Back to Journals » Journal of Inflammation Research » Volume 16
The Systemic Immune Inflammatory Response Index Can Predict the Clinical Prognosis of Patients with Initially Diagnosed Coronary Artery Disease
Authors Li Y, Bai G, Gao Y, Guo Z, Chen X, Liu T, Li G
Received 21 August 2023
Accepted for publication 24 October 2023
Published 2 November 2023 Volume 2023:16 Pages 5069—5082
DOI https://doi.org/10.2147/JIR.S432506
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Tara Strutt
Yuqing Li,* Geng Bai,* Yi Gao,* Ziqiang Guo, Xiaolin Chen, Tong Liu, Guangping Li
Tianjin Key Laboratory of Logic-Molecular Function of Cardiovascular Disease, Department of Cardiology, Tianjin Institute of Cardiology, The Second Hospital of Tianjin Medical University, Tianjin, 300211, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guangping Li, Tel + 86-22-88328648, Fax + 86-22-28261158, Email [email protected]
Background: Recently, the systemic immune inflammatory response index (SIIRI), a novel and expanded inflammatory response marker, has been an independent predictor of lesion severity in patients with acute coronary syndrome (ACS). However, its predictive role in patients with initially diagnosed coronary artery disease (CAD) remains to be explored.
Patients and Methods: We evaluated 959 patients with CAD undergoing an initial coronary intervention. Each patient had laboratory measurements, including blood cell counts, taken after admission and before interventional treatment. The primary endpoint was major cardiovascular events (MACEs), defined as cardiovascular death, nonfatal myocardial infarction(MI), and nonfatal stroke. The secondary endpoints included MACEs and readmission for congestive heart failure(HF).
Results: During a mean follow-up period of 33.3± 9.9 months, 229 (23.9%) MACEs were recorded. ROC curve analysis displayed that the best cut-off value of SIIRI for predicting MACEs was 247.17*1018/L2. Kaplan-Meier survival curve analysis showed that the survival rate of the low SIIRI group was higher than that of the high SIIRI group (P< 0.001). Compared with the low SIIRI group, the high SIIRI group had a significantly higher risk of MACEs (187 cases (39.53%) vs.42 patients (8.64%), P< 0.001). Univariate and multivariate Cox regression analyses displayed that high SIIRI levels were independently associated with the occurrence of MACEs in patients with initially diagnosed CAD undergoing percutaneous coronary intervention (PCI) (adjusted hazard ratio [HR]: 3.808, 95% confidence interval [CI%]: 2.643– 5.486, P< 0.001). Adding SIIRI to conventional risk factor models improved the predictive value of MACEs.
Conclusion: Elevated SIIRI is associated with adverse cardiovascular prognosis in patients with initially diagnosed CAD. SIIRI can be a simple and practical index to identify high-risk patients with CAD after PCI.
Keywords: systemic immune inflammatory response index, coronary artery disease, inflammation, markers, blood cell count
Introduction
According to the evidence from the World Health Organization, although the mortality rate of CAD has decreased in recent decades, it is still one of the leading causes of death in the global population. It brings a heavy economic burden.1 Approximately 50% of the mortality reduction can be attributed to managing ACS and related complications, effectively implementing primary and secondary prevention strategies, and effectively revascularizing patients with chronic coronary syndromes.2 Identifying high-risk patients with CAD is helpful for clinical treatment and prognosis management.
As an inflammatory disease of large arteries, atherosclerosis plays an indispensable role in the occurrence and development of CAD. Traditional factors such as hypertension, diabetes, dyslipidemia, and smoking have been proven to be closely related to the progression of atherosclerosis, and inflammation is considered to play a crucial role in it.3 Inflammatory markers are associated with the severity of CAD and poor cardiovascular prognosis. These represent laboratory measures readily available in resource-poor clinical Settings.4,5 Inflammatory markers are essential in monitoring the progression of CAD. However, applying many biomarkers in the clinical setting is challenging due to the high cost and processing time.6 Developing an ideal indicator that can be easily measured with high precision is vital to predicting the clinical prognosis of patients with CAD.
Considering the complex pathophysiological relationship between inflammation and the occurrence and development of atherosclerosis, the relationship between inflammatory markers and the clinical prognosis of patients with CAD deserves further study. Inflammation has been associated with chronic HF, metabolic disorders, cancer, and cardiovascular disease.7–10 Blood cell analysis has recently received extensive attention as a routine laboratory test. These indicators are based on platelet count and leukocyte subtype (neutrophil, monocyte, and lymphocyte) counts, including neutrophil to lymphocyte ratio (NLR), monocyte to lymphocyte ratio (MLR), platelet to lymphocyte ratio (PLR), systemic inflammation index (SII) and systemic inflammatory response index (SIRI). When two or three different cell line values that interact with each other are combined, the overall predictive value for clinical prognosis and mortality in patients with cardiovascular disease increases synergistically.11–13
NLR is associated with the severity of CAD. With the increase of NLR stratification level, the incidence of cardiovascular prognosis events gradually increases.14 NLR can also be used as an independent predictor of cardiac mortality in stable CAD patients.15 MLR can be used as an independent predictor of MACEs in patients with CAD undergoing PCI,16 and it can improve the predictive power of mortality in patients with CAD.17 Elevated PLR can be used as an independent predictor of CAD in elderly patients (> 55 years) and is associated with the severity of CAD and the increased risk of MACEs within five years in patients with stable CAD.18–20 SII and SIRI use three blood cell subtypes, reflecting the balance between inflammation and immune response. SII is better than traditional risk factors in predicting major cardiovascular events in patients with CAD and positively correlates with the severity of CAD.12,21 SII has also been described as a predictor of poor clinical prognosis in ACS patients with chronic kidney disease.22 During a 10-year follow-up study, SIRI was associated with incident CAD and an increased risk of all-cause death and stroke but not MI.23 Similarly, higher SIRI was associated with more severe disease status in patients with ACS undergoing PCI, and the predictive value was synergistically increased when combined with the GRACE risk score.24
Several novel inflammatory markers have also shown the ability to predict CAD. A high C-reactive protein to albumin ratio is an independent predictor of the severity and extent of ischemia in patients with coronary artery disease.25 CAD patients with diabetes mellitus and a high fibrinogen-to-albumin ratio have a poor 5-year clinical prognosis, which is helpful in identifying high-risk patients.26
Patients diagnosed with CAD and undergoing diagnostic coronary angiography must take dual antiplatelet and statin lipid-lowering drugs routinely after surgery, which is helpful for patients’ clinical prognosis and disease outcome.27 The anti-inflammatory effect of statins helps them play an active role in the progression of atherosclerosis.28 Platelets are essential in thrombosis, acute MI, and acute stroke. Antiplatelet drugs also inhibit the progression of atherosclerosis due to their anti-inflammatory effects.29 Therefore, the new inflammatory markers may be more accurate in predicting the clinical prognosis of patients with initially diagnosed CAD who have not taken antiplatelet and statin drugs.
Recently, Mangalesh et al designed a new inflammatory marker, SIIRI, through the combination of four cells and showed that SIIRI was an independent predictor of the severity of lesions in patients with ACS. Its overall predictive effect was better than that of previous inflammatory markers.6 Similarly, previous inflammatory markers, including NLR, PLR, and MLR, have been shown to be predictive in patients with CAD.14–20 As proved by various cohort studies and meta-analyses, SII, as a new inflammatory marker composed of three blood cell subtypes, can more comprehensively evaluate the inflammatory status of patients with CAD compared with the inflammatory markers composed of two cell subtypes (NLR, PLR, and MLR).30 Unfortunately, there is a lack of studies on the poor clinical prognosis of patients with SIIRI and initially diagnosed CAD. Early identification of high-risk patients, effective treatment, and prognosis management can improve the long-term clinical prognosis of patients with CAD.
Therefore, this study aimed to investigate the predictive value of SIIRI for clinical prognosis in patients with the same initial diagnosis of CAD.
Materials and Methods
Study Population
We retrospectively collected 959 patients with initially diagnosed CAD who underwent diagnostic coronary angiography in the Second Hospital of Tianjin Medical University from January 2019 to April 2021 in Tianjin, China. Each patient had never had symptoms related to coronary artery disease, including chest pain and shortness of breath, and had never undergone coronary CT angiography or coronary angiography before the presentation. Each patient was required to obtain complete demographic, clinical laboratory, and prognostic follow-up results. Before a hospitalization, each patient was tested for COVID-19 virus twice on different days, and the two results were negative. Patients who tested positive for COVID-19 were not included in the study because they were referred to specific healthcare facilities. Each patient signed written informed consent and participated in the study. Informed consent was obtained from the patients at the time of readmission, outpatient visits, and home visits.
The primary endpoint was MACEs, defined as cardiovascular death, nonfatal MI, and nonfatal stroke. The secondary endpoints included MACEs and readmission for congestive HF. Study exclusion criteria were as follows: (1) active tumor or paraneoplastic syndrome, (2) acute infection, (3) severe renal insufficiency (eGFR<30mL/min /1.73m2), (4) severe liver failure, (5) known inflammatory/autoimmune disease, (6) active cerebrovascular disease, (7) Use of statins, steroids, antiplatelet and anticoagulant drugs.
Clinical and Laboratory Data
Electronic medical record systems collected data on demographic characteristics and laboratory test results. Gaps in medical records were obtained by asking the patient on admission. Results of the first venous blood sample and complete blood count were obtained from all hospitalized patients before diagnostic coronary angiography. Regarding biomarkers, NLR is the ratio of neutrophil count to lymphocyte count, PLR is the ratio of platelet count to lymphocyte count, MLR is the ratio of monocyte count to lymphocyte count, SII is defined as platelet count * neutrophil count/lymphocyte count, SIRI as monocyte count * neutrophil count/lymphocyte count, and SIIRI as platelet count * monocyte count * neutrophil count/lymphocyte count. According to the 2021 European Society of Hypertension practice guideline, hypertension is blood pressure ≥140/90mmHg measured in the office.31 According to the 2019 ESC guidelines for prediabetes, diabetes combined with cardiovascular risk factor criteria finally defined diabetes: A fasting blood glucose value ≥7.0mmol/L (126mg/dL), or patients with symptoms of hyperglycemia (eg, frequent urination, polydipsia, fatigue, acetone breathing, and nausea) combined with a random blood glucose value ≥11.1mmol/L (200mg/mL); Or at least 11.1mmol/L (200mg/dL) after a 2-hour glucose tolerance test.32 Following the recommendations of the 2019 ESC/EAS guidelines for managing dyslipidemia, patients whose lipid levels did not meet the respective risk stratification treatment goals were defined as newly diagnosed dyslipidemia.33 Diagnostic coronary angiography was performed with either radial or coronary access. Coronary angiography is the preferred method to assess lesion severity in patients with CAD, following the 2021 ACC/AHA/SCAI guideline recommendations.34 Three independent cardiologists evaluated the coronary angiographic results of each patient. The severity of lesions was quantified using the CASSC score. The degree of stenosis of the main coronary artery (left anterior descending artery, left circumflex artery, and right coronary artery) >70% was assigned 1 point, and the degree of stenosis of the left main coronary artery >50% was assigned 2 points. The final results of 0–3 scores were included in the database to show the severity of lesions in patients with CAD during the switch.
Statistical and Analysis
Continuous variables were demonstrated in mean ± standard deviation (SDs) or median (25th to 75th percentile) form and compared using t-tests or Wilcoxon rank-sum tests when appropriate. Categorical variables are displayed as frequencies and percentages, using Fisher’s exact or chi-square test, as suitable to determine the significance of categorical variables between the two groups. The receiver operating characteristic (ROC) curve determined the optimal cut-off value. Kaplan-Meier curve was used for survival analysis to analyze the prognosis differences and event-free survival rates of patients in different SIIRI groups. Primary and secondary clinical prognoses were presented as percentages and proportions with 95% confidence intervals (CIs). After adjusting for individual risk factors, univariate and multivariate Cox regression analyses were exploited to evaluate the hazard ratios (HRs) for combined and individual endpoints with 95% confidence intervals (CIs). The multivariate analysis included baseline clinical factors that differed significantly between the two groups (P<0.005). To assess whether adding SIIRI would improve the ability of a basic model of known risk factors (gender, age, hypertension, diabetes, newly diagnosed hyperlipidemia, smoking history) to predict adverse cardiovascular events, calculated as C-index, net reclassification improvement (NRI), and integrated discrimination improvement (IDI). Two-tailed P values of within 0.05 were thought statistically necessary. All statistical analyses were performed employing SPSS 27.0, R 4.2.2, and GraphPad Prism 8.0.
Results
Baseline Characteristics
During a mean follow-up period of 33.3±9.9 months, 959 patients (mean age 61.4±10.8 years; 51.8% male) with the initial diagnosis of CAD underwent diagnostic coronary angiography and were retrospectively enrolled in this study. Figure 1 shows the screening flow chart of the study. MACEs, including cardiac death, nonfatal MI, and nonfatal stroke, determined the optimal cut-off value of SIIRI. As shown in Figure 2, ROC curve analysis evaluated the optimal SIIRI cut-off point (247.17*1018/L2) for predicting MACEs. We divided the study population into two groups based on the optimal cut-off value of SIIRI. Table 1 emerges the baseline characteristics of the patients after grouping by SIIRI. Compared with the low SIIRI group, the high SIIRI group had a higher proportion of male patients and a higher proportion of hypertension and smoking history.
 |
Table 1 Baseline Characteristics of 959 Patients with Initially Diagnosed CAD |
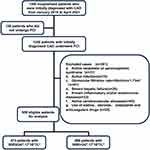 |
Figure 1 Flowchart of the study cohort. |
Regarding laboratory tests, hemoglobin, white blood cell, neutrophil, monocyte, and platelet count were higher, and lymphocyte count was lower in the high SIIRI group. The glycosylated hemoglobin levels, fasting blood glucose, and triglyceride levels were higher, and the stories of albumin, total cholesterol, and high-density lipoprotein were lower on admission. Regarding coronary angiography results, the proportion of multivessel lesions, branch lesions, and CASSC scores was higher in the high SIIRI group. The two groups had no significant difference in medication history before admission.
Clinical Prognosis in Different Groups Stratified by SIIRI
The clinical prognosis of patients in the low SIIRI and high SIIRI groups are shown in Table 2. During a mean follow-up of 33.3 months, there were 27 cardiac deaths, 134 nonfatal MI, 68 nonfatal strokes, 139 hospitalizations for congestive HF, 182 repeat coronary revascularization procedures, and 230 secondary outcome events. The prevalence of MACEs was significantly higher in the high SIIRI group than in the low SIIRI group [187 (39.53%) vs 42 (8.64%), P<0.001]. The incidences of nonfatal MI, nonfatal stroke, hospitalization for congestive HF, and revascularization were higher in patients with high SIIRI than in those with low SIIRI. There was no significant difference in the incidence of cardiac death between the two groups. The K-M survival curve and Log-rank sum test also showed that patients in the high SIIRI group had a higher incidence of MACEs (Figure 3A), nonfatal MI (Figure 3C), nonfatal stroke (Figure 3D), and readmission for congestive HF (Figure 3E) and repeat coronary-artery revascularization (Figure 3F). There was no significant difference in cardiac death between the two groups (Figure 3B). These results indicate that high SIIRI is associated with an increased risk of cardiovascular events.
 |
Table 2 Clinical Prognosis in Patients According to SIIRI Score |
Subgroup, Univariate, and Multivariate Analyses of Clinical Prognosis in Patients with Initial CAD
Univariate and multivariate Cox regression analyses were executed to recognize independent indicators of MACEs in patients with an initial diagnosis of CAD (Table 3). According to Cox regression analysis, SIIRI≥247.17*1018/L2 (HR: 3.808; 95% CI: 2.643–5.486; P<0.001) were the independent predictors associated with MACEs. Age, diabetes mellitus, newly diagnosed dyslipidemia, albumin, and polyangiopathy were independent predictors. Cox proportional-hazards regression model analyses were performed using three separate models to detect independent predictors of clinical prognosis (Table 4). The results showed that after controlling for all independent predictors except SIIRI in multivariate analysis, high SIIRI was associated with an increased risk of MACEs (HR: 3.830; 95% CI: 2.674–5.487; P<0.001), increased risk of nonfatal MI (HR: 4.689; 95% CI: 2.881–7.631; P<0.001), nonfatal stroke 4.559; 95% CI: 2.819–7.373; P<0.001), readmission for congestive HF (HR: 4.559; 95% CI: 2.819–7.373; P<0.001), and increased risk of repeat coronary revascularization (HR: 2.470; 95% CI: 1.732–3.521; P<0.001) and an increased risk of secondary end events (HR: 3.625; 95% CI: 2.547–5.161; P<0.001). In subgroup analyses, we determined whether the incidence of MACEs in different subgroups was affected by other covariates (Figure 4). There was no significant interaction between SIIRI and sex, age, hypertension, diabetes, newly diagnosed dyslipidemia, smoking, and CASSC score. MACEs in the high SIIRI group remained consistent across subgroups. Subgroup analyses further determined the robustness of the association between high SIIRI and major adverse cardiovascular events.
 |
Table 3 Cox Regression Analysis |
 |
Table 4 The Association of High SIIRI (≥247.17×1018 /L2) and Future Adverse Events in Patients |
 |
Figure 4 The Association of high SIIRI (≥247.17×1018 /L2) and future adverse events in patients. |
Adding SIIRI to the Baseline Model Had Additional Predictive Effects on Adverse Cardiovascular Events
Adding SIIRI to the base model with conventional risk factors increased the risk of MACEs (P<0.001), nonfatal MI (P=0.002), nonfatal stroke (P=0.007), readmission for congestive HF (P=0.004), and repeat coronary revascularization (P<0.001) and of secondary end events (P<0.001). As shown in Table 4. In addition, the addition of SIIRI improved the predictive power of MACEs by 0.135 (P<0.001) in integrated discrimination improvement (IDI) and 18.7% (P<0.001) in net reclassification improvement (NRI). As shown in Table 5. These results suggest that the addition of SIIRI is superior to conventional risk factors in predicting adverse cardiovascular prognosis in patients with initially diagnosed CAD.
 |
Table 5 Evaluation of Predictive Models for Cardiovascular Events |
Discussion
SIIRI is a novel inflammatory marker based on the composition of neutrophils, lymphocytes, monocytes, and platelets in blood cell count. In this study, SIIRI was an independent predictor of poor clinical prognosis in patients with initially diagnosed CAD, and adding SIIRI to conventional risk factor models improved the predictive power of the basic model.
SII, composed of neutrophils, lymphocytes, and platelets, was first developed by Hu et al in 2014 to evaluate the poor clinical prognosis of patients undergoing liver cancer resection.35 SII has been studied in disease states such as cancer, rheumatoid arthritis, ischemic stroke, diabetes, and depression and has been used to predict poor clinical prognosis.36–38 Recently, SII has also been used in the diagnosis and prognosis of various cardiovascular diseases.39–41 In addition to predicting poor clinical prognosis in patients with CAD and mortality in patients with ACS, SII is a potential marker for predicting atrial fibrillation in patients with ischemic stroke.42 SII has also been shown to be a prognostic marker for MACEs in patients with advanced chronic HF with renal insufficiency,43 and it has a predictive role for acute kidney injury in patients undergoing coronary angiography.44 A recent study has shown that SII is a risk factor for all-cause mortality in sufferers with hypertrophic cardiomyopathy. SII, combined with other risk factors, can be applied to risk stratification of death in patients with hypertrophic cardiomyopathy.45 A recent meta-analysis also showed that SII might be a potential biomarker for cardiovascular disease, and increased SII may increase the risk of cardiovascular disease. However, the level of evidence is generally low, and the optimal cut-off value and applicable population still need to be determined.46
SIRI, which replaces platelet count with monocyte count instead of SII, was first developed by Qi et al in 2016 to predict postoperative survival in pancreatic cancer patients receiving chemotherapy.47 SIRI is an independent risk factor for MACEs in patients with ACS undergoing PCI and plays an additional role in predicting MACEs when combined with the GRACE risk score.24 SIRI is related to the severity of CAD and can predict the long-term clinical prognosis of patients with type B aortic dissection after vascular endothelial repair.11,48 SIRI also predicted mortality in patients undergoing off-pump coronary artery bypass grafting.49
Previous studies have demonstrated that NLR, PLR, and MLR show the pro-inflammatory role of different cell types in atherosclerosis and are practical factors for predicting cardiovascular disease and mortality in patients with CAD.50–52 Therefore, the novel inflammatory marker SIIRI, which combines the currently available four cellular subtypes, should theoretically also be able to predict poor clinical prognosis in patients with CAD. SIIRI ultimately resulted in an improvement in IDI and NRI by 0.135 and 18.7%, respectively.
Neutrophils play a role in activating the inflammatory response, producing reactive oxygen species and protein lyase, driving the activation and chemotaxis of macrophages, monocytes, and dendritic cells, and promoting the development of atherosclerosis.53 The migration and maturation of monocytes into macrophages in the arterial wall are the initial events of atherosclerosis.54 Leukocytosis, especially neutrophilia, is an independent risk factor for the development of atherosclerosis. Leukocytosis is predictive of adverse cardiovascular events.55 Lymphocytes are involved in the process of immune regulation. Studies have found that lymphopenia positively correlates with MACEs, HF, and adverse cardiovascular events in patients with ACS.53,56 Elevated platelet counts accelerate the progression of atherosclerosis, destabilize atherosclerotic plaques, and are associated with an increased long-term incidence of adverse cardiac events.57 On the other hand, platelets can regulate the recruitment of white blood cells to atherosclerotic lesions and play an immunomodulatory role.58 A recent study showed that NLR, PLR, SII, and SIRI can predict the occurrence and severity of pneumonia in patients with intracerebral hemorrhage.59 New inflammatory markers, such as SII, SIRI, and SIIRI, seem to be powerful indicators of systemic inflammatory response in patients with CAD. Further exploration of the additional synergistic effect of SIIRI combined with other predictors is conducive to increasing the clinical relevance, practicability, and predictive value.
In this study, SIIRI remained an independent predictor of adverse cardiovascular events in patients with initially diagnosed CAD after adjusting for relevant covariates. Age, newly diagnosed dyslipidemia, smoking, albumin level, and presence of polyangiopathy are independent predictors, and their predictive value needs further exploration.
The best predictive value of SIIRI was 247.17*1018/L2, and the model’s predictive value could be improved when it was included in the basic model of traditional risk factors. Similarly, the predictive value of SIIRI in patients with CAD complicated with hypertension, diabetes, and cerebrovascular disease needs to be further explored.
Limitations
This study has certain limitations. First, the study is a single-center, retrospective, observational study, which may be subject to selection bias and limits generalizations. Secondly, the follow-up of patients in this study was not all completed by patients in the hospital, and the telephone follow-up may have subjective bias of patients and understanding bias of follow-up personnel. In patients with CAD, the severity of the lesion was based on coronary angiography and CASSC score, which could not address the effect of coronary plaque stability. Finally, our study included a relatively wide range of exclusion criteria, and further studies are needed to investigate the changes in the diagnostic performance of SIIRI under these criteria to obtain more general results. In addition, the value of SIIRI needs to be explored in future studies involving patients with other cardiovascular disease subgroups, including HF, atrial fibrillation, hypertrophic cardiomyopathy, and infective endocarditis.
Conclusion
Elevated SIIRI is associated with adverse cardiovascular outcomes in patients with initially diagnosed CAD, suggesting that SIIRI may be a valuable predictor of poor prognosis in patients with initially diagnosed CAD. The predictive role of SIIRI needs to be verified in more extensive clinical trials.
Ethics Statement
The study complied with the Declaration of Helsinki and was permitted by the Second Affiliated Hospital of Tianjin Medical University (IRB number 2023-05-B023). Patients/participants all signed informed consent before enrollment. Permission was obtained from the patient/participant at each follow-up visit.
Acknowledgments
We gratefully acknowledge the assistance of the investigators of the Second Hospital of Tianjin Medical University and the participant’s support.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82100342) and the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-029A).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dayan N, Filion KB, Okano M, et al. Cardiovascular risk following fertility therapy: systematic review and meta-analysis. J Am Coll Cardiol. 2017;70(10):1203–1213. doi:10.1016/j.jacc.2017.07.753
2. Ralapanawa U, Sivakanesan R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J Epidemiol Glob Health. 2021;11(2):169–177. doi:10.2991/jegh.k.201217.001
3. Violi F, Cammisotto V, Bartimoccia S, Pignatelli P, Carnevale R, Nocella C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat Rev Cardiol. 2023;20(1):24–37. doi:10.1038/s41569-022-00737-2
4. Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–2494. doi:10.1056/NEJMra071014
5. Collet JP, Thiele H, Barbato E, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;42(14):1289–1367.
6. Mangalesh S, Dudani S, Mahesh NK. Development of a novel inflammatory index to predict coronary artery disease severity in patients with acute coronary syndrome. Angiology. 2023:33197231151564.
7. Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17(5):269–285. doi:10.1038/s41569-019-0315-x
8. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
9. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi:10.1038/nature05485
10. Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126(9):1260–1280. doi:10.1161/CIRCRESAHA.120.315937
11. Dziedzic EA, Gąsior JS, Tuzimek A, et al. Investigation of the associations of novel inflammatory biomarkers-systemic inflammatory index (SII) and systemic inflammatory response index (SIRI)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. 2022;23(17):9553. doi:10.3390/ijms23179553
12. Yang Y-L, Wu C-H, Hsu P-F, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5):e13230. doi:10.1111/eci.13230
13. Chen Y, Chen S, Han Y, Xu Q, Zhao X. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are important indicators for predicting in-hospital death in elderly AMI patients. J Inflamm Res. 2023;16:2051–2061. doi:10.2147/JIR.S411086
14. Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225(2):456–460. doi:10.1016/j.atherosclerosis.2012.09.009
15. Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008;395(1–2):27–31. doi:10.1016/j.cca.2008.04.019
16. Song FH, Zheng YY, Tang JN, et al. A correlation between monocyte to lymphocyte ratio and long-term prognosis in patients with coronary artery disease after PCI. Clin Appl Thromb Hemost. 2021;27:1076029621999717. doi:10.1177/1076029621999717
17. Gijsberts CM, Ellenbroek GH, Ten Berg MJ, et al. Routinely analyzed leukocyte characteristics improve prediction of mortality after coronary angiography. Eur J Prev Cardiol. 2016;23(11):1211–1220. doi:10.1177/2047487315621832
18. Trakarnwijitr I, Li B, Adams H, Layland J, Garlick J, Wilson A. Age modulates the relationship between platelet-to-lymphocyte ratio and coronary artery disease. Int J Cardiol. 2017;248:349–354. doi:10.1016/j.ijcard.2017.06.127
19. Qiu Z, Jiang Y, Jiang X, et al. Relationship between platelet to lymphocyte ratio and stable coronary artery disease: meta-analysis of observational studies. Angiology. 2020;71(10):909–915. doi:10.1177/0003319720943810
20. Bressi E, Mangiacapra F, Ricottini E, et al. Impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio on 5-Year clinical outcomes of patients with stable coronary artery disease undergoing elective percutaneous coronary intervention. J Cardiovasc Transl Res. 2018;11(6):517–523. doi:10.1007/s12265-018-9829-6
21. Candemir M, Kiziltunç E, Nurkoç S, Şahinarslan A. Relationship Between Systemic Immune-Inflammation Index (SII) and the severity of stable coronary artery disease. Angiology. 2021;72(6):575–581. doi:10.1177/0003319720987743
22. Shi S, Kong S, Ni W, et al. Association of the systemic immune-inflammation index with outcomes in acute coronary syndrome patients with chronic kidney disease. J Inflamm Res. 2023;16:1343–1356. doi:10.2147/JIR.S397615
23. Jin Z, Wu Q, Chen S, et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131–140. doi:10.2147/JIR.S283835
24. Han K, Shi D, Yang L, et al. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann Med. 2022;54(1):1667–1677. doi:10.1080/07853890.2022.2083671
25. Sabanoglu C, Chang H-P, Chang R-Y, Tai H-Y, Huang Y-W, Lee P-C. H Inanc I. C-reactive protein to albumin ratio predicts for severity of coronary artery disease and ischemia. Eur Rev Med Pharmacol Sci. 2022;26(20):7631–7632. doi:10.26355/eurrev_202210_30039
26. Peizhi W, Deshan Y, Ce Z, et al. High fibrinogen-to-albumin ratio with type 2 diabetes mellitus is associated with poor prognosis in patients undergoing percutaneous coronary intervention: 5-year findings from a large cohort. Cardiovasc Diabetol. 2022;21(1):46. doi:10.1186/s12933-022-01477-w
27. Bergmark BA, Mathenge N, Merlini PA, Lawrence-Wright MB, Giugliano RP. Acute coronary syndromes. Lancet. 2022;399(10332):1347–1358doi:10.1016/S0140-6736(21)02391-6
28. Satny M, Hubacek JA, Vrablik M. Statins and Inflammation. Curr Atheroscler Rep. 2021;23(12):80. doi:10.1007/s11883-021-00977-6
29. Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. 2018;122(2):337–351. doi:10.1161/CIRCRESAHA.117.310795
30. Quixuan L, Xiaoteng M, Qiaoyu S, et al. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients. Front cardiovasc med. 2022;3(9):811790.
31. Stergiou GS, Palatini P, Parati G, et al. European society of hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39(7):1293–1302. doi:10.1097/HJH.0000000000002843
32. Cosentino F, Grant PJ, Aboyans V, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;41(2):255–323.
33. Mach F, Baigent C, Catapano AL, et al. ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;41(1):111–188.
34. Lawton JS, Tamis-Holland JE, Bangalore S, et al. ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;145(3):e18–e114. doi:10.1161/CIR.0000000000001038
35. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
36. Wang J, Zhou D, Dai Z, Li X. Association between systemic immune-inflammation index and diabetic depression. Clin Interv Aging. 2021;16:97–105. doi:10.2147/CIA.S285000
37. Geraghty JR, Lung TJ, Hirsch Y, et al. Systemic immune-inflammation index predicts delayed cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2021;89(6):1071–1079. doi:10.1093/neuros/nyab354
38. Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999–2018. Arthritis Res Ther. 2023;25(1):34. doi:10.1186/s13075-023-03018-6
39. Xu M, Chen R, Liu L, et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: the Dongfeng-Tongji cohort study. Atherosclerosis. 2021;323:20–29. doi:10.1016/j.atherosclerosis.2021.02.012
40. Xu JP, Zeng RX, Zhang YZ, et al. Systemic inflammation markers and the prevalence of hypertension: a NHANES cross-sectional study. Hypertens Res. 2023;46(4):1009–1019. doi:10.1038/s41440-023-01195-0
41. Wang P, Guo X, Zhou Y, et al. Monocyte-to-high-density lipoprotein ratio and systemic inflammation response index are associated with the risk of metabolic disorders and cardiovascular diseases in general rural population. Front Endocrinol. 2022;13:944991. doi:10.3389/fendo.2022.944991
42. Lin KB, Fan FH, Cai MQ, et al. Systemic immune inflammation index and system inflammation response index are potential biomarkers of atrial fibrillation among the patients presenting with ischemic stroke. Eur J Med Res. 2022;27(1):106. doi:10.1186/s40001-022-00733-9
43. Wang Z, Qin Z, Yuan R, et al. Systemic immune-inflammation index as a prognostic marker for advanced chronic heart failure with renal dysfunction. ESC Heart Fail. 2023;10(1):478–491. doi:10.1002/ehf2.14217
44. Jiang H, Li D, Xu T, et al. Systemic immune-inflammation index predicts contrast-induced acute kidney injury in patients undergoing coronary angiography: a cross-sectional study. Front Med. 2022;9:841601. doi:10.3389/fmed.2022.841601
45. Wang Z, Ruan H, Li L, et al. Assessing the relationship between systemic immune-inflammation index and mortality in patients with hypertrophic cardiomyopathy. Ups J Med Sci. 2021;126. doi:10.48101/ujms.v126.8124
46. Ye Z, Hu T, Wang J, et al. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: a systematic review and meta-analysis. Front cardiovasc med. 2022;9:933913. doi:10.3389/fcvm.2022.933913
47. Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167. doi:10.1002/cncr.30057
48. Zhao Y, Hong X, Xie X, et al. Preoperative systemic inflammatory response index predicts long-term outcomes in type B aortic dissection after endovascular repair. Front Immunol. 2022;13:992463. doi:10.3389/fimmu.2022.992463
49. Urbanowicz T, Michalak M, Olasińska-Wiśniewska A, et al. Neutrophil counts, neutrophil-to-lymphocyte ratio, and Systemic Inflammatory Response Index (SIRI) predict mortality after off-pump coronary artery bypass surgery. Cells. 2022;11(7):1124. doi:10.3390/cells11071124
50. Adamstein NH, MacFadyen JG, Rose LM, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42(9):896–903. doi:10.1093/eurheartj/ehaa1034
51. Hua Y, Sun JY, Lou YX, Sun W, Kong XQ. Monocyte-to-lymphocyte ratio predicts mortality and cardiovascular mortality in the general population. Int J Cardiol. 2023;379:118–126. doi:10.1016/j.ijcard.2023.03.016
52. Chen T, Yang M. Platelet-to-lymphocyte ratio is associated with cardiovascular disease in continuous ambulatory peritoneal dialysis patients. Int Immunopharmacol. 2020;78:106063. doi:10.1016/j.intimp.2019.106063
53. Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020;17(6):327–340. doi:10.1038/s41569-019-0326-7
54. Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. Arterioscler Thromb Vasc Biol. 2011;31(7):1506–1516. doi:10.1161/ATVBAHA.110.221127
55. Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Sci NY. 2013;339(6116):161–166. doi:10.1126/science.1230719
56. Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. 2022;19(2):177–191.
57. Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102(08):248–257. doi:10.1160/TH09-03-0192
58. von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100(1):27–40. doi:10.1161/01.RES.0000252802.25497.b7
59. Wang RH, Wen WX, Jiang ZP, et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. 2023;14:1115031. doi:10.3389/fimmu.2023.1115031
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


