Back to Journals » Journal of Inflammation Research » Volume 15
CCL20/CCR6 Mediated Macrophage Activation and Polarization Can Promote Adenoid Epithelial Inflammation in Adenoid Hypertrophy
Authors Ye C, Guo X, Wu J, Wang M, Ding H, Ren X
Received 17 September 2022
Accepted for publication 20 December 2022
Published 23 December 2022 Volume 2022:15 Pages 6843—6855
DOI https://doi.org/10.2147/JIR.S390210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Chenchen Ye,1,2 Xinxue Guo,2 Jiani Wu,2 Minhua Wang,2 Haiyan Ding,2 Xianzhi Ren1,3,4
1The First Clinical College, Nanjing University of Chinese Medicine, Nanjing, 210046, People’s Republic of China; 2Department of Pediatrics, Yixing Hospital of Traditional Chinese Medicine, Yixing, 214200, People’s Republic of China; 3Department of Pediatrics, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, 210046, People’s Republic of China; 4Department of Pediatrics, Jiangsu Provincial Hospital of Traditional Chinese Medicine, Nanjing, 210046, People’s Republic of China
Correspondence: Xianzhi Ren, Department of Pediatrics, Jiangsu Provincial Hospital of Traditional Chinese Medicine, Nanjing, 210046, People’s Republic of China, Email [email protected]
Background: Adenoid hypertrophy (AH) is a chronic or acute obstruction-related ailment of the upper respiratory tract that arises as an inflammatory response to exposure of bacteria, viruses or allergies. Activation and polarization of macrophages are key processes in inflammation-related disorders like AH and CCL20/CCR6 axis is a critical therapeutic target.
Purpose: To determine that CCL20/CCR6 mediated macrophage activation and polarization can promote adenoid epithelial inflammation in AH.
Methods: To support this claim, CCL20 and CCR6 expressions were studied in clinical AH samples. In addition, the expressions of cytokines such as TNF-α, IL-1β, IL-6, IL-17, IL-10 and TGF-β were analysed. In vitro, human adenoid epithelial cells were co-cultured with polarized THP-1 and T lymphocyte H9 cells to study the expressions of several inflammatory markers.
Results: The expressions of M1 macrophage markers CD86 and IL-17 were significantly increased, whereas the expressions of M2 macrophage markers CD206 and FOXP3 were significantly decreased. The THP-1 cells were successfully polarized to M0, M1 and M2 macrophages. The survival of macrophages improved after 24 hr of induction and enhanced TGF-β expression was observed. The expressions of the inflammatory cytokines IL-6, TNF-α, IL-1β and CCL20 increased significantly.
Conclusion: Collectively, these results suggest that the CCL20/CCR6 mediated macrophage activation and polarization into M1-type macrophages can promote adenoid epithelial inflammation in AH. Further studies are warranted to determine the roles of inflammatory markers in the pathophysiology of AH and identifying potential targets.
Keywords: adenoid hypertrophy, CCL20/CCR6 axis, inflammation, macrophages, markers
Introduction
Adenoids are condensed forms of lymphoepithelial tissues existing behind the nose in the posterosuperior region of nasopharynx, medial to the Eustachian tube orifices and are responsible for enhanced immunological memory recognized amidst children.1,2 Adenoid hypertrophy (AH) is a chronic or acute obstruction-related ailment that arises as an inflammatory response and is observed as a blockage of flow of air via the upper nasal region.3 It is commonly witnessed among children of ages less than 16, where the adenoids enlarge reaching its peak at age 6.4 Several viral and bacterial pathogens have been identified to be infectious signatures for AH.5 Passive or involuntary exposure to cigarette smoke, occurrences of allergy and gastroesophageal reflux are associated with non-infectious risk factors for AH.6 Since changes in the immunological barrier can be critical among children who suffer from obstruction of the upper nasal region, sleep apnea and otitis media, diagnosis remains significant for observing the adenoids. Procedures such as posterior rhinoscopy, nasal endoscopy and investigations using radiological examinations such as computed tomography and magnetic resonance imaging are usually preferred for the diagnosis of obstructive AH. Other objective tests like computed rhinomanometry and acoustic rhinometry are also used to serve this purpose. Adenoidectomy is considered quite a familiar surgical procedure for management of the condition.7–9
Monocytes and macrophages form the core of innate immunity and maintaining the homeostasis among these cells can protect us from self- and external antigens. These immune cells can engulf bacteria by phagocytosis via the secretion of mediator components responsible for pro-inflammatory and antimicrobial effects.10,11 Monocytes can spot danger signals and differentiate into terminal macrophages which can ingest pathogenic microbes and their toxins and yield the processed antigens to other cells of the immune system.12 In addition, studies conducted on clinical adenoid samples of AH patients indicate that elevated serum enzyme or protein levels that resemble the activation of monocyte/macrophage and myeloid cells like eosinophils have been attained.13 Interestingly, bacterial pathogens that can cause infections of the upper respiratory tract were found intracellularly in monocytes/macrophages of adenoids from children with AH.14 Also, macrophages and monocytes that display pro-inflammatory markers like CD14 and CD64 are observed extensively in children with AH.15,16
Macrophage inflammatory protein 3 (MIP-3α), better known as Chemokine CCL20, is upregulated under the action of inflammatory mediators, thereby regulating the immune function of the body.17 Moreover, CCR6 is a G protein-coupled receptor expressed in various immune cells and has the ability to specifically interact with its ligand CCL20.18 CCL20-CCR6 axis is extensively studied in inflammation-related diseases like cancer, diabetes and several other disorders, whereas, less studied with regard to AH.19–21 The effect of CCL20 on its receptor CCR6 in T lymphocytes can induce cell differentiation into Th17, and the increase of Th17/Treg ratio is recognized as an inducer of AH.22,23 However, the effect of macrophage activation and polarization mediated by CCL20/CCR6 axis on adenoid epithelial inflammation and lymphocyte differentiation in AH has not been reported so far.
Therefore, in the present report, we will first detect the levels of CCL20, CCR6 and macrophage marker proteins (different activation and polarization phenotypes) and verify the actual expression of these factors in diseased samples. Following this, the expressions of the above factors was regulated by human intervention via assays using cell line models so as to verify the relevant influencing mechanisms and relationships.
Materials and Methods
Collection of Clinical Samples
All procedures and protocols were approved by the Ethics Committee of Affiliated Hospital of Nanjing University of Chinese Medicine and Yixing Hospital of Traditional Chinese Medicine. All procedures employed in the study using human participants complied with the Helsinki Declaration and its amendments. Free and informed consent to contribute to this study was acquired from the parents or legal guardian for the 19 children under legal age to consent. Diagnosis was done by electronic nasopharyngoscopy or sella turcica lateral X-ray examination. The study participants or children with hypertrophy underwent bilateral tonsil adenoidectomy for obstructive complications such as snoring, nasal congestion, sleep apnea, open mouth breathing or adenoid visage. The excised adenoids were stored at −80°C or fixed with 4% polyoxymethylene for further use. The general information of clinical adenoid samples acquired from AH patients is shown in Table 1.
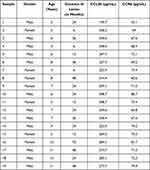 |
Table 1 Relevant Information of the Clinical Samples from AH Patients |
ELISA
To examine the expressions of CCL20 and CCR6 en route of detecting macrophage polarization among adenoid tissues, ELISA test was performed. Subsequently, 5 samples with the high CCL20 expressions (designated as HE group with enlarged adenoids) and 5 samples with the low expressions of CCL20 (designated as LE group with comparatively lesser enlargement of the adenoids) were selected for further pathological examination. CCL20/CCR6 protein expression profiles in the obtained adenoids were quantified by adopting enzyme-linked immunosorbent assay (ELISA) with human CCL20 and CCR6 kits adhering to the instructions provided by the manufacturer (Jianglaibio, China). The absorbance was read using a Multiscan Ex ELISA microplate reader (Thermo Fisher). The expressions of IL-1β, IL-6, IL-17, IL-10, TNF-α and TGF-β were quantified through equivalent kits obtained from Beyotime Inc., China.
Hematoxylin and Eosin Staining
The adenoids were initially fixed with 4% polyoxymethylene for 24 hr at 37°C. Later, the tissues were paraffin-embedded, sectioned into slices of 5 µm thickness and stained with the two histological stains obtained from Nanjing Jiancheng Bioengineering Institute, China. The sections were dried out, resin-sealed and randomly selected fields were used for capturing the images by Nikon inverted fluorescent microscope (200X).
Immunohistochemical (IHC) Staining
The expressions of macrophage markers (CD86 and CD206) and Treg and Th17-associated markers (IL-17A and FOXP3) in the adenoid tissues were evaluated by IHC according to established protocols. Paraffin sections were prepared by heating in an oven for 1 hr. The sections were later directed towards xylene-based deparaffinization followed by ethanol-centred rehydration and heated by means of microwave oven using sodium citrate buffer. The slides were later allowed for cooling at room temperature and immersed in 3% H2O2 for 20 min. The tissue segments were smeared with blocking buffer containing 3% BSA and incubated with 50 µL of primary antibodies overnight at 4°C. After a PBS wash, the sections were incubated with secondary antibodies for 20 min at 37°C. The samples were then colored by diaminobenzidine (DAB), washed and stained with hematoxylin, diluted for 30 secs with HCl, dehydrated, sealed and observed under a microscope.
Cell Culture
Human adenoid epithelial cells were taken from human adenoids and cultured with RPMI1640 containing 10% FBS containing 1% penicillin plus streptomycin (Gibco; Thermo Fisher Scientific, Inc., USA) under humidified atmosphere of 5% CO2 at 37 °C. Human THP-1 and T lymphocyte H9 cells (American Type Culture Collection) were cultured under similar conditions as mentioned before. To induce the polarization of THP-1 cells, it was first treated with PMA (10ng/mL, Sigma-Aldrich). The THP-1 cells were later processed with IFN-γ (20 ng/mL, Sigma-Aldrich) and lipopolysaccharide (LPS, 100 ng/mL, Sigma-Aldrich) for M1 polarization. For M2 polarization, the cells were treated with IL-4 (20 ng/mL, Sigma-Aldrich) and IL-13 (20 ng/mL, Sigma-Aldrich). The control group did not receive any special processing. The polarized cell culture medium was used to cultivate H9 cells, and the cells were grouped into control, M0, M1 and M2 groups. Accordingly, the polarized cell culture medium was used to cultivate adenoid epithelial cells, and the cells induced by M1 medium were treated with CCR6 inhibitors. The cells were grouped into control, M0, M1, M2 and M1+CCR6 inhibitor groups.
Cell Counting Kit-8 (CCK-8) Cell Viability Assay
H9 and adenoid epithelial cells (8×103/well) were cultured and after induction with corresponding conditioned medium, 10 μL of CCK-8 reagent obtained from Dojindo Laboratories, Japan, was appended and incubated for 2 hr. The absorbance was later measured at 450 nm using a microplate reader (Thermo Scientific, USA).
Western Blotting
Cultured cells were lysed in cell RIPA lysis buffer, and a bicinchoninic acid (BCA) kit was used to detect the protein concentration (Beyotime Inc., China). Equal amounts of protein (20 μg) were sorted using 10% SDS-PAGE and moved on to PVDF membranes (Invitrogen; Thermo Fisher Scientific, Inc., USA). Later, the membranes were incubated with the following primary antibodies at 4°C overnight: anti-CCR6 (1:1000, ab227036, Abcam); anti-NLRP3 (1:1000, 263899, Abcam); anti-cleaved-Caspase-1 (1:1000, 4199, CST); anti- Caspase-1 (1:1000, 98033, CST); anti-GSDMD-N (1:1000, ab215203, Abcam); anti-GAPDH (1:1000, ab9485, Abcam). Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) (1:5000, ab150077, Abcam) were then used to treat the membrane for 1 hr at room temperature. Thereafter, the bands of proteins were envisaged using ECL-prime kit and analyzed with ImageJ program.
Immunofluorescence (IF) Staining
THP-1 cells were treated with PMA, IFN-γ + LPS and IL-4 + IL-13, respectively, and affixed with 4% polyoxymethylene (Sigma-Aldrich) at 4°C for 15 min. Subsequently, the cells were treated with 1% Triton X-100 in 0.01 M PBS for 15 min. Antibodies for CD14, CD11b, CD86 and CD206 (Abcam, 1:50, Cambridge, MA) were incubated with the cells at 4°C the full night. After treating with the primary antibodies, Alexa Fluor 546 conjugated goat anti-rabbit IgG (1:200; Invitrogen) was used to visualize the samples using a fluorescence microscope (Olympus, IX71, Japan).
Statistical Analyses
The difference among groups were represented statistically as mean ± SD. One-way analysis of variance (ANOVA) by means of Tukey’s test was used as a tool to make multiple comparisons. P value <0.05 was considered to be statistically significant among the triplicates tested. All experiments were performed in triplicate, and the data are presented as mean ± SD.
Results
The CCL20/CCR6 Axis Mediated Macrophage Polarization and Lymphocyte Differentiation in AH Samples
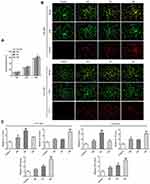
To examine the expressions of CCL20 and CCR6 en route of detecting macrophage polarization among adenoid tissues, ELISA test was performed. It was interesting to find that the samples with high CCL20 content had obvious AH as observed via enlarged adenoids, while the AH patients with low CCL20 expressions had comparatively lesser enlargement of the adenoids (Figure 1A). Subsequently, 5 samples with the high CCL20 expressions (HE group) and 5 samples with the low expressions of CCL20 (LE group) were selected for further pathological examination. To accomplish this purpose, the expression of macrophage polarization and T lymphocyte infiltration markers CD86 and CD206, IL-17A and FOXP3 were detected by IHC. The outcomes exhibited that in comparison to the LE group, the expressions of M1 macrophage marker CD86 and IL-17 as a marker of Th17 cells were significantly increased, whereas, the expressions of M2 macrophage marker CD206 and FOXP3 expression as a marker of Treg cells were significantly decreased in the HE group (Figure 1B).
 |
Figure 1 (A) H&E staining was used to detect the pathological state of the adenoids of AH patients. (B) IHC based detection of the expressions of CD86, CD206, IL-17A and FOXP3. |
CCL20/CCR6 Mediated Macrophage Polarization into M1-Type Cells so as to Promote the Differentiation of Lymphocytes into Th17 Cells in vitro in AH Lymphocytes
After the pro-inflammatory and alternative stimuli leading to cytokine-induced polarization of THP-1 cells, IF was used to detect the cell polarization status, and the results displayed that THP-1 cells were successfully polarized to M0, M1 and M2 macrophages (Figure 2A and B). The polarized M0, M1 and M2 macrophages were used to culture H9 cells, and the cells were grouped into control, M0, M1 and M2 groups. Initially, CCK-8 assay revealed no significant differences in cell survival rate between the control group and M0 group. Compared to M0, the cell survival rate of M1 group significantly increased 24 hr and 48 hr post-induction. The cell survival rate of M2 group increased after 48 hr of induction (Figure 3A).
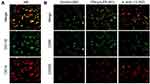 |
Figure 2 (A and B) Detection of polarization of macrophages by IF. |
The analysis of expressions of marker factors by IF involving the Th17 cells and Treg cells showed that in comparison to the M0 group, IL-17 expression in M1 group was significantly increased, while, FOXP3 expression was not significantly changed; however, IL-17 expression in M2 group was not significantly changed, but FOXP3 expression was significantly increased (Figure 3B). Supporting these outcomes, ELISA results showed that the trend of expression of IL-17 was compatible with or resembled the effects reported by IF. The expression results of Treg cell markers showed that in comparison with M0, the expression of IL-10 in M2 was significantly increased, whereas, no significant changes were observed in M1. Compared with M0, TGF-β expression was significantly increased in both M1 and M2 (Figure 3C). Therefore, CCL20/CCR6 mediated macrophage polarization into M1-type can stimulate the differentiation of AH lymphocytes into Th17 cells.
CCL20/CCR6 Mediated Macrophage Polarization into M1-Type Cells so as to Promote Adenoid Epithelial Inflammation in vitro in AH Cells
After identifying the roles of CCL20/CCR6 axis in polarization and differentiation of lymphocytes into Th17 cells, adenoid epithelial cells were cultured with polarized M0, M1, M2 macrophage medium, and treated with CCR6 inhibitor. The cells were grouped into control, M0, M1, M2 and M1+CCR6 inhibitor. CCK-8 results revealed that compared with M0, the cellular activities of M1 and M2 groups were significantly increased 24 hr and 48 hr after induction, and an increasing trend observed in the M1 group was more significant. Compared with the M1 group, cell activity was significantly decreased in M1+CCR6 inhibitor group (Figure 4A).
ELISA was used to distinguish the expressions of inflammatory cytokines and CCL20. The outcomes disclosed that compared to M0, the expressions of IL-6, TNF-α, IL-1β and CCL20 in M1 group were markedly greater, and the expressions of these cytokines in M2 group also showed an upward trend. But this upward trend was not as obvious as that of the M1. In addition, the expressions were significantly decreased in M2+CCR6 inhibitor group in comparison to the M2 group (Figure 4B). Nevertheless, the expression trend of CCR6 was consistent with that of CCL20 (Figure 5A). Western blot analysis of expressions of the inflammatory pathway proteins showed that compared with M0 group, the expressions of NLRP3, cleaved-caspase 1, and GSDMD-N were significantly increased in M1 group, but this was not obvious in M2 group. Compared with M1 group, expression of the components of inflammasome-induced apoptosis decreased significantly in M1+CCR6 inhibitor group (Figure 5B). Collectively, these results suggest that the CCL20/CCR6 mediated macrophage activation and polarization into M1-type macrophages can promote adenoid epithelial inflammation in AH.
Discussion
AH may be caused by bacteria, viruses or allergy. It is usually presented as the proliferation or increase in size of loose lymphoid connective tissues and their follicles or of the mucosal epithelial cells.24 The proliferation process is also accompanied by a diverse group of immune cells enriched in the meantime or on the surface, further aggravating the stimulation. For the inflammatory hyperplasia of epithelial cells on the surface of the adenoid, the secreted products are more likely to invade forward to cause rhinosinusitis, and invade the eustachian tube on both sides to lead to otitis media.25 In addition, previous research and published studies have found that the number of Th17 cells in the adenoid tissue or peripheral blood of AH patients is significantly increased, and Th17/Treg ratio is related to adenoid size, so the increase of Th17/Treg ratio is also an inducer of AH.23,26 Therefore, in our experiment, the symptoms of AH were determined by the detection of adenoid epithelial cell inflammation and Th17/Treg ratio in T lymphocytes.
Macrophages have remarkable plasticity and are able to alter their functional phenotypes in response to the tissue microenvironment, thus performing a range of different functions necessary for host defense and tissue repair.27 It has been acknowledged that there is a cooperative relationship between monocytes, macrophages and granulocytes in the adenoids to control the nasopharyngeal bacterial community as a whole.28 Monocytes and macrophages produce inflammatory mediators such as leukotrienes that can promote the symptoms of AH.29 Macrophages play a key pathogenic role in AH instigated by bacterial infections and inflammations that arise during allergies.30 Besides, immunological reactions and their responses play critical roles in AH physiology and the increased levels of cytokines and the macrophage markers are commonly observed in AH tissues.31
Certain members of aerobic gram-positive Streptococcus, Staphylococcus and Enterococcus genus and gram-negative organisms like Moraxella catarrhalis and Haemophilus influenza have been identified to be present in adenoid tissues of patients with AH.32,33 Among these organisms, gram-positive pathogens produce lipoteichoic acid known to regulate macrophages and activate leukocytes to involve in creating an inflammatory reaction.34–38 Also, the above-mentioned gram-negative pathogens can produce or constitute lipopolysaccharides.39,40 Both lipoteichoic acid and lipopolysaccharides can activate monocytes/macrophages, act as M1 macrophage signals and generate an inflammatory response by causing a cytokine trigger.41–45
Hence, polarization of macrophages is an essential process for the formation of their phenotypes, namely, classically activated M1 and alternatively activated M2 macrophages specific to a stimulus. This process is necessary for tissue repair and maintaining homeostasis and the polarized macrophages possess unique surface markers and cytokines for a specified function.46 Macrophages are classically activated for an M1 response by treatment with IFN-γ and LPS and characterized by elevated expressions of CD86, whereas, the M2 response is alternatively activated by treatment with IL-4 and IL-13 and characterized by elevated expressions of CD206.47 Typically, M1 macrophages demonstrate pro-inflammatory and antimicrobial properties, whereas, M2 macrophages possess anti-inflammatory properties.46
Therefore, infecting polarized macrophages with pathogenic microbes can result in the enhanced release of pro-inflammatory cytokines.48 The cytokines IL-1β, IL-6, IL-17, IL-10, TNF-α and TGF-β are involved in pro-inflammatory activities, resisting pathogens, pathologizing a number of inflammation-related disorders and enhancing the polarization of macrophages.49–59 Also, FOXP3 and IL-17A are considered important Treg and Th17-related markers.60,61 Adding to this, CCL20 is expressed by both lymphoid and non-lymphoid tissues to maintain homeostasis and immune functions. Under an array of conditions characterized by inflammation or hypoxia, cells infected by bacteria and viruses along with cells that release pro-inflammatory cytokines induce CCL20 production. Several cells that engage in inflammation including endothelial cells that form the lining of blood vessels, cells of the innate immune system like neutrophils and natural killer cells, and cells that mediate humoral immunity such as effector Th17 cells and B lymphocytes can secrete CCL20. Remarkably, dendritic cells, Langerhans cells and macrophages can also produce the chemokine.62–64 TGF-β regulates the expression of CCR6 in Th17 cells, and these pro-inflammatory cells are dependent on CCR6 for promoting the improved migratory properties and engagement of Th17 and Treg cells to the tissues at the site of inflammation.65 In our study, we establish that in AH disease, macrophages can polarize into M1-type macrophages to promote lymphocyte differentiation, increase the Th17/Treg ratio and epithelial cell inflammation, ultimately promoting the development of AH disease.
CCL20 is the only known ligand with high affinity that binds CCR6 and drives the migration of CCR6+ cells in tissues.66 It has been known that macrophages can secrete the chemokine CCL20 after activation, and CCL20 can bind to receptor CCR6 in T lymphocytes to induce the lymphocytes to differentiate into Th17 cells and increase the Th17/Treg cell ratio.67,68 Th17/Treg ratio is a critical factor in the pathogenesis of AH.26 In addition, it has been reported that the expression of CCL20 is increased by multiple fold accredited to conditions that relate to inflammation, and the expression of CCR6 in such inflammatory diseases is strongly correlated with how severe the disease is.69
Hence, it was thought-provoking to guesstimate that there are some interesting relationships between CCL20/CCR6 axis and AH. To gain a basic understanding of how the CCL20-CCR6 axis interacts with AH and attains a relationship, we continued with our experiment and it was found that the expressions of CCL20 and CCR6 were significantly elevated in samples obtained from AH patients. The more obvious the AH was, the higher the expressions of CCL20 and CCR6 were. In addition, CCL20/CCR6 mediated lymphocyte differentiation and macrophage polarization can aggravate inflammation.70 Likewise, the recruitment and participation of CCL20-CCR6 axis can promote the infiltration, activation and migration of macrophages.71,72 In our experiment, we found that the inhibition of CCR6 expression could inhibit the expression of CCL20, thus reversing the promoting effect of M1-type macrophage polarization on epithelial cell inflammation.
NLRP3 inflammasome is brought together in responding to microbial encounters and infections. Gasdermin D (GSDMD) is a vital factor or constituent of inflammasomes and when it can activate macrophages, is recruited for cleaving by caspase-1 to form N-GSDMD. These fragments oligomerize across the plasma membrane, resulting in the formation of pores leading to improved permeability of membranes, mediating pyroptosis and engage in releasing IL-1β.73,74 The upregulated expressions of these factors observed in this study indicate the roles of these components in the possible cell killing.
Conclusion
To summarize, our research illustrates that CCL20/CCR6 mediated macrophage activation and polarization into M1-type macrophages can promote adenoid epithelial inflammation in AH.
Acknowledgments
This study was supported by the Traditional Chinese Medicine Research Project of Wuxi Health Commission (ZYZD201808); Social Development Guidance Project of Yixing Science and Technology Bureau (2020).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Geiger Z, Gupta N. Adenoid Hypertrophy. Treasure Island (FL): StatPearls Publishing; 2021.
2. Rout MR, Mohanty D, Vijaylaxmi Y, Bobba K, Metta C. Adenoid hypertrophy in adults: a case series. Indian J Otolaryngol Head Neck Surg. 2013;65(3):269–274. doi:10.1007/s12070-012-0549-y
3. Pereira L, Monyror J, Almeida FT, et al. Prevalence of adenoid hypertrophy: a systematic review and meta-analysis. Sleep Med Rev. 2018;38:101–112. doi:10.1016/j.smrv.2017.06.001
4. Yildirim N, Şahan M, Karslioğlu Y. Adenoid hypertrophy in adults: clinical and morphological characteristics. J Int Med Res. 2008;36(1):157–162. doi:10.1177/147323000803600120
5. Buzatto G, Tamashiro E, Proenca-Modena JL, et al. The pathogens profile in children with otitis media with effusion and adenoid hypertrophy. PLoS One. 2017;12(2):e0171049. doi:10.1371/journal.pone.0171049
6. Niu X, Wu Z-H, Xiao X-Y, Chen X. The relationship between adenoid hypertrophy and gastroesophageal reflux disease: a meta-analysis. Medicine. 2018;97(41):114.
7. Brambilla I, Pusateri A, Pagella F, et al. Adenoids in children: advances in immunology, diagnosis, and surgery. Clin Anat. 2014;27(3):346–352. doi:10.1002/ca.22373
8. Retcheski AJ, Silva NP, Leite F, Nouer PR. Reliability of adenoid hypertrophy diagnosis by cephalometric radiography. RGO-Revista Gaúcha de Odontologia. 2014;62:275–280. doi:10.1590/1981-8637201400030000071762
9. Jyothirmai A, Sadhana O, Chandra TS, Murthy P. Assessment of adenoid hypertrophy with clinical grading versus radiology and endoscopy-A cross-sectional study. IP J Otorhinolaryngol Allied Sci. 2021;3(4):130–135. doi:10.18231/j.ijoas.2020.028
10. Hirayama D, Iida T, Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci. 2017;19(1):92. doi:10.3390/ijms19010092
11. Parihar A, Eubank TD, Doseff AI. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun. 2010;2(3):204–215. doi:10.1159/000296507
12. Chiu S, Bharat A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr Opin Organ Transplant. 2016;21(3):239. doi:10.1097/MOT.0000000000000313
13. Onal M, Onal O, Turan A. Can secondary lymphoid organs exert a favorable effect on the mild course of COVID-19 in children? Acta Otolaryngol. 2021;141:83–84. doi:10.1080/00016489.2020.1814965
14. Stępińska M, Olszewska-Sosińska O, Lau-Dworak M, Zielnik-Jurkiewicz B, Trafny E. Identification of intracellular bacteria in adenoid and tonsil tissue specimens: the efficiency of culture versus fluorescent in situ hybridization (FISH). Curr Microbiol. 2014;68(1):21–29. doi:10.1007/s00284-013-0436-0
15. Pagella F, De Amici M, Matti E, Pusateri A, Benazzo M, Ciprandi G. CD64 expression on monocytes in children with adenoid hypertrophy. Asian Pacific J Allergy Immunol. 2013;31(2):132. doi:10.12932/AP0294.31.2.2013
16. Peker BC, Mustafa A, Şahin M. Identification of the immune receptor CD14 in hypertrophic adenoids. ENT Updates. 2015;5(3):93–96. doi:10.2399/jmu.2015003003
17. Lee AY, Phan TK, Hulett MD, Körner H. The relationship between CCR6 and its binding partners: does the CCR6–CCL20 axis have to be extended? Cytokine. 2015;72(1):97–101. doi:10.1016/j.cyto.2014.11.029
18. Lu M-Y, Lu -S-S, Chang S-L, Liao F. The phosphorylation of CCR6 on distinct Ser/Thr residues in the carboxyl terminus differentially regulates biological function. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.00415
19. Kadomoto S, Izumi K, Mizokami A. The CCL20-CCR6 axis in cancer progression. Int J Mol Sci. 2020;21(15):5186. doi:10.3390/ijms21155186
20. Frick VO, Rubie C, Keilholz U, Ghadjar P. Chemokine/chemokine receptor pair CCL20/CCR6 in human colorectal malignancy: an overview. World J Gastroenterol. 2016;22(2):833. doi:10.3748/wjg.v22.i2.833
21. Das M, Tang X, Han JY, et al. CCL20-CCR6 axis modulated traumatic brain injury-induced visual pathologies. J Neuroinflammation. 2019;16(1):1–12. doi:10.1186/s12974-019-1499-z
22. Lyu M, Li Y, Hao Y, et al. CCR6 defines a subset of activated memory T cells of Th17 potential in immune thrombocytopenia. Clin Exp Immunol. 2019;195(3):345–357. doi:10.1111/cei.13233
23. Sade K, Fishman G, Kivity S, DeRowe A, Langier S. Expression of Th17 and Treg lymphocyte subsets in hypertrophied adenoids of children and its clinical significance. Immunol Invest. 2011;40(6):657–666. doi:10.3109/08820139.2011.575426
24. Ju L, Yu M, Zhu L, Jia Z, Zhang M, Chen J. Chronic toxicity of Multi-walled carbon nanotubes in human pleural mesothelial cells. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2021;39(3):173–177. doi:10.3760/cma.j.cn121094-20190919-00382
25. Bowers I, Shermetaro C. Adenoiditis. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
26. Ni K, Zhao L, Wu J, Chen W, Li X, Li X. Th17/Treg balance in children with obstructive sleep apnea syndrome and the relationship with allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2015;79(9):1448–1454. doi:10.1016/j.ijporl.2015.06.026
27. Funes SC, Rios M, Escobar‐Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154(2):186–195. doi:10.1111/imm.12910
28. Ivarsson M, Lundberg C. Phagocytosis in the nasopharyngeal secretion by cells from the adenoid. Acta Otolaryngol. 2001;121(4):517–522. doi:10.1080/000164801300366697
29. Kar M, Altıntoprak N, Muluk NB, Ulusoy S, Bafaqeeh SA, Cingi C. Antileukotrienes in adenotonsillar hypertrophy: a review of the literature. Eur Arch Oto Rhino Laryngol. 2016;273(12):4111–4117. doi:10.1007/s00405-016-3983-8
30. De Amici M, Ciprandi G, Marseglia A, et al. Adenoid hypertrophy: definition of some risk factors. J Biol Regul Homeost Agents. 2012;26(1 Suppl):S1–7.
31. Gao K, Li Y, Yue Z, Han J, Zhou X, Wang X. Down‐regulation of anti‐inflammatory TIPE2 may aggravate adenoidal hypertrophy in children. FEBS Open Bio. 2020;10(5):761–766. doi:10.1002/2211-5463.12821
32. Rajeshwary A, Rai S, Somayaji G, Pai V. Bacteriology of symptomatic adenoids in children. N Am J Med Sci. 2013;5(2):113. doi:10.4103/1947-2714.107529
33. McClay JE. Resistant bacteria in the adenoids: a preliminary report. Arch Otolaryngol Head Neck Surg. 2000;126(5):625–629.
34. Hong SW, Baik JE, Kang -S-S, Yun C-H, Seo D-G, Han SH. Lipoteichoic acid of Streptococcus mutans interacts with Toll-like receptor 2 through the lipid moiety for induction of inflammatory mediators in murine macrophages. Mol Immunol. 2014;57(2):284–291. doi:10.1016/j.molimm.2013.10.004
35. Leemans JC, Heikens M, van Kessel KP, Florquin S, van der Poll T. Lipoteichoic acid and peptidoglycan from Staphylococcus aureus synergistically induce neutrophil influx into the lungs of mice. Clin Vaccine Immunol. 2003;10(5):950–953.
36. Kim W, Lee EJ, Bae I-H, et al. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J Extracell Vesicles. 2020;9(1):1793514. doi:10.1080/20013078.2020.1793514
37. Gao JJ, Xue Q, Zuvanich EG, Haghi KR, Morrison DC. Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect Immun. 2001;69(2):751–757. doi:10.1128/IAI.69.2.751-757.2001
38. Wang S, Liu K, Seneviratne CJ, et al. Lipoteichoic acid from an Enterococcus faecalis clinical strain promotes TNF-α expression through the NF-κB and p38 MAPK signaling pathways in differentiated THP-1 macrophages. Biomed Rep. 2015;3(5):697–702. doi:10.3892/br.2015.495
39. Schweda EK, Richards JC, Hood DW, Moxon ER. Expression and structural diversity of the lipopolysaccharide of Haemophilus influenzae: implication in virulence. Int J Med Microbiol. 2007;297(5):297–306. doi:10.1016/j.ijmm.2007.03.007
40. Peng D, Hong W, Choudhury BP, Carlson RW, Gu -X-X. Moraxella catarrhalis bacterium without endotoxin, a potential vaccine candidate. Infect Immun. 2005;73(11):7569–7577. doi:10.1128/IAI.73.11.7569-7577.2005
41. Huang Z, Kraus VB. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev Rheumatol. 2016;12(2):123–129. doi:10.1038/nrrheum.2015.158
42. Tucureanu MM, Rebleanu D, Constantinescu CA, et al. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor. Int J Nanomedicine. 2018;13:63. doi:10.2147/IJN.S150918
43. Cox KH, Cox ME, Woo-Rasberry V, Hasty DL. Pathways involved in the synergistic activation of macrophages by lipoteichoic acid and hemoglobin. PLoS One. 2012;7:e47333. doi:10.1371/journal.pone.0047333
44. Kwak MS, Lim M, Lee YJ, et al. HMGB1 binds to Lipoteichoic acid and enhances TNF-a and IL-6 production through HMGB1-mediated transfer of Lipoteichoic acid to CD14 and TLR2. J Innate Immun. 2015;7(4):405–416. doi:10.1159/000369972
45. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6. doi:10.12703/P6-13
46. Yao Y, Xu X-H JL, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol. 2019;10:792. doi:10.3389/fimmu.2019.00792
47. Smith TD, Tse MJ, Read EL, Liu WF. Regulation of macrophage polarization and plasticity by complex activation signals. Integr Biol (Camb). 2016;8(9):946–955. doi:10.1039/c6ib00105j
48. Buchacher T, Ohradanova-Repic A, Stockinger H, Fischer MB, Weber V. M2 polarization of human macrophages favors survival of the intracellular pathogen Chlamydia pneumoniae. PLoS One. 2015;10(11):e0143593. doi:10.1371/journal.pone.0143593
49. Moratal C, Raffort J, Arrighi N, et al. IL-1β-and IL-4-polarized macrophages have opposite effects on adipogenesis of intramuscular fibro-adipogenic progenitors in humans. Sci Rep. 2018;8(1):1–13. doi:10.1038/s41598-017-17765-5
50. Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22(4):189–195.
51. Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One. 2014;9(4):e94188. doi:10.1371/journal.pone.0094188
52. Miller JE, Ahn SH, Marks RM, et al. IL-17A modulates peritoneal macrophage recruitment and M2 polarization in endometriosis. Front Immunol. 2020;11:108. doi:10.3389/fimmu.2020.00108
53. Lopes RL, Borges TJ, Zanin RF, Bonorino C. IL-10 is required for polarization of macrophages to M2-like phenotype by mycobacterial DnaK (heat shock protein 70). Cytokine. 2016;85:123–129. doi:10.1016/j.cyto.2016.06.018
54. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev. 2012;32(1):101.
55. Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79(1):541–566. doi:10.1146/annurev-physiol-022516-034339
56. Andrade RM, Wessendarp M, Portillo J-AC, et al. TNF receptor-associated factor 6-dependent CD40 signaling primes macrophages to acquire antimicrobial activity in response to TNF-α. J Immunol. 2005;175(9):6014–6021. doi:10.4049/jimmunol.175.9.6014
57. Masli S, Turpie B. Anti‐inflammatory effects of tumour necrosis factor (TNF)‐α are mediated via TNF‐R2 (p75) in tolerogenic transforming growth factor‐β‐treated antigen‐presenting cells. Immunology. 2009;127(1):62–72. doi:10.1111/j.1365-2567.2008.02933.x
58. Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9(4):447–453. doi:10.1016/j.coph.2009.04.008
59. Zhang F, Wang H, Wang X, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7(32):52294. doi:10.18632/oncotarget.10561
60. Agrawal S, Parkash O, Palaniappan AN, et al. Efficacy of T regulatory cells, Th17 cells and the associated markers in monitoring tuberculosis treatment response. Front Immunol. 2018;9:157. doi:10.3389/fimmu.2018.00157
61. Milovanovic J, Arsenijevic A, Stojanovic B, et al. Interleukin-17 in chronic inflammatory neurological diseases. Front Immunol. 2020;11:947. doi:10.3389/fimmu.2020.00947
62. Li X, Syrovets T, Simmet T. The serine protease plasmin triggers expression of the CC-chemokine ligand 20 in dendritic cells via Akt/NF-κB-dependent pathways. J Biomed Biotechnol. 2012;2012. doi:10.1155/2012/186710
63. Lee AY, Eri R, Lyons AB, Grimm MC, Korner H. CC chemokine ligand 20 and its cognate receptor CCR6 in mucosal T cell immunology and inflammatory bowel disease: odd couple or axis of evil? Front Immunol. 2013;4:194. doi:10.3389/fimmu.2013.00194
64. Kennedy-Crispin M, Billick E, Mitsui H, et al. Human keratinocytes’ response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. J Investig Dermatol. 2012;132(1):105–113. doi:10.1038/jid.2011.262
65. Yamazaki T, Yang XO, Chung Y, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181(12):8391–8401. doi:10.4049/jimmunol.181.12.8391
66. Lee AYS, Korner H. CCR6/CCL20 chemokine axis in human immunodeficiency virus immunity and pathogenesis. J Gen Virol. 2017;98(3):338–344. doi:10.1099/jgv.0.000691
67. Almanzar G, Klein M, Schmalzing M, et al. Disease manifestation and inflammatory activity as modulators of Th17/Treg balance and RORC/FoxP3 methylation in systemic sclerosis. Int Arch Allergy Immunol. 2016;171(2):141–154. doi:10.1159/000450949
68. Lee AY, Körner H. The CCR6-CCL20 axis in humoral immunity and TB cell immunobiology. Immunobiology. 2019;224(3):449–454. doi:10.1016/j.imbio.2019.01.005
69. Meitei HT, Jadhav N, Lal G. CCR6-CCL20 axis as a therapeutic target for autoimmune diseases. Autoimmun Rev. 2021;20(7):102846. doi:10.1016/j.autrev.2021.102846
70. Wunderlich CM, Ackermann PJ, Ostermann AL, et al. Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat Commun. 2018;9(1):1–16. doi:10.1038/s41467-018-03773-0
71. Chen Z, Chen G, Zhao H. FDPS promotes glioma growth and macrophage recruitment by regulating CCL20 via Wnt/β‐catenin signalling pathway. J Cell Mol Med. 2020;24(16):9055–9066. doi:10.1111/jcmm.15542
72. Kadomoto S, Izumi K, Hiratsuka K, et al. Tumor-associated macrophages induce migration of renal cell carcinoma cells via activation of the CCL20-CCR6 axis. Cancers. 2019;12(1):89. doi:10.3390/cancers12010089
73. He W-T, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi:10.1038/cr.2015.139
74. Karmakar M, Minns M, Greenberg EN, et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun. 2020;11(1):1–14. doi:10.1038/s41467-020-16043-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.