Back to Journals » Journal of Inflammation Research » Volume 16
Chronic Inflammatory and Immune Microenvironment Promote Hepatocellular Carcinoma Evolution
Authors Chen S, Zhang L, Chen Y, Zhang X, Ma Y
Received 14 August 2023
Accepted for publication 7 November 2023
Published 15 November 2023 Volume 2023:16 Pages 5287—5298
DOI https://doi.org/10.2147/JIR.S435316
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Shimin Chen,1– 4 Long Zhang,1– 4 Yukun Chen,1– 4 Xuzhi Zhang,1– 4 Yi Ma1– 4
1Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, 510080, People’s Republic of China; 2Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, 510080, People’s Republic of China; 3Guangdong Provincial Key Laboratory of Organ Donation and Transplant Immunology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, 510080, People’s Republic of China; 4Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation), The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, 510080, People’s Republic of China
Correspondence: Xuzhi Zhang; Yi Ma, Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, 510080, People’s Republic of China, Tel +86 13112274921, Email [email protected]; [email protected]
Abstract: Hepatocellular carcinoma (HCC) is among the most prevalent and dangerous cancers in the world, which is associated with hepatitis and fibrosis resulting from different etiologies, such as hepatitis B and C virus, alcohol and diabetes. Chronic inflammation is suggested to promote tumorigenesis and progression by producing inflammatory cytokines and free radicals, triggering malignant transformation of cells, promoting tumor cells proliferation and inducing tumor angiogenesis. Immunosuppressive microenvironment established in the liver also plays a vital role in HCC development. The mechanisms include activation of cancer-associated fibroblasts, upregulation of pattern recognition receptors, inhibition of effector T cells, recruitment of regulatory T cells and myeloid-derived suppressor cells, activation of hepatic stellate cells and immunosuppressive M2 macrophages. In this review, we will summarize the most recent studies and discuss the advanced mechanisms of chronic inflammatory microenvironment and immunosuppressive microenvironment promoting HCC development, aiming to explore potential therapeutic targets.
Keywords: chronic inflammation, immunosuppression, tumor microenvironment, hepatocellular carcinoma
Introduction
Primary liver cancer is the seventh most commonly diagnosed cancer and the third major cause of cancer-associated mortality worldwide,1 of which hepatocellular carcinoma (HCC) accounts for approximately 80%. Active hepatitis C and B continue to drive most of the burden of HCC.2 The usual sequence of liver injury, chronic inflammation, fibrosis, cirrhosis and HCC is clearly related to the inflammatory response caused by various immune cells.3 In acute inflammation, some of the cytokines can stimulate liver regeneration and recruit more macrophages to clear necrotic cell debris making space for new hepatocytes.4 After tissue repair or the external stimulus is eliminated, the acute inflammatory response is resolved. However, chronic inflammation is a pathological condition characterized by continued active inflammation response and tissue destruction, which can trigger malignant transformation of cells and plays a vital role in liver tumorigenesis and progression.5 Chronic hepatitis can be divided into viral and non-viral ones. Most of viral chronic hepatitis are caused by hepatitis B and C virus (HBV, HCV), while the etiologies of non-viral chronic hepatitis include obesity, alcohol, autoimmunity and so on. The infectiousness, pathological changes, rate of progression and therapies of two different hepatitis are various, but both of them take the risk of developing into HCC. Free radicals produced in inflammation can damage DNA and increase the frequency of mutations. Simultaneously, several cytokines induced by chronic inflammation, such as TNF-α and IL-6, directly promote cancer cell proliferation and tumor angiogenesis. Furthermore, chronic inflammation induces an immunosuppressive microenvironment in the liver, allowing malignant cells to escape from immune surveillance, including recruiting regulatory T (Tregs) cells and myeloid-derived suppressor cells (MDSCs), activation of innate immune receptors, activating cancer-associated fibroblasts (CAFs) and hepatic stellate cells (HSCs) and promoting polarization of tumor-associated macrophages (TAMs) from M1 to M2. In this review, we will discuss the role of the hepatic chronic inflammatory and immunosuppressive microenvironment in HCC development, and explore potential therapeutic targets.
Chronic Inflammatory Microenvironment Mediates HCC Transformation
Inflammation-Induced Free Radicals
Chronic inflammation persistently damages hepatocytes, which induces compensatory cell proliferation due to the high regenerative capacity of liver. Simultaneously, the inflammatory response increases the release of free radicals, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), contributing to DNA damage and oncogenic mutations.6 Accumulation of aberrant hepatocytes proliferation and mutations induces HCC transformation (Figure 1).
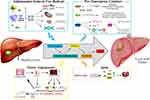 |
Figure 1 Chronic inflammatory microenvironment mediates HCC transformation. |
At the site of inflammation, increased free radicals activity is associated with the activation of the neutrophil NADPH oxidase and/or the uncoupling of a variety of redox systems, including endothelial cell xanthine dehydrogenase.7 Activation of phagocytes directly releases ROS such as superoxide, hydrogen peroxide, hypohalous acid, and hydroxyl radicals.8 Some pro-inflammatory cytokines, such as IL-6 and TNF-α, can also trigger the generation of free radicals.9–12
Increased free radicals induce damage of DNA, by producing DNA base and sugar products, single- and double-strand breaks, 8.5’-cyclopurine-2’-deoxynucleosides, tandem lesions, clustered sites and DNA-protein cross-links.12 Cell oxidative stress caused by redundant-free radicals also contributes to mitochondria dysfunction. In response to oxidative stress, the mitokines FGF21 and GDF15 are upregulated during the mitochondrial unfolded protein response, and their levels are positively correlated with liver cancer development, progression and metastasis.13 Additionally, free radicals induce structure or function modifications of cancer-related proteins and gene mutations, including those related to signal transduction, cell-cycle control, apoptosis, lipid peroxidation, and DNA repair. Jaiswal found that the expression of iNOS and 8-oxodeoxyguanine formation in primary sclerosing cholangitis patients, and RNS can inhibit DNA repair.14 There is also evidence of an association between NO and p53 mutations. NO induces p53 posttranslational modifications, leading to an increase in p53 transcriptional targets and a G(2)M cell cycle checkpoint.15 Increased iNOS expression and G:C to A:T transition mutations in p53 appear in stomach, brain and breast cancers.6 Cells with mutated p53 show an accelerated tumor growth associated with an increased expression of vascular endothelial growth factor (VEGF) and neovascularization.16 Therefore, increased ROS and RNS caused by chronic inflammation participate in liver carcinogenesis. Some substances have been reported to inhibit the progression of HCC by reducing production of free radicals, promoting removal of free radicals, or mediating the signaling pathway downstream of free radicals, which can reduce oncogene mutations and trigger apoptosis of HCC cells.
Production of Pro-Tumorigenic Cytokines
Both immune cells and stromal cells can synthesize cytokines. Cytokines mediate cell-to-cell communication, as well as regulating cell growth and differentiation, function maintenance, inflammatory response, immune response, tumor growth or decline, and cell death. Cytokines can participate in immune surveillance and prevent tumor development. However, in chronic inflammation and immunosuppressive microenvironment, they can promote the growth and progression of cancer17(Figure 1).
TNF-α is an important pro-inflammatory cytokine, related to carcinogenesis. Various types of cells can synthesize TNF-α, including activated macrophages, natural killer (NK) cells, and T cells. It is reported that high concentration of TNF-α indirectly and immunologically dependently induces an antitumor response against murine sarcoma.18 High level of TNF-α has anti-angiogenic activity, and may regulate immune responses and trigger apoptosis of tumor cells.19 However, a low level of TNF-α in chronic inflammation can act as an endogenous tumor promoter, by activating NF-κB and JNK-mediated AP-1 signaling pathway.20 TNF-α may exacerbate apoptosis, compensatory proliferation and carcinogenesis.21 ROS and RNS induced by TNF-α can damage DNA and promote tumorigenesis.11
IL-6 is another pro-inflammatory and pro-tumorigenic cytokine. IL-6 acts as an inducer of acute phase response and infection defense, as well as a potent hepatocyte mitogen in the liver, which is essential for hepatocyte homeostasis, metabolic function and liver regeneration.22 However, persistently increased IL-6 in chronic inflammation contributes to the development of HCC. In a prospective study, it was reported that higher concentrations of IL-6 were associated with an increased risk of HCC.23 IL-6 binds to gp130 and activates JAK-STAT signaling pathway, then phosphorylates STATs (STAT1 and STAT3), which function as transcriptional factors related to tumorigenesis. IL-6 induces compensatory proliferation of hepatocytes, prevents cellular senescence, and promotes tumor angiogenesis and lymphangiogenesis.24 Furthermore, IL-6 is found to be a crucial regulator of MDSCs accumulation and activation.25 IL-6 produced by TAM can promote the expansion of human hepatocellular carcinoma stem cells.26
The role of TGF-β is complex in liver tumorigenesis. TGF-β activates SMAD signaling pathway, which is crucial for liver architecture and biliary morphogenesis, and maintenance of liver homeostasis. In the early stages of tumor development, TGF-β acts as a tumor suppressor by inducing cell cycle arrest and apoptosis.27 However, it later mediates an immunosuppressive microenvironment and contributes to tumor progression. TGF-β in chronic liver inflammation activates HSCs to myofibroblasts, exacerbating liver fibrosis and hepatocarcinogenesis.12 TGF-β induces epithelial mesenchymal transition (EMT), associated with invasiveness and metastasis of tumor cells.28 TGF-β inhibits anti-tumor immune by directly upregulating the transcriptional expression of PD-1 in cancer cells, promoting the differentiation of M2-type macrophages, suppressing the activities of CD8+T cells, NK cells, DCs, and increasing the activity of CD4+T cells.29
IL-10 may have pro- or anti-tumorigenesis effects. IL-10 is synthesized by TAM, lymphocytes, and tumor cells. IL-10 controls inflammatory processes by suppressing NF-κB signaling.30 IL-10 mediates the activation of NK cells and CTL cells,31 and STAT3-IL10-IL6 autocrine-paracrine loop enables macrophages to maintain their scavenging ability,32 contributing to tumor inhibition. However, it was reported that serum IL-10 is elevated significantly with liver disease progression and has a positive correlation coefficient with transaminase values, suggesting its role in chronic inflammation leading to HCC.33 IL-10 induces sustained STAT3 phosphorylation, related to tumor cell proliferation, genomic instability and migration.32 IL-10 can also reduce antigen presentation of APCs facilitating tumor escape.31
Inflammation and Tumor Angiogenesis
HCC is one of the most vascular solid tumors, and angiogenesis is crucial in HCC development, progression and metastasis.34 New vessels provide nutrients to tumor cells. Neovascularization plays a pivotal role in collateral circulation formation of the portal veins, mesenteric congestion, and high perfusion, contributing to the progression from liver fibrosis to HCC.35 Additionally, the vessels in sustained angiogenesis are structurally and functionally abnormal, tortuous with uneven diameter and irregular branches, and immature with high leakiness, which may contribute to interstitial hypertension, severe hypoxia and necrosis.35,36 Tumor angiogenesis also participates in the development of an immunosuppressive microenvironment. Angiogenesis factors directly suppress APCs and immune effector cells, or enhance the effect of Treg, MDSCs and TAMs. Those suppressive immune cells can also drive angiogenesis37(Figure 1).
Inflammation is a key element in the angiogenesis of HCC. Persistent inflammation impairs oxygen diffusion to hepatocytes, promotes vascular permeability, and enhances the recruitment of various leukocytes, which release numerous pro-angiogenic cytokines.38 Degradation of basement and membrane and extracellular matrix in inflammation promotes the synthesis of cytokines.39 Activated HSCs in the tumor microenvironment also produce growth factors. Various pro-angiogenic factors mediate the proliferation and migration of endothelial cells (ECs), and the formation of new vessels. Excess of inflammatory cells contributes to a local hypoxia state in the liver, and hypoxia is another critical stimulant of angiogenesis.40 Oxidative stress and ROS produced in inflammation also initiate angiogenesis. Neovascularization enables the continuous recruitment of inflammatory cells and release of pro-angiogenic cytokines. These series of positive feedback loops ultimately create a vicious cycle.41
Vascular endothelial growth factor (VEGF) is one of the most important pro-angiogenic cytokines. VEGF-A binds to VEGFR-2, which causes an increase of vascular permeability and vasodilatation by inducing NO production. VEGF-A also promotes ECs survival by activating phosphatidylinositol-3-kinase pathway and production of anti-apoptotic proteins.42
Other pro-angiogenic factors synthesized in inflammation include fibroblast growth factors (FGF), angiopoietins, platelet-derived growth factor (PDGFs), integrins, and cadherins.43 Activation of various signaling pathways in inflammation, such as STAT3, NF-κB, PI3K/Akt, and p38 MAPK, are proved to be associated with angiogenesis in tumor development.39 Thrombospondin-1 (TSP-1), endostatin, angiostatin can inhibit angiogenesis through interference with VEGF, FGF and integrins.43
Anti-angiogenic therapy is a promising treatment of HCC. Some drugs, such as Sorafenib, can block the receptors that trigger angiogenesis, including VEGFR2, PDGFR, FGFR and so on. Moreover, Bevacizumab can bind to VEGF as antibody, and thus reverse pro-angiogenic microenvironment and inhibit progression of HCC.
Inflammation and Aging
Uncontrolled chronic, low-grade inflammation drives carcinogenesis in aging. Inflammation is one of the hallmarks of aging. In the process of aging, it shows dysregulation of the inflammatory response as well as an imbalance between inflammatory and anti-inflammatory mechanisms,44 which is known as “inflamm-aging”. The expression levels of some cytokines and chemokines increases, such as IL-1β, IL-6, IL-8, TNF-α,45,46 are significantly elevated with activation of NF-κB and STAT3 signaling pathways.47 Serum C reactive protein (CRP) also increases in the elder.48 Inflamm-aging participates in immunosenescence and formation of an immunosuppressive microenvironment, resulting in reduction of the capacity to cope with a variety of stressors and inducing carcinogenesis.49
Chronic inflammation is related to some aging-related diseases. Pro-inflammatory cytokines may play a major role in the higher risk of cardiovascular events, such as atherosclerosis, while dysregulation of the inflammatory pathway may also be involved in the pathophysiological mechanisms of neurodegenerative disorders, such as Alzheimer's disease.46,50 It is reported that liver aging is associated with increased necroptosis, and necroptosis contributes to chronic inflammation in the liver, which contributes to liber fibrosis, CLD, and possibly carcinogenesis.51(Figure 1) However, it remains to be illustrated how chronic inflammation contributes to aging-related HCC development.
Immune Microenvironment Mediates HCC Development
Exposed to foreign molecules in the portal blood constantly, the liver maintains in an anti-inflammatory and immunotolerant state. However, the liver has strong self-defense mechanisms and is able to mount a rapid immune response. The innate immune cells, including DCs, NK cells, NKT cells, monocytes, and liver-resident macrophages (Kupffer cells), as well as adaptive immune cells, including CD4+T cells and CD8+T cells, are involved in liver immunity. They can remove harmful substances in liver and eliminate early cancer cells.52,53 Targeted therapy, and immunotherapy mainly concerned with PD-1 and Chimeric Antigen Receptor T-cell Immunotherapy (CAR-T) have been widely used in clinical practice. However, chronic inflammation can inhibit anti-tumor immunity, and induce an immunosuppressive microenvironment, allowing malignant cells to escape from immune surveillance (Figure 2).
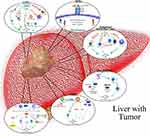 |
Figure 2 Immunosuppressive microenvironment mediates HCC development. |
Activation of Cancer-Associated Fibroblasts
The activation of cancer-associated fibroblasts (CAFs) is vital to HCC development, progression and metastasis. CAFs originate from HSCs, bone marrow mesenchymal stem cells or other precursor cells. Tumor cells of HCC can induce HSCs into CAFs by secreting CXCL6 and TGF-β,54 forming exosomal miRNA-21 and upregulating PDK1/AKT signaling.55 The feedback loop accelerates tumor progression.
CAFs can directly communicate with tumor cells. BGN and VCAN secreted by CAFs might bind to TLR2 on HCC cells, while CD81 on CAFs might bind to GPC3 on HCC cells, initiating signal transduction.56 CAFs also product various of pro-tumorigenic cytokines and remodel ECM, indirectly involved in HCC deterioration. CAFs secrete lots of ECM proteins and lead to matrix degradation, deposition and stiffening, which can promote tumor cells proliferation and chemotherapeutic resistance.57 CAFs can promote the angiogenesis of HCC. CAFs are reported to produce VEGF and upregulate EZH2/VASH1 pathway.58 Another study shows CAFs highly expressed CD90 can generate placental growth factor (PGF) and facilitates neoangiogenesis.59 Some signals, such as HGF, EGF, PDGF, TGF-β, produced by CAFs and other tumor-associated stromal cells, can induce epithelial–mesenchymal transition (EMT), which is responsible for HCC progression and metastasis.60
CAFs induce the forming of an immunosuppressive microenvironment. CAFs-derived PGE2 and indoleamine 2.3-dioxygenase (IDO) inhibit NK cells activation and decrease the cytotoxicity.61 Endosialin expressed by CAFs can interact with CD86 and recruit macrophages, while CAFs-derived GAS6 can mediate TAMs polarization to M2, promoting HCC progression.62 CAFs in HCC can induce neutrophils recruitment and decrease their spontaneous apoptosis through IL-6-STAT3-PDL1 signaling, and enhance the inhibitory effect of neutrophils on T cells, leading to immune suppression.63 Another study shows CAFs in HCC can promote the generation of regulatory DCs and DCs suppress T-cell function and facilitate Tregs proliferation by producing IDO. CAFs also enhance immune suppression by inducing MDSC generation and activation, for which CAFs-derived IL-6 and SDF-1a, as well as STAT3 activation may be responsible.64
Activation of Pattern Recognition Receptors
The innate immune responses mediate by pattern recognition receptors (PRRs) contribute to the development of HCC. PRRs, including Toll-like receptors (TLRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs), RIG-I-like receptors (RLRs) and AIM2-like receptors (ALRs), are activated by microbe-associated molecular patterns (MAMPs) from the gut, which induce proinflammatory responses.
The activation of TLRs is suggested to play a role in HCC development. TLRs are expressed on different cells in liver. Kupffer cells express TLR2,3,4 and 9. Hepatocytes express TLR2,3,4 and 5. HSCs express TLR4 and 9. Liver sinusoidal endothelial cells (LSEC) express TLR4. NK cells express TLR2,3,5,8 and 9.65 TLRs are activated by signal through myeloid differentiation factor 88 (MyD88), then upregulate NF-κB, JNK, p38, ERK1/2 signaling pathway, and mediate the production of various proinflammatory cytokines, such as TNF-α, IL-1, IL-6, and IL-8, which may contribute to hepatocarcinogenesis.65–67
Activation of TLR4 is significant in liver cancer development. It is reported that strong cytoplasmic TLR4 expression is an independent factor for poor prognosis in HCC.68 Mice deficient in TLR4 and MyD88, but not TLR2, have a marked decrease in the incidence, size, and number of diethylnitrosamine-induced liver cancer.69 Chronic inflammation is associated with changes in gut microbiome composition. Gut-derived LPS and TLR4 are proved to be critical for HCC promotion.70 TLR4 signaling induced by LPS can promote epithelial–mesenchymal transition in HCC.71 Isolated stem cell-like cells (TICs) exhibit TLR4-dependent upregulation of NANOG. TLR4-NANOG pathway induces the expression of Igf2bp3 and Yap1, which inhibit TGF-β tumor suppressor pathway, and contribute to TICs’ tumorigenic activity.72 TLR4-NANOG also cooperates with leptin receptor-Pstat3 signaling pathway, resulting in liver tumorigenesis through an exaggerated mesenchymal phenotype with prominent Twist1-expressing TICs.73 Furthermore, TLR4 controls telomere and maintains telomeres length through heterochromatin protein 1 isoforms, and thus promotes the malignant growth of liver cancer cells.74
Increased expression of TLR2 is also shown in the hepatic inflammation-fibrosis-carcinoma sequence. Gene silencing of TLR2 inhibits the proliferation of human liver cancer cells and secretion of inflammatory cytokines, indicating its contribution to HCC development.75 It has been demonstrated that extracellular HSP70-peptide complexes promote the proliferation of HCC cells through activation of TLR2 and TLR4 and the JNK1/2/MAPK pathway.76 During hypoxia, high mobility group box 1 (HMGB1) binds to TLR9, leading to activation of p38 and phosphorylation of PGC-1α, which result in upregulation of mitochondrial biogenesis and promote HCC survival and proliferation.77 Increased TLR5 expression is also an independent risk factor for poor HCC prognosis.78 These studies indicate that activation of various TLRs promotes HCC development through multiple mechanisms.
It has been reported that the activation of NLRs (NOD1 and NOD2) can regulate proinflammatory responses mediated by TLRs and thus affects the development of HCC.79
Adaptive Immune Cells in Liver Carcinogenesis
During early tumor growth, adaptive immune cells play a critical role in surveilling and killing tumor cells. However, dysregulation of immune surveillance is exhibited in the tumor microenvironment.
The effect of CD8+T cells on HCC development is paradoxical. On the one hand, CD8+T cells play antitumorigenic roles as tumor-infiltrating lymphocytes (TILs). CD8+T cells and CD4+T cells can secrete IFN-γ and suppress the development of cancer. Cytotoxic CD8+T cells also produce perforin and directly mediate tumor cells death.80 It is reported that chronic inflammation and fibrosis in humans and mice with NASH is accompanied by accumulation of immunoglobulin-A-producing (IgA+) plasma cells, which express programmed death ligand 1 (PD-L1) and IL-10, and promote tumor growth by directly suppressing cytotoxic CD8+T cells activation.81 Continuous tumor antigen exposure may lead to CD8+T cells exhaustion and dysfunction, resulting in reduced cell proliferation and cytotoxic capacity. Exhausted CD8+T cells are correlated with poor clinical outcome in HCC.82 It is induced by activation of immune checkpoint pathways,83 and characterized by overexpression of inhibitory receptors, such as programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).84 Immune checkpoint inhibitors (ICIs) are widely used clinically. ICIs can block the interaction of checkpoint proteins and their ligands as antibodies, especially between PD1 and PDL1, CTLA4 and CD80/86, and thus reduce the inactivation of cytotoxic T cells. Additionally, the combination of the anti-PDL1 antibody Atezolizumab and the VEGF antibody Bevacizumab has been shown to have stronger antitumor effects. Other ICIs, such as LAG3 and TIM3, remain to be potential antitumor targets. On the other hand, activated CD8+T cells have protumorigenic effects in HCC by activating NF-κB pathway and producing LIGHT, lymphotoxin-α and lymphotoxin-β.85 Furthermore, accumulation of lymphocytes and formation of ectopic lymphoid-like structures (ELSs) are associated with poor prognosis for HCC. ELSs function as microniches for tumor progenitor cells.86
CD4+CTLs can produce IFN-γ and play a role in immune surveillance in HCC. In NAFLD-induced HCC, disruption of mitochondrial function by linoleic acid generates higher levels of ROS, and mediates the selective loss of intrahepatic CD4+T lymphocytes, leading to accelerated hepatocarcinogenesis.87 Further analysis shows that the progressive deficit in circulating and tumor-infiltrating CD4+CTLs induced by increased FoxP3+Treg is correlated with poor survival and high recurrence rates in HCC patients.88 In HCC, the function of CD4+T follicular helper (Tfh) cells is impaired, with dysfunction in IL-21 secretion and induction of B cell maturation, contributing to reduced survival time.89 On the other hand, CD4+Th17 cells antagonize the differentiation and function of IFN-γ producing Th1 cells. IL-17 secreted by Th17 can activate IL-6-Stat3 signaling pathway and thus promote HCC growth.90
Interaction between tumor-infiltrating B cells and T cells enhances the local immune response and limits the progression of HCC, leading to a better prognosis.91 It is indicated that T cells prevent initial tumor formation, while B cells critically limit the growth of established tumors.92 On the contrary, the levels of serum B cell-activating factor (BAFF) are higher in NASH patients than in simple steatosis patients, and higher levels are associated with the presence of hepatocyte ballooning and fibrosis,93 indicating the likely contribution of B cells to HCC development.
Recruitment of Tregs and MDSCs
Tregs and MDSCs can inhibit tumor immune surveillance and induce an immunosuppressive microenvironment.
CD4+CD25+Foxp3+T cells, termed regulatory T cells (Tregs), play a crucial role in promoting HCC development. Tregs can secrete anti-inflammatory cytokines, such as TGF-β, IL-10 and IL-35, and suppress antigen-specific T-cell responses via inhibitory molecules such as CTLA-4 and Lag-3.94 Tregs also directly induce the apoptosis of effector cells via granzyme and perforin.95 Additionally, Tregs impair T cells proliferation via IL-2 signaling, regulate the maturation and function of dendritic cells (DCs), and produce nucleotides.96,97 Studies have suggested that Tregs are accumulated in HCC and increased Tregs are correlated with CD8+T cells impairment and poor survival in HCC patients.98 Chimeric Antigen Receptor T-Cell Immunotherapy (CAR-T) has also been applied in clinic. It is reported that Tregs account for HBV-induced HCC by inducing and maintaining the liver’s suppressive microenvironment.97 Tregs and neutrophils extracellular trap interaction also contributes to NASH-induced HCC.99
MDSCs are a heterogenic population of immature myeloid cells, including progenitors of macrophages, DCs, and granulocytes. MDSCs can suppress T cells responses by producing TGF-β, IL-10, ROS, RNS, arginase 1, indoleamine oxidase,100,101 and by depleting cystine and cysteine.102 MDSCs also affect T cell function, survival and trafficking in HCC through diverse mechanisms. MDSCs can induce Treg expansion.103 Higher levels of MDSCs are shown in HCC patients.104 The overexpression of inflammatory cytokines, such as IL-1β, IL-6, prostaglandin E2 (PGE2), granulocyte macrophage colony-stimulating factor (GM-CSF) and vascular endothelial growth factor (VEGF) in tumor microenvironment can recruit MDSCs to the site of inflammation and activate them.105 The activation of HSCs can also recruit MDSCs.
Polarization of TAMs
Tumor-associated macrophages play a critical role in HCC development, growth and metastasis. In the early tumor stage, Type 1 macrophages (M1) infiltrate and mediate pro-inflammatory response. M1 macrophages are induced by Th1 cytokines such as INF-γ, GM-CSF and LPS at the site of inflammation. M1 macrophages can secrete pro-inflammatory cytokines, such as IL-6, IL-12, and TNF-α.96 M1 macrophages also enhance anti-tumor immune by presenting antigens via MHC molecules, and producing CXCL19 and CXCL10 to attract and promote T cells and NK cells development.17 M1 macrophages are cytotoxic by releasing ROS or toxic intermediates.106 Overall, M1 macrophages contribute to the elimination of tumor cells in the early stage of HCC development. Interestingly, TNF-α produced by TAMs activates NF-κB which protects hepatocytes from apoptosis and promotes tumor growth.103
However, TAMs polarize to a more Type 2 macrophages (M2) state in the advanced stage of tumorigenesis. The M2 polarization of TAMs is stimulated by CSF-1, IL-4 and lactate in hypoxic tumor tissue.107 M2 macrophages release anti-inflammatory factors, such as IL-10, IL-23, PGE-2 and TGF-β.96 M2 macrophages lack sufficient MHC expression and induce an immunosuppressive environment by producing chemokines such as CCL17, CCL22 and CCL24, favoring Treg recruitment and development.17 Furthermore, M2 macrophages can promote cancer proliferation, angiogenesis and ECM remodeling.108 M2 macrophages secrete growth factors including TGF-β, VEGF, EGF, PDGF, angiogenic factor thymidine phosphorylase, angiogenesis-modulating enzymes COX-2 and MMPs, promoting migration of endothelial cells and angiogenesis in HCC. M2 macrophages promote HCC invasion by remodeling the matrix.103
Activation of HSCs
The activation of HSCs promotes HCC development. HSCs are important stromal cells in the tumor microenvironment and can be activated by various immune cells during chronic inflammation, such as NK cells and TAMs. Mediators that activate HSCs include TGF-β, PDGF, connective tissue growth factor (CCN2), VEGF, viral infection, FAK-MMP9 signaling, p53/21, PI3K/AKT, MAPK/ERK, IL-6/Stat3 signaling pathways.109
Activated HSCs accelerate liver fibrosis and thus induce HCC. HSCs can secrete extracellular matrix (ECM) and form scar tissue, which protects the liver from further damage. However, in chronic inflammation, HSCs are persistently activated into a myofibroblast-like phenotype. Excess collagen and other ECM components are deposited as the liver generates a wound-healing response to encapsulate injuries, which contribute to liver fibrogenesis, even further cirrhosis and carcinoma.110
Activated HSCs can induce immunosuppression and promote HCC development. HSCs inhibit lymphocyte infiltration in tumors, induce apoptosis of infiltrating mononuclear cells, and enhance the expression of Tregs.111 HSCs secrete cytokines which induce MDSCs expansion and induce MDSCs from peripheral blood monocytes in a CD44-dependent fashion.112 HSCs also induce polarization of TAMs from inflammatory M1 macrophages to immunosuppressive M2 macrophages, contributing to the poor prognosis in HCC.113 Additionally, HSCs mediate T cells apoptosis by enhanced expressing of B7-H1, and inhibit T cell responses.114 Furthermore, activated HSCs secrete angiogenic growth factors such as VEGF to induce tumor angiogenesis.109 Overall, activation of HSCs helps the tumor to escape from immune surveillance and promote HCC growth.
Conclusion
In the tumor microenvironment of HCC, chronic inflammation triggers malignant transformation of cells and promotes tumor growth, while the formation of an immunosuppressive microenvironment allows tumor cells to escape from immune surveillance, which makes the tumor microenvironment one of the targets for cancer therapy. Therefore, it may be effective to develop strategies to block tumor-promoting chronic inflammation, or to transform the immunosuppressive state into an anti-tumor immunity state. Targeting tumor microenvironment, such as tumor angiogenesis, has been widely used in treatment of HCC for its advantages of high selectivity, effectiveness due to its unique mechanism, low toxicity to normal cells and various potential targets. However, it meets plenty of challenges, including development of drug resistance, difficulties in developing drugs due to the characteristics of some targets and so on. Tumor immunotherapy is also an effective and promising strategy in clinic, especially in the advanced stage of HCC, including CAR-T and ICIs. However, the existence of cold tumors which lack of CILs and are in hypoimmune state, and the low response rate due to tumor heterogeneity and variability of microenvironment bring challenges. The combination therapies and individualized therapies should be further studied. Furthermore, a better understanding of inflammatory and immune signals is required to find more effective therapy targets.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Natural Science Foundation of Guangdong Province (2023A1515011805 and 2022A1515011052), the National Natural Science Foundation of China (81873591), the Science and Technology Planning Project of Guangdong Province (2018A050506030), the Science and Technology Program of Guangzhou (201704020073), the Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007, 2017B030314018, and 2020B1212060026), and the Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002 and 2020A0505020003).
Disclosure
The authors declare no conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156(2):477–491.e471. doi:10.1053/j.gastro.2018.08.065
3. Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1–61. doi:10.1016/bs.acr.2020.10.001
4. Woolbright BL, Jaeschke H. Sterile inflammation in acute liver injury: myth or mystery? Expert Rev Gastroenterol Hepatol. 2015;9(8):1027–1029. doi:10.1586/17474124.2015.1060855
5. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi:10.1155/2014/149185
6. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3(4):276–285. doi:10.1038/nrc1046
7. Winrow VR, Winyard PG, Morris CJ, Blake DR. Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull. 1993;49(3):506–522. doi:10.1093/oxfordjournals.bmb.a072627
8. Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB J. 1995;9(2):200–209. doi:10.1096/fasebj.9.2.7540156
9. Yang D, Elner SG, Bian ZM, Till GO, Petty HR, Elner VM. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007;85(4):462–472. doi:10.1016/j.exer.2007.06.013
10. Han F, Li S, Yang Y, Bai Z. Interleukin-6 promotes ferroptosis in bronchial epithelial cells by inducing reactive oxygen species-dependent lipid peroxidation and disrupting iron homeostasis. Bioengineered. 2021;12(1):5279–5288. doi:10.1080/21655979.2021.1964158
11. Ferlat S, Favier A. Tumor necrosis factor (TNF) and oxygen free radicals: potential effects for immunity. C R Seances Soc Biol Fil. 1993;187(3):296–307.
12. Crosas-Molist E, Bertran E, Fabregat I. Cross-talk between TGF-β and NADPH oxidases during liver fibrosis and hepatocarcinogenesis. Curr Pharm Des. 2015;21(41):5964–5976. doi:10.2174/1381612821666151029112126
13. Lee HY, Nga HT, Tian J, Yi HS. Mitochondrial metabolic signatures in hepatocellular carcinoma. Cells. 2021;10(8). doi:10.3390/cells10081901
14. Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. 2001;120(1):190–199. doi:10.1053/gast.2001.20875
15. Hofseth LJ, Saito S, Hussain SP, et al. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci U S A. 2003;100(1):143–148. doi:10.1073/pnas.0237083100
16. Ambs S, Merriam WG, Ogunfusika MO, et al. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat Med. 1998;4(12):1371–1376. doi:10.1038/3957
17. Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7(5):651–658. doi:10.7150/ijbs.7.651
18. Havell EA, Fiers W, North RJ. The antitumor function of tumor necrosis factor (TNF), I. Therapeutic action of TNF against an established murine sarcoma is indirect, immunologically dependent, and limited by severe toxicity. J Exp Med. 1988;167(3):1067–1085. doi:10.1084/jem.167.3.1067
19. Zidi I, Mestiri S, Bartegi A, Amor NB. TNF-alpha and its inhibitors in cancer. Med Oncol. 2010;27(2):185–198. doi:10.1007/s12032-009-9190-3
20. Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25(3):409–416. doi:10.1007/s10555-006-9005-3
21. Cubero FJ, Zhao G, Nevzorova YA, et al. Haematopoietic cell-derived Jnk1 is crucial for chronic inflammation and carcinogenesis in an experimental model of liver injury. J Hepatol. 2015;62(1):140–149. doi:10.1016/j.jhep.2014.08.029
22. Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64(6):1403–1415. doi:10.1016/j.jhep.2016.02.004
23. Aleksandrova K, Boeing H, Nöthlings U, et al. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology. 2014;60(3):858–871. doi:10.1002/hep.27016
24. Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26(1):54–74. doi:10.1016/j.smim.2014.01.001
25. Weber R, Groth C, Lasser S, et al. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol. 2021;359:104254. doi:10.1016/j.cellimm.2020.104254
26. Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147(6):1393–1404. doi:10.1053/j.gastro.2014.08.039
27. Fabregat I, Moreno-Càceres J, Sánchez A, et al. TGF-β signalling and liver disease. Febs j. 2016;283(12):2219–2232. doi:10.1111/febs.13665
28. Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–172. doi:10.1038/cr.2009.5
29. Chen J, Gingold JA, Su X. Immunomodulatory TGF-β signaling in hepatocellular carcinoma. Trends Mol Med. 2019;25(11):1010–1023. doi:10.1016/j.molmed.2019.06.007
30. Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS. Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274(45):31868–31874. doi:10.1074/jbc.274.45.31868
31. Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015;367(2):103–107. doi:10.1016/j.canlet.2015.07.009
32. Campana L, Starkey Lewis PJ, Pellicoro A, et al. The STAT3-IL-10-IL-6 pathway is a novel regulator of macrophage efferocytosis and phenotypic conversion in sterile liver injury. J Immunol. 2018;200(3):1169–1187. doi:10.4049/jimmunol.1701247
33. El-Emshaty HM, Nasif WA, Mohamed IE. Serum cytokine of IL-10 and IL-12 in chronic liver disease: the immune and inflammatory response. Dis Markers. 2015;2015:707254. doi:10.1155/2015/707254
34. Morse MA, Sun W, Kim R, et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 2019;25(3):912–920. doi:10.1158/1078-0432.CCR-18-1254
35. Li H. Angiogenesis in the progression from liver fibrosis to cirrhosis and hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2021;15(3):217–233. doi:10.1080/17474124.2021.1842732
36. Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–3736. doi:10.1158/0008-5472.CAN-04-0074
37. Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. 2019;25(18):5449–5457. doi:10.1158/1078-0432.CCR-18-1543
38. Kukla M. Angiogenesis: a phenomenon which aggravates chronic liver disease progression. Hepatol Int. 2013;7(1):4–12. doi:10.1007/s12072-012-9391-2
39. Zhou W, Yang L, Nie L, Lin H. Unraveling the molecular mechanisms between inflammation and tumor angiogenesis. Am J Cancer Res. 2021;11(2):301–317.
40. Paternostro C, David E, Novo E, Parola M. Hypoxia, angiogenesis and liver fibrogenesis in the progression of chronic liver diseases. World J Gastroenterol. 2010;16(3):281–288. doi:10.3748/wjg.v16.i3.281
41. Kim YW, West XZ, Byzova TV. Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med. 2013;91(3):323–328. doi:10.1007/s00109-013-1007-3
42. Kroll J, Waltenberger J. Regulation of the endothelial function and angiogenesis by vascular endothelial growth factor-A (VEGF-A. Z Kardiol. 2000;89(3):206–218. doi:10.1007/s003920050472
43. Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31(2):146–162. doi:10.1111/j.1478-3231.2010.02369.x
44. Bottazzi B, Riboli E, Mantovani A. Aging, inflammation and cancer. Semin Immunol. 2018;40:74–82. doi:10.1016/j.smim.2018.10.011
45. Hager K, Machein U, Krieger S, Platt D, Seefried G, Bauer J. Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiol Aging. 1994;15(6):771–772. doi:10.1016/0197-4580(94)90066-3
46. Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877–882. doi:10.1016/j.jamda.2013.05.009
47. Chung HY, Lee EK, Choi YJ, et al. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011;90(7):830–840. doi:10.1177/0022034510387794
48. Verma S, Szmitko PE, Ridker PM. C-reactive protein comes of age. Nat Clin Pract Cardiovasc Med. 2005;2(1):29–36; quiz 58. doi:10.1038/ncpcardio0074
49. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi:10.1111/j.1749-6632.2000.tb06651.x
50. Vasto S, Candore G, Balistreri CR, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128(1):83–91. doi:10.1016/j.mad.2006.11.015
51. Mohammed S, Thadathil N, Selvarani R, et al. Necroptosis contributes to chronic inflammation and fibrosis in aging liver. Aging Cell. 2021;20(12):e13512. doi:10.1111/acel.13512
52. Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol. 2018;36:247–277. doi:10.1146/annurev-immunol-051116-052415
53. Liaskou E, Wilson DV, Oo YH. Innate immune cells in liver inflammation. Mediators Inflamm. 2012;2012:949157. doi:10.1155/2012/949157
54. Song M, He J, Pan QZ, et al. Cancer-associated fibroblast-mediated cellular crosstalk supports hepatocellular carcinoma progression. Hepatology. 2021;73(5):1717–1735. doi:10.1002/hep.31792
55. Zhou Y, Ren H, Dai B, et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37(1):324. doi:10.1186/s13046-018-0965-2
56. Huang P, Kong Q, Gao W, et al. Spatial proteome profiling by immunohistochemistry-based laser capture microdissection and data-independent acquisition proteomics. Anal Chim Acta. 2020;1127:140–148. doi:10.1016/j.aca.2020.06.049
57. Zhang J, Gu C, Song Q, et al. Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell Biosci. 2020;10(1):127. doi:10.1186/s13578-020-00488-y
58. Huang B, Huang M, Li Q. Cancer-associated fibroblasts promote angiogenesis of hepatocellular carcinoma by VEGF-Mediated EZH2/VASH1 pathway. Technol Cancer Res Treat. 2019;18:1533033819879905. doi:10.1177/1533033819879905
59. Liu Z, Chen M, Zhao R, et al. CAF-induced placental growth factor facilitates neoangiogenesis in hepatocellular carcinoma. Acta Biochim Biophys Sin. 2020;52(1):18–25. doi:10.1093/abbs/gmz134
60. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi:10.1172/JCI39104
61. Li T, Yang Y, Hua X, et al. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318(2):154–161. doi:10.1016/j.canlet.2011.12.020
62. Yang F, Wei Y, Han D, et al. Interaction with CD68 and regulation of GAS6 expression by endosialin in fibroblasts drives recruitment and polarization of macrophages in hepatocellular carcinoma. Cancer Res. 2020;80(18):3892–3905. doi:10.1158/0008-5472.CAN-19-2691
63. Cheng Y, Li H, Deng Y, et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9(4):422. doi:10.1038/s41419-018-0458-4
64. Cheng JT, Deng YN, Yi HM, et al. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5(2):e198–e198. doi:10.1038/oncsis.2016.7
65. Testro AG, Visvanathan K. Toll-like receptors and their role in gastrointestinal disease. J Gastroenterol Hepatol. 2009;24(6):943–954. doi:10.1111/j.1440-1746.2009.05854.x
66. O’Neill LA, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24(6):286–290. doi:10.1016/S1471-4906(03)00115-7
67. French SW, Oliva J, French BA, Li J, Bardag-Gorce F. Alcohol, nutrition and liver cancer: role of Toll-like receptor signaling. World J Gastroenterol. 2010;16(11):1344–1348. doi:10.3748/wjg.v16.i11.1344
68. Kairaluoma V, Kemi N, Huhta H, Pohjanen VM, Helminen O. Prognostic role of TLR4 and TLR2 in hepatocellular carcinoma. Acta Oncol. 2021;60(4):554–558. doi:10.1080/0284186X.2021.1877346
69. Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. doi:10.1126/science.1140485
70. Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504–516. doi:10.1016/j.ccr.2012.02.007
71. Jing YY, Han ZP, Sun K, et al. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med. 2012;10:98. doi:10.1186/1741-7015-10-98
72. Tsukamoto H, Mishra L, Alcohol MK. TLR4-TGF-β antagonism, and liver cancer. Hepatol Int. 2014;8(Suppl 2):408–412. doi:10.1007/s12072-013-9489-1
73. Uthaya Kumar DB, Chen CL, Liu JC, et al. TLR4 signaling via NANOG cooperates with stat3 to activate twist1 and promote formation of tumor-initiating stem-like cells in livers of mice. Gastroenterology. 2016;150(3):707–719. doi:10.1053/j.gastro.2015.11.002
74. Zheng Q, Xu J, Lin Z, et al. Inflammatory factor receptor Toll-like receptor 4 controls telomeres through heterochromatin protein 1 isoforms in liver cancer stem cell. J Cell Mol Med. 2018;22(6):3246–3258. doi:10.1111/jcmm.13606
75. Huang Y, Cai B, Xu M, et al. Gene silencing of Toll-like receptor 2 inhibits proliferation of human liver cancer cells and secretion of inflammatory cytokines. PLoS One. 2012;7:7.
76. Zhe Y, Li Y, Liu D, Su DM, Liu JG, Li HY. Extracellular HSP70-peptide complexes promote the proliferation of hepatocellular carcinoma cells via TLR2/4/JNK1/2MAPK pathway. Tumour Biol. 2016;37(10):13951–13959. doi:10.1007/s13277-016-5189-5
77. Tohme S, Yazdani HO, Liu Y, et al. Hypoxia mediates mitochondrial biogenesis in hepatocellular carcinoma to promote tumor growth through HMGB1 and TLR9 interaction. Hepatology. 2017;66(1):182–197. doi:10.1002/hep.29184
78. Kairaluoma V, Kemi N, Huhta H, Pohjanen VM, Helminen O. Toll-like receptor 5 and 8 in hepatocellular carcinoma. Apmis. 2021;129(8):470–479. doi:10.1111/apm.13142
79. Omaru N, Watanabe T, Kamata K, Minaga K, Kudo M. Activation of NOD1 and NOD2 in the development of liver injury and cancer. Front Immunol. 2022;13:1004439. doi:10.3389/fimmu.2022.1004439
80. Guidotti LG, Inverso D, Sironi L, et al. Immunosurveillance of the liver by intravascular effector CD8(+) T cells. Cell. 2015;161(3):486–500. doi:10.1016/j.cell.2015.03.005
81. Shalapour S, Lin XJ, Bastian IN, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551(7680):340–345. doi:10.1038/nature24302
82. Ma J, Zheng B, Goswami S, et al. PD1(Hi) CD8(+) T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J Immunother Cancer. 2019;7(1):331. doi:10.1186/s40425-019-0814-7
83. Hsu CL, Ou DL, Bai LY, et al. Exploring markers of exhausted CD8 T cells to predict response to immune checkpoint inhibitor therapy for hepatocellular carcinoma. Liver Cancer. 2021;10(4):346–359. doi:10.1159/000515305
84. McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi:10.1146/annurev-immunol-041015-055318
85. Haybaeck J, Zeller N, Wolf MJ, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16(4):295–308. doi:10.1016/j.ccr.2009.08.021
86. Finkin S, Yuan D, Stein I, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol. 2015;16(12):1235–1244. doi:10.1038/ni.3290
87. Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531(7593):253–257. doi:10.1038/nature16969
88. Fu J, Zhang Z, Zhou L, et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. 2013;58(1):139–149. doi:10.1002/hep.26054
89. Jia Y, Zeng Z, Li Y, et al. Impaired function of CD4+ T follicular helper (Tfh) cells associated with hepatocellular carcinoma progression. PLoS One. 2015;10:2.
90. Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464. doi:10.1084/jem.20090207
91. Garnelo M, Tan A, Her Z, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66(2):342–351. doi:10.1136/gutjnl-2015-310814
92. Schneider C, Teufel A, Yevsa T, et al. Adaptive immunity suppresses formation and progression of diethylnitrosamine-induced liver cancer. Gut. 2012;61(12):1733–1743. doi:10.1136/gutjnl-2011-301116
93. Miyake T, Abe M, Tokumoto Y, et al. B cell-activating factor is associated with the histological severity of nonalcoholic fatty liver disease. Hepatol Int. 2013;7(2):539–547. doi:10.1007/s12072-012-9345-8
94. von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6(4):338–344. doi:10.1038/ni1180
95. Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174(4):1783–1786. doi:10.4049/jimmunol.174.4.1783
96. Sprinzl MF, Galle PR. Immune control in hepatocellular carcinoma development and progression: role of stromal cells. Semin Liver Dis. 2014;34(4):376–388. doi:10.1055/s-0034-1394138
97. Li W, Han J, Wu H. Regulatory T-cells promote hepatitis B virus infection and hepatocellular carcinoma progression. Chronic Dis Transl Med. 2016;2(2):67–80. doi:10.1016/j.cdtm.2016.09.001
98. Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi:10.1053/j.gastro.2007.03.102
99. Wang H, Zhang H, Wang Y, et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021;75(6):1271–1283. doi:10.1016/j.jhep.2021.07.032
100. Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168(2):689–695. doi:10.4049/jimmunol.168.2.689
101. Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65(8):3044–3048. doi:10.1158/0008-5472.CAN-04-4505
102. Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70(1):68–77. doi:10.1158/0008-5472.CAN-09-2587
103. Wan S, Kuo N, Kryczek I, Zou W, Welling TH. Myeloid cells in hepatocellular carcinoma. Hepatology. 2015;62(4):1304–1312. doi:10.1002/hep.27867
104. Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73(8):2435–2444. doi:10.1158/0008-5472.CAN-12-3381
105. Nagai N, Kudo Y, Aki D, Nakagawa H, Taniguchi K. Immunomodulation by inflammation during liver and gastrointestinal tumorigenesis and aging. Int J Mol Sci. 2021;22(5):2238. doi:10.3390/ijms22052238
106. Brittenden J, Heys SD, Ross J, Eremin O. Natural killer cells and cancer. Cancer. 1996;77(7):1226–1243. doi:10.1002/(SICI)1097-0142(19960401)77:7<1226::AID-CNCR2>3.0.CO;2-G
107. Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563.
108. Tahmasebi Birgani M, Carloni V. Tumor microenvironment, a paradigm in hepatocellular carcinoma progression and therapy. Int J Mol Sci. 2017;18(2):405. doi:10.3390/ijms18020405
109. Barry AE, Baldeosingh R, Lamm R, et al. Hepatic stellate cells and hepatocarcinogenesis. Front Cell Dev Biol. 2020;8:709. doi:10.3389/fcell.2020.00709
110. Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25(2):195–206. doi:10.1016/j.bpg.2011.02.005
111. Zhao W, Zhang L, Yin Z, et al. Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int J Cancer. 2011;129(11):2651–2661. doi:10.1002/ijc.25920
112. Höchst B, Schildberg FA, Sauerborn P, et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J Hepatol. 2013;59(3):528–535. doi:10.1016/j.jhep.2013.04.033
113. Ji J, Eggert T, Budhu A, et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology. 2015;62(2):481–495. doi:10.1002/hep.27822
114. Yu MC, Chen CH, Liang X, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40(6):1312–1321. doi:10.1002/hep.20488
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Decaprenyl Diphosphate Synthase Subunit 1 (PDSS1): A Potential Prognostic Biomarker and Immunotherapy-Target for Hepatocellular Carcinoma
Yang Y, Li J, Tang M, Nie B, Huang W
Cancer Management and Research 2022, 14:1627-1639
Published Date: 3 May 2022
Infiltration of a Unique CD8+CD274+ Cell Subgroup in Hepatocellular Carcinoma is Associated with Poor Clinical Outcomes
Zhang Y, Cui K, Yang Y, Liu B, Zhu M, Chen H, Zhao C, Zhou Y, Nie Y
Journal of Hepatocellular Carcinoma 2023, 10:1051-1067
Published Date: 8 July 2023
Development and Validation of a Propionate Metabolism-Related Gene Signature for Prognostic Prediction of Hepatocellular Carcinoma
Xiao J, Wang J, Zhou C, Luo J
Journal of Hepatocellular Carcinoma 2023, 10:1673-1687
Published Date: 2 October 2023
Construction of a Prognostic Model for Hepatocellular Carcinoma Based on Macrophage Polarization-Related Genes
Chen H, Li J, Cao D, Tang H
Journal of Hepatocellular Carcinoma 2024, 11:857-878
Published Date: 11 May 2024
