and IL-22. These cytokines act on keratinocytes, triggering the production of additional inflammatory mediators such as TNF-α, IL-23, IL-6, and IL-8, thereby sustaining and amplifying the immune-inflammatory response.5,22 Previous studies had shown that the levels of IL-6 and IL-8 in joint effusion and peripheral blood of patients with PsA were significantly higher than those of patients with psoriasis vulgaris.26,27 Similarly, our study revealed a significant association between serum IL-6 levels and the development of PsA, while serum IL-8 levels did not exhibit a significant association.
The exact role of IL-6 in the pathogenesis of PsA remains unclear. IL-6 promotes synovitis mainly by inducing angiogenesis, inflammatory cell infiltration and synovial hyperplasia. Additionally, IL-6 induces osteoclast formation and contributes to bone resorption and cartilage degradation through NF-ΚB signaling pathway.28 A phase IIb clinical trial showed that clazakizumab, a monoclonal antibody against IL‑6, may be an effective treatment option for musculoskeletal aspects of PsA. However, Madureira et al29 reported that the efficacy of tocilizumab, an IL‑6 receptor blockade, in the treatment of PsA was conflicting. This discrepancy could be attributed to disease heterogeneity, as IL-6 may drive the pathogenesis in certain patients.30 In this study, serum IL-6 levels were found to be higher in patients with early PsA than non-early PsA. Therefore, we hypothesized that the serum IL-6 levels might be associated with different stages of PsA, with increased levels in early PsA potentially linked to IL-6 involvement in early osteoclast activity. However, IL-6 gradually decreased in the later stages of osteogenesis. Moreover, IL-6 is widely recognized as a marker of systematic inflammation and an important factor in the production of CRP by hepatocyte.24 In this study, serum IL-6 levels were shown to positively correlate with ESR, CRP and the number of joints with swelling and pain in patients with PsA, which was consistent with literature reports.11,27 These findings suggest that IL-6 could serve as a useful marker for evaluating PsA disease activity.
In recent years, there has been a growing number of studies investigating the role of NLR and PLR in the diagnosis and assessment of inflammatory diseases. Lymphocytes are thought to be the main immune cells involved in the pathogenesis of psoriasis, and abnormalities in neutrophils may also contribute to innate immune disorders, potentially contributing to the development of psoriasis.31 Shao et al17 discovered that neutrophil activation can trigger the release of neutrophil extracellular traps (NETs) and activate Toll-like receptors on keratinocytes. This downstream signaling pathway may play a role in the pathogenesis of psoriasis. Moreover, patients with psoriasis experience ongoing platelet consumption due to increased clotting responses. Consequently, larger and less mature platelets are released into the peripheral circulation, and these abnormal platelets participate in the immune regulation of psoriasis by releasing chemokines, cytokines, and expressing adhesion molecules and surface receptors.16,32
NLR, PLR and SII, as a combination of innate and acquired immune components, are less susceptible to fluctuations during the detection process, providing better stability and reliability compared to single blood cell values. In this study, the PLR of patients with PsA was significantly higher than that of patients with psoriasis vulgaris (p< 0.05), and the elevated PLR was identified as an independent risk factor for PsA, whereas NLR and SII showed no significant correlation with PsA. Kim et al18 discovered that NLR and PLR were higher in peripheral blood of patients with PsA than those in patients with psoriasis vulgaris, and elevated NLR (OR=3.351, 95% CI 1.785–6.292) and PLR (OR=1.102, 95% CI 1.003–1.021) were risk factors for PsA. Similarly, Asahina et al33 found significantly higher NLR and PLR values in patients with PsA compared to those with psoriasis vulgaris. Moreover, although ESR and CRP are commonly used to assess disease severity in PsA patients, elevated levels are only observed in approximately 50% of patients,6 indicating suboptimal sensitivity. Therefore, NLR and PLR may be more specific than CRP for systemic inflammation response.33 In this study, NLR and PLR exhibited a positive correlation with ESR and CRP in patients with PsA, consistent with the existing literature.18,33 Therefore, NLR and PLR can be used as alternative markers of PsA disease activity. However, due to the lack of consensus regarding normal range or cut-off values for NLR and PLR, these biomarkers may be more effectively utilized within individual patients to monitor treatment efficacy or assess subclinical inflammation change.
As a systemic disease, psoriasis, particularly PsA, is associated with metabolic syndrome, diabetes, dyslipidemia, hyperuricemia, obesity and other diseases.21,34,35 Additionally, inflammatory factors such as IL-6, IL-8, NLR, and PLR are influenced by blood lipid metabolism, blood glucose, and uric acid.27,36,37 In this study, the influence of age, gender, skin lesions, and comorbidities was adjusted using multivariable logistic regression, revealing that nail psoriasis, elevated serum IL-6, and PLR were independent risk factors for PsA. Furthermore, a 10-fold cross-validation was performed to assess the predictive correlation between the combination of IL-6, PLR, and nail psoriasis and the early diagnosis of PsA. The results showed a well-fitted model, suggesting that a combination of these risk factors can serve as a reliable predictor and screening tool for early PsA.
In summary, our results showed that the serum IL-6 and PLR elevation in patients with early PsA were significantly higher than those in patients with plaque psoriasis. And nail involvements, elevation of serum IL-6 and elevation in PLR were independent risk factors for PsA. Although IL-6 and PLR are not exclusive inflammatory factors for PsA, they still exhibit a certain level of specificity and sensitivity in differentiating PsA from plaque psoriasis. Thus, the combination of serum IL-6, PLR, and nail psoriasis holds potential as markers for the early diagnosis of PsA in patients with psoriasis. However, additional research is required to determine how these values can be used in clinical practice.
The limitations of our study included the single-center design, the small number of cases and the lack of matching of patients in terms of age, sex and psoriasis severity. In our hospital, there were more patients with severe psoriasis than patients with mild to moderate psoriasis. Therefore, a large sample, multi-center, long-term prospective study should be conducted to further validate the specificity and sensitivity of the combination of IL-6, PLR and nail psoriasis for the early diagnosis of PsA. However, this study provided a theoretical basis for the early diagnosis of PsA in patients with cutaneous psoriasis by combining clinical features with traditional laboratory tests.
Ethical Approval
The study protocol was reviewed by the Ethics Committee of Peking University People’s Hospital (No. 2021PHB459-001). The study was conducted according to the ethical principles of the Declaration of Helsinki. Informed consent was obtained from each patient in writing before study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. 2019;15(3):153–166. doi:10.1038/s41584-019-0175-0
2. Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–735. doi:10.1016/j.jaad.2013.07.023
3. Tillett W, Charlton R, Nightingale A, et al. Interval between onset of psoriasis and psoriatic arthritis comparing the UK Clinical Practice Research Datalink with a hospital-based cohort. Rheumatology. 2017;56(12):2109–2113. doi:10.1093/rheumatology/kex323
4. Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74(6):1045–1050. doi:10.1136/annrheumdis-2013-204858
5. Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci. 2018;19(1):179. doi:10.3390/ijms19010179
6. FitzGerald O, Ogdie A, Chandran V, et al. Psoriatic arthritis. Nat Rev Dis Primers. 2021;7(1):59. doi:10.1038/s41572-021-00293-y
7. Mease PJ, Gottlieb AB, Berman A, et al. The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a Phase IIB study of adults with active psoriatic arthritis. Arthritis Rheumatol. 2016;68(9):2163–2173. doi:10.1002/art.39700
8. Stober C. Pathogenesis of psoriatic arthritis. Best Pract Res Clin Rheumatol. 2021;35(2):101694. doi:10.1016/j.berh.2021.101694
9. Alenius GM, Eriksson C, Rantapaa Dahlqvist S. Interleukin-6 and soluble interleukin-2 receptor alpha-markers of inflammation in patients with psoriatic arthritis? Clin Exp Rheumatol. 2009;27(1):120–123.
10. Chandran V. Soluble biomarkers may differentiate psoriasis from psoriatic arthritis. J Rheumatol Suppl. 2012;89:65–66. doi:10.3899/jrheum.120247
11. Fitzgerald O, Chandran V. Update on biomarkers in psoriatic arthritis: a report from the GRAPPA 2010 annual meeting. J Rheumatol. 2012;39(2):427–430. doi:10.3899/jrheum.111241
12. Abdelaal NH, Elhefnawy NG, Abdulmonem SR, Sayed S, Saleh NA, Saleh MA. Evaluation of the expression of the stromal cell-derived factor-1 alpha (CXCL 12) in psoriatic patients after treatment with Methotrexate. J Cosmet Dermatol. 2020;19(1):253–258. doi:10.1111/jocd.12994
13. El-Leithy S, Sherif N, El-Arousy NH, El-Hilaly R, Shakweer MM. Cutaneous immunohistochemical expression of interleukin-23 receptor (IL-23R) in psoriasis and psoriatic arthritis patients: relation to musculoskeletal ultrasound findings. Egypt Rheumatol. 2020;42(4):313–318. doi:10.1016/j.ejr.2020.02.007
14. Bos F, Capsoni F, Molteni S, et al. Differential expression of interleukin-2 by anti-CD3-stimulated peripheral blood mononuclear cells in patients with psoriatic arthritis and patients with cutaneous psoriasis. Clin Exp Dermatol. 2014;39(3):385–390. doi:10.1111/ced.12251
15. Szentpetery A, Heffernan E, Gogarty M, et al. Abatacept reduces synovial regulatory T-cell expression in patients with psoriatic arthritis. Arthritis Res Ther. 2017;19(1):158. doi:10.1186/s13075-017-1364-3
16. Fan Z, Wang L, Jiang H, Lin Y, Wang Z. Platelet dysfunction and its role in the pathogenesis of psoriasis. Dermatology. 2021;237(1):56–65. doi:10.1159/000505536
17. Shao S, Fang H, Dang E, et al. Neutrophil extracellular traps promote inflammatory responses in psoriasis via activating epidermal TLR4/IL-36R crosstalk. Front Immunol. 2019;10:746. doi:10.3389/fimmu.2019.00746
18. Kim DS, Shin D, Lee MS, et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol. 2016;43(3):305–310. doi:10.1111/1346-8138.13061
19. Gladman DD. Early psoriatic arthritis. Rheum Dis Clin North Am. 2012;38(2):373–386. doi:10.1016/j.rdc.2012.05.005
20. Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–265 e219. doi:10.1016/j.jaad.2018.06.027
21. Karmacharya P, Chakradhar R, Ogdie A. The epidemiology of psoriatic arthritis: a literature review. Best Pract Res Clin Rheumatol. 2021;35(2):101692. doi:10.1016/j.berh.2021.101692
22. Ritchlin CT, Colbert RA, Gladman DD, Longo DL. Psoriatic Arthritis. N Engl J Med. 2017;376(10):957–970. doi:10.1056/NEJMra1505557
23. Yang Q, Qu L, Tian H, et al. Prevalence and characteristics of psoriatic arthritis in Chinese patients with psoriasis. J Eur Acad Dermatol Venereol. 2011;25(12):1409–1414. doi:10.1111/j.1468-3083.2011.03985.x
24. Mulder MLM, van Hal TW, Wenink MH, et al. Clinical, laboratory, and genetic markers for the development or presence of psoriatic arthritis in psoriasis patients: a systematic review. Arthritis Res Ther. 2021;23(1):168. doi:10.1186/s13075-021-02545-4
25. Castellanos-Gonzalez M, Joven BE, Sanchez J, et al. Nail involvement can predict enthesopathy in patients with psoriasis. J Dtsch Dermatol Ges. 2016;14(11):1102–1107. doi:10.1111/ddg.12989
26. Fiocco U, Sfriso P, Oliviero F, et al. Synovial effusion and synovial fluid biomarkers in psoriatic arthritis to assess intraarticular tumor necrosis factor-α blockade in the knee joint. Arthritis Res Ther. 2010;12(4):R148. doi:10.1186/ar3090
27. Pietrzak A, Chabros P, Grywalska E, et al. Serum concentration of interleukin 6 is related to inflammation and dyslipidemia in patients with psoriasis. Postepy Dermatol Alergol. 2020;37(1):41–45. doi:10.5114/ada.2018.78028
28. Ogata A, Kumanogoh A, Tanaka T. Pathological role of interleukin-6 in psoriatic arthritis. Arthritis. 2012;2012:713618. doi:10.1155/2012/713618
29. Madureira P, Pimenta SS, Bernardo A, Brito JS, Bernardes M, Costa L. Off-label use of tocilizumab in psoriatic arthritis: case series and review of the literature. Acta Reumatol Port. 2016;41(3):251–255.
30. FitzGerald O. Spondyloarthropathies: IL-6 blockade in psoriatic arthritis - a new therapeutic option? Nat Rev Rheumatol. 2016;12(6):318–319. doi:10.1038/nrrheum.2016.84
31. Wang WM, Jin HZ. Role of Neutrophils in Psoriasis. J Immunol Res. 2020;2020:3709749. doi:10.1155/2020/3709749
32. Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017;36(2):195–198. doi:10.1007/s10555-017-9677-x
33. Asahina A, Kubo N, Umezawa Y, Honda H, Yanaba K, Nakagawa H. Neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and mean platelet volume in Japanese patients with psoriasis and psoriatic arthritis: response to therapy with biologics. J Dermatol. 2017;44(10):1112–1121. doi:10.1111/1346-8138.13875
34. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397(10281):1301–1315. doi:10.1016/s0140-6736(20)32549-6
35. Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: review and update. Clin Immunol. 2020;214:108397. doi:10.1016/j.clim.2020.108397
36. Di Y, Wang J, Chen Y, et al. Elevated interleukin 1β and interleukin 6 levels in the serum of children with hyperuricemia. J Clin Rheumatol. 2018;24(2):65–69. doi:10.1097/RHU.0000000000000611
37. Chen M, Zhu Y, Wang J, Wang G, Wu Y. The predictive value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio levels of diabetic peripheral neuropathy. J Pain Res. 2021;14:2049–2058. doi:10.2147/JPR.S304595
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

