Back to Journals » Journal of Inflammation Research » Volume 17
Updates on the Role of Periodontitis-Related Epigenetics, Inflammation, Oral Microbiome, and Treatment in Cardiovascular Risk
Received 13 November 2023
Accepted for publication 20 January 2024
Published 7 February 2024 Volume 2024:17 Pages 837—851
DOI https://doi.org/10.2147/JIR.S449661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Bei Men, Yongjun Li, Shu Jiang
Department of Prosthodontics and Implantation, Dazhong Stomatological Hospital, Wuhan, 430000, People’s Republic of China
Correspondence: Bei Men, Department of Prosthodontics and Implantation, Dazhong Stomatological Hospital, Wuhan, 430000, People’s Republic of China, Email [email protected]
Abstract: Substantial evidence has recently been gathered to substantiate the epidemiological correlation between this oral inflammatory ailment and several systemic health disorders, such as cardiovascular diseases (CVDs). It is worth noting that certain shared factors exist among individuals with periodontal disease (PD) and CVDs, like obesity or overweight, minimal physical activities, smoking habits, male gender, low socioeconomic position, advanced age, and limited educational attainment. Moreover, recent research suggests a distinct and separate relationship between PD and CVDs. This literature review discusses the association between CVDs and PD and their underlying mechanisms and connections. Current literature strongly confirms a correlation between cardiovascular risk and PD. The underlying mechanisms involve inflammation, epigenetics, epithelial dysfunction, oral microbiome dysbiosis, and the efficacy of periodontitis treatment. Future investigations are required to comprehend these complex interactions and develop targeted interventions for oral health improvement and reduction in cardiovascular risk.
Keywords: periodontal disease, cardiovascular disease, periodontitis, gingivitis, oral health, markers, inflammation
Introduction
Periodontal disease (PD) develops as a progressive and chronic inflammatory disorder affecting the tissues supporting the teeth, eventually resulting in tooth loss. The condition is well recognized as a multifactorial illness,1 in which an imbalance in the oral microbiota is believed to be a significant contributing component.2 It is known to occur in those with a predisposition, mostly due to plaque deposition. This plaque buildup triggers a reversible initial inflammatory response known as gingivitis, affecting the teeth-adjacent soft tissues and gums. It induces gingival irritation, erythema, edema, and hemorrhage.3,4 Gingivitis does not result in the loss of bone tissue.3,4 However, it may advance to periodontitis, a condition that cannot be reversed and causes damage to the bone, cementum, and periodontal ligament if left untreated. This finally results in the last stage of the illness, characterized by teeth loss. Inflammatory mediators, including interleukin (IL)-1β, IL-6, C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α), may be observed early within the circulation.5 PD imposes a significant financial burden, as it is the leading cause of masticatory impairment resulting from tooth loss.6,7 Furthermore, it affects over 50% of the global population, making it the second most frequent oral illness globally. Substantial evidence has recently been gathered to substantiate the epidemiological correlation between this oral inflammatory ailment and several systemic health disorders, such as cardiovascular diseases (CVDs).8
CVDs are the commonest non-communicable disorders and the primary etiology owing to disability and death worldwide and have substantial societal and economic repercussions.9 Although heredity has a role in the onset and progression of CVD, lifestyle choices also play a role. Inappropriate dietary intake, physical inactivity, tobacco use, and alcohol use are the most controllable risk factors.10 These disorders cause systemic inflammation, which is important to CVD development.11 The pathophysiological processes are unknown, although during the past two decades, a growing body of data links oral and CVDs.8,12–14
It is worth noting that certain shared factors exist among individuals with PD and CVDs, like obesity or overweight, minimal physical activities, smoking habits, male gender, low socioeconomic position, advanced age, and limited educational attainment.15,16 Moreover, recent research suggests a distinct and separate relationship between PD and CVDs.17 The basic underpinnings of the relationship between periodontal inflammation and CVDs have been postulated to have direct and indirect mechanistic connections. Proinflammatory bacteria are carried directly from the mouth to the circulation, where they may infiltrate the cardiovascular system. However, indirect methods activate systemic immune pathways, causing prolonged inflammation with nonspecific effects. This literature review will focus on the correlation between PD and CVDs, as well as the many mechanistic explanations for this correlation. This will include a broad discussion on the roles of inflammation, endothelial dysfunction, oral microbiome, and epigenetics in CVD pathogenesis.
Methods
This literature review discussed the correlation between CVDs and PD and how different mechanistic approaches might be involved in this association based on evidence from human and animal investigations. Accordingly, a comprehensive search was employed via various databases, including Scopus, Web of Science, and PubMed to find the relevant research data from the relevant original human and animal studies with the relevant keywords from previous investigations. Some of these keywords include (periodontitis, coronary artery disease, Periodontal disease, gingivitis, CVD, atherosclerosis, cardiovascular disease, inflammation, inflammatory markers, oral dysbiosis, treatment, hypertension, and epithelial dysfunction). These keywords were combined via Boolean operators based on the terms announced on each searched database. The search was conducted from inception till May 2023, which identified 108 relevant articles. We updated the search on December 2023 and identified additional 9 relevant articles. Moreover, a comprehensive search was conducted for all subsection formulated in the review. The researchers used a conventional blinded and discussion-based approach for searching and selecting relevant information. The relevant databases were thoroughly examined using various search methodologies and keyword-combinations. Title/abstract screening was the first stage of research selection for citations deemed possibly relevant. Following the removal of duplicate entries, the subsequent screening stage included a comprehensive assessment of the content inside the articles. Furthermore, reference list examination of the included papers was conducted to collect any other article that were not retrieved via the original search strategy, and resulted in the identification of two additional papers. Subsequently, 119 articles were finally included. All of the included studies were published in English.
CVD and PD Association
The Impact of Periodontitis on CVD Risk
Numerous clinical Investigations consistently showed a correlation between periodontitis and CVD (Figure 1).18–28 In 2012, a scientific statement was issued by the American Heart Association affirming CVD and periodontitis correlation but acknowledging the absence of a definitive causative connection.29 Moreover, a systematic review by Dietrich et al, which included 12 papers, yielded a similar conclusion on the correlation between periodontitis and atherosclerotic CVD. Nevertheless, the authors included a cautionary note about the generalizability of their results, suggesting that they may apply to certain groups only.30 A comprehensive case-control study conducted in Sweden examined periodontitis and coronary disease correlation within 805 individuals. A significant increase in myocardial infarction risk among periodontitis individuals was revealed, with an odds ratio of 1.28. This increased risk persisted even after accounting for confounding factors like marital status, education years, diabetes, and smoking.31
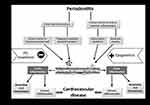 |
Figure 1 A summary of how different periodontitis-related factors contribute to cardiovascular disease risk. |
Sen et al31 conducted a more recent investigation and found that periodontitis subjects had more than double the risk of thrombotic and cardioembolic stroke as those who keep their gums healthy. The link between CVD and periodontitis was examined by Herrera et al.32 The goal of this study was to lend credence to the 2020 consensus report put forth by the World Heart Federation and the European Federation of Periodontology. The study’s authors found that those with periodontitis had a higher chance of developing coronary disease and having a heart attack. Nonetheless, consensus on whether or not periodontitis contributes to later atherosclerotic CVD events is still lacking.32,33
Periodontitis Management and CVD Risk
Self-reported rates of brushing, better oral hygiene, dental visit frequency, and periodontal treatment results were evaluated in a Cochrane review and the 2020 consensus report of interventional studies. The analysis concluded that these variables are linked to cardiovascular incidents. The study’s authors concluded that periodontal therapy, which includes education on good oral hygiene and more regular dental checkups, may have an effect on atherosclerotic cardiovascular disease development beyond that seen with the management of conventional risk factors for CVD alone. Although periodontitis treatment may be effective in decreasing or postponing atherosclerotic CVD events, the consensus report found that there is a lack of meaningful evidence to support or refute this possibility.33,34
Support for a causal link between periodontal disease and CVDs is provided by the positive impacts of periodontal management on many indicators of metabolic control, cardiovascular prognostic markers, and systemic inflammation.35–37 An increasing body of clinical and translational research indicates that the enhancement of oral microbiome composition by periodontal therapy, namely mechanical biofilm removal, results in a drop in disease-associated bacteria and a reduction in inflammatory markers.38,39 Blood pressure, WBCs, lipid profile, fibrinogen, and CRP are some of the surrogate markers of cardiovascular disease that have been studied in interventional studies to evaluate the effect of periodontal treatment.23,40–44 Bokhari et al44 examined those who had both periodontitis and coronary disease. The study’s goal was to examine the differences in results between a group who underwent scaling and root planing and a control group that did not. Following scaling and root planing, the researchers found a decrease in WBCs, fibrinogen, and CRP levels. Six months into their periodontal treatment, Caula et al42 assessed and monitored their progress. The study’s authors draw the conclusion that inflammatory markers like C-reactive protein and triglyceride levels may be lowered with periodontal treatment. Systolic blood pressure was shown to be lower after periodontal treatment in research by Houcken et al.40 Furthermore, a comprehensive analysis and synthesis of relevant studies indicated that periodontal treatment decreases the biomarkers associated with atherosclerotic CVD and enhances endothelial function.45 Moreover, the findings of a recent comprehensive survey conducted on a national scale by our research team demonstrate a negative correlation between home oral hygiene habits, specifically the frequency and method of tooth brushing, and an individual’s blood pressure profile. This correlation remains significant even after accounting for relevant factors that may influence the results, thereby establishing the initial evidence of a link between blood pressure and oral hygiene practices.46 Large longitudinal research of 161,286 individuals found that maintaining good oral hygiene through regular toothbrushing and professional dental cleaning was correlated with a diminished heart failure and atrial fibrillation risk.47
Comparably, a research investigation involving a cohort of 247,696 individuals deemed to be in good health, aged 40 years or older, and without pre-existing CVDs was conducted as part of an oral health screening initiative. The results of this research showed that the frequency with which people brushed their teeth was remarkably correlated with a diminished cardiovascular events risk such as mortality, heart failure, stroke, and infarction. Moreover, the risk reduction was even more pronounced if the participants reported receiving regular professional oral hygiene care.39 According to the findings of a longitudinal study involving 256 patients who were diagnosed with coronary artery disease, it was observed that subjects having more teeth at the beginning of the study had a greater likelihood of surviving CVD. Additionally, it was noted that individuals who demonstrated better oral hygiene had notable survival advantages concerning mortality caused by CVDs.48
To examine the potential systemic effects of periodontal therapy timing, Graziani et al49 compared root planing and scaling administration within a 24-hour timeframe to root planing and scaling done over four weeks. The impact of both treatment regimens on inflammatory markers was additionally assessed. The findings suggest that whole root planing and mouth scaling within a 24-hour timeframe leads to an elevated acute phase response, as seen by higher CRP levels and IL-6. Nevertheless, the outcomes were temporary, and eventually, both treatment approaches yielded comparable results. However, further research is required to ascertain if the heightened acute phase response influences the risk of CVD.49 The facts given in this study align with the findings of the previously discussed 2020 consensus report. The paper provides evidence suggesting that periodontal therapy can decrease systemic, low-grade inflammation. Herrera et al32 emphasized in their review that there is a scarcity of research examining periodontal therapy impact on CVD outcomes.
CVD Impact on Periodontitis
The putative connection between CVD and periodontitis has received great attention. The effects of CVD on periodontitis, however, have received less attention. In light of this, the 2020 consensus report concludes that there is currently insufficient evidence to support the hypothesis that CVD is a risk factor for periodontitis.33 The link between CVD and periodontitis has been studied extensively, and the results are clear. Tobacco use, being older, low socioeconomic status, and being overweight are all risk factors for both periodontitis and cardiovascular disease; this may suggest that the underlying pathophysiology of both conditions is similar.35,49
Periodontic Events and Cardiovascular Risk
Epigenetics
Emerging data suggests that epigenetics is crucial in the association between PD and cardiovascular risk.50 Oral epithelial cells, the body’s initial defense line against infectious microbes, may undergo epigenome modifications when exposed to bacteria and bacterial metabolites. Modifications to signaling networks and gene expression result in alterations to the inflammatory cell’s dynamic and function. Gene expression may be limited in response to environmental cues known as epigenetic processes and changes. Therefore, DNA molecules do not include information on the modifications. Epigenetics still dictates a comprehensive chromatin remodeling by subsequent chemical changes, resulting in gene expression-related repression or activation. Some of the most critical environmental elements that influence epigenetic dynamics include one’s habits, and exposure to smoking, toxic chemicals and radiation, and infections.
Non-coding RNA (such as micro-RNA)-based gene expression control, DNA methylation, and post-transcriptional alternations to histones impacting chromatin composition are all important epigenetic processes. Single nucleotide polymorphisms provide another layer of complexity by interacting with epigenetic processes that direct gene expression. The pathophysiology of CVDs is heavily influenced by epigenetic changes and micro-RNA, both of which contribute to the creation and vulnerability of atherosclerotic plaque.51 A recent study outlined the critical epigenetic processes.52 The first deals with how human cells react to their external environment, which may lead to mutations in the DNA. Then, using a combination of the epigenetic initiator and epigenetic maintainer, the alterations are conserved throughout cell divisions. The persistent inflammation and gram-negative bacteria in the mouth associated with poor dental hygiene may affect patterns of DNA methylation and histone protein modification in people with periodontitis.53 Cytokines, like IL-1 and −6, are essential players for periodontitis development, and epigenetic pathways regulate their effects. Environmental variables are essential because of their involvement in causing epigenetic alterations relevant to disease development.54 This might affect an individual’s propensity to acquire CVD risk factors.
Inflammation and Endothelial Dysfunction
The pathogenesis, progression, and clinical manifestation of CVD are all influenced by inflammation.55 Endothelial nitrite oxidase synthase expression is downregulated, endothelial nicotinamide dinucleotide phosphate oxidase synthesis is upregulated, and endothelial cell adhesion molecule expression is induced by inflammatory mediators, like pro-inflammatory cytokines, as shown in numerous clinical studies.56,57 There may be a causative link among proinflammatory cytokines and risk of CVD, as subjects with changes in these inflammatory indicators might possess increased risk regardless of whether the levels of low-density lipoprotein were lowered.58 In addition, elevated CRP levels seemed to mediate the connection between low tooth count at baseline and poor CVD survival,59 demonstrating an inverse relationship between tooth count and CVD mortality. The monoclonal antibody canakinumab blocks IL-1β and reduces cardiovascular events without changing low-density lipoprotein cholesterol, further supporting the idea that inflammation is a key player in the development of CVD.60,61 The clinical effect of canakinumab was shown to be directly correlated with the degree to which circulating CRP or IL-6 levels were reduced, as demonstrated by the CANTOS study. After myocardial infarction, patients were analyzed in the Colchicine Cardiovascular Outcomes Trial (COLCOT).62 Both studies concluded that low-dose colchicine was safe and effective in treating patients. Consequently, recent research suggests that specific suppression of inflammation further decreases CVD risk-related events.63,64
The significance of periodontal inflammation in vascular disease has continually been emphasized, but the precise biomolecular pathways remain incompletely understood.65 Vascular endothelial cells, which are essential for preserving the integrity of the vascular wall and whose dysfunction is a significant factor in CVDs, can release a variety of factors that can modulate processes such as smooth muscle cell migration and proliferation, cell adhesion and migration, vessel wall inflammation, and vascular thrombosis.66 In the presence of unfavorable stimuli, the phenotypic of vascular endothelial cells transforms into an active state, often referred to as endothelial dysfunction.66 Endothelial dysfunction contributes to developing and advancing several vascular and metabolic disorders, including hypertension, diabetes, atherosclerosis, and hypercholesterolemia. Vascular inflammation encompasses a series of signaling cascades initiated by endothelial mediators, resulting in heightened synthesis of cell adhesion molecules, chemokines, and cytokines, flaring up inflammation.66 A remarkable indication of CVDs prognosis and risk is dysfunctional endothelium, developing prior to the discovery of morphological vessel wall alterations.66
Inflammatory indicators such pro-inflammatory cytokines, fibrinogen, WBCs, and CRP are all seen in higher concentrations in people with CVD and PD. It is also worth noting that many of the risk factors for both diseases are the same. These include tobacco use, poor oral hygiene, obesity, diabetes, stress, and lack of exercise. Those with PD have an increased risk of hypertension, according to a number of studies looking at blood pressure monitoring and oral health concerns.67–69 Evidence for a positive linear link between both illnesses is mounting, as was noted in a paper published jointly by the Italian Society of Hypertension and the Italian Society of Periodontology and Implantology. The systolic blood pressure reading was shown to be strongly affected by the periodontitis severity.70 The conclusions from a recently conducted pilot research add to the growing body of data linking oral and cardiovascular health. These results suggest that people with PD who have higher amounts of several bacterial species—specifically F. nucleatum, P. intermedia, and A. actinomycetemcomitans—may be more prone to develop high blood pressure than those without PD.71 The presence of P. gingivalis, an indicator of an unbalanced oral microbiota, has been found in studies of arterial plaques in people with cardiovascular disease.72,73
Compared to those without PD, subjects diagnosed with severe periodontitis and heightened levels of pro-inflammatory agents, like IL-1 and-6, CRP, and fibrinogen, exhibited an augmented presence of neutrophils in their peripheral blood.33,74 Neutrophils are drawn towards tissues via the influence of several chemoattractants, such as leukotrienes A4 and B4. These chemoattractants also have implications in the development of atherosclerosis, as shown by the presence of leukotrienes A4 hydrolase, 5-LO-activating protein, and 5-lipoxygenase in symptomatic human atherosclerotic plaques.74 Several disorders linked to oral health problems, such as CVDs, exhibit changes to blood microbiota makeup together with the circulating neutrophils morphologies.74 Indeed, during their oral presence, these microorganisms with the related byproducts might influence neutrophils functions, which may subsequently migrate into the peripheral circulation, indirectly influencing overall systemic health outcomes. The variability of endotoxin derived from oral microorganisms is contingent upon the composition of bacterial communities and the state of oral health. Consequently, this variability has varied impacts on the mechanisms of cellular tolerance towards lipopolysaccharides (LPS), contributing to periodontitis-related characteristic neutrophil phenotype.74 The creation of extracellular traps created by may be triggered by interactions between microbes and their byproducts, such as endothelial cells, activated platelets, together with pro-inflammatory cytokines, and LPS. Regarding periodontitis, these DNA-based traps are aided with antimicrobial peptides and help with tissue injury infliction.74 Further evidence suggests that extracellular neutrophil traps contribute to atherosclerosis and thrombosis development.74
Periodontitis is linked to endothelial dysfunction, as evidenced by epidemiological studies and clinical evidence.75 Long-term research of a sizable population found that periodontitis significantly affects patients’ ability to dilate their blood vessels.76 A recent pilot investigation found a correlation between tooth mobility and endothelial dysfunction, as measured by reactive hyperemia-peripheral arterial tonometry. This correlation was true across all ages and glycosylated hemoglobin levels.77 Previous research has shown that patients with periodontitis had lower levels of functional capillary density, capillary widths, resting red blood cell velocity, post-ischemic peak flow, and endothelium-independent vasodilatation.75 It has been shown that the presence of P. gingivalis in periodontal infections affects matrix metalloproteinase-2/tissue inhibitor of metalloproteinases 2 complex, inflammatory mediators production, chemokines like CX3C chemokine ligand 1, IL-8 and monocyte chemotactic protein-1, and myeloperoxidase within vascular endothelial cells.75 Because of this, leukocytes and monocytes are better able to migrate to and adhere to the endothelium of blood arteries. These immune cells have also been demonstrated to release extra inflammatory factors while transporting periodontal bacteria into the arterial wall. Endothelial inflammation worsens as a result of this procedure.75 Elevated blood fibrinogen levels are associated with periodontitis and have been shown to stimulate the production of inflammatory mediators such IL-6, and-8, TNF-alpha, and matrix metalloproteinases like metalloproteinase-1 and −9, which in turn may exacerbate endothelial inflammation.78
Increased reactive oxidative stress generation in endothelial cells and reduced NO availability have also been linked to periodontitis.79 When it comes to endothelial dysfunction, reactive oxidative stress is a major factor. The buildup of excessive reactive oxidative stress hinders the proper functioning of the nitric oxide (NO) signaling system, resulting in a decrease in NO availability. This, in turn, leads to endothelial dysfunction by diminishing the capacity of the endothelium to relax in response to various stimuli.79 Endothelial cells affected by P. gingivalis showed an augmentation in generating mitochondrial reactive oxidative stress.80 Therefore, the significance of salivary NO levels as a critical connection between periodontitis and endothelial dysfunction has been shown.20
Oral Microbiome
The mouth is home to many different kinds of microorganisms called the oral microbiome, which include bacteria, fungus, viruses, archaea, and protozoa.81 Dysbiosis of the oral microbiota is associated with the onset and progression of different conditions, including CVDs and PDs (Figures 2 and 3).82,83 At the same time, there is mounting evidence connecting oral dysbiosis to CVDs.8,83–86 For instance, nitrate-nitrite-nitric oxide entero-salivary pathway alternations during oral dysbiosis may explain the link between chronic periodontitis and CVD.87 In 1994, two separate studies reported the production of NO in the stomach from nitrite found in saliva in humans.88,89 This mechanism does not need the action of nitric oxide synthase (NOS), but rather includes the circulation of inorganic nitrate via the digestive system and saliva. Dietary nitrate is quickly assimilated in the upper gastrointestinal system. Within the bloodstream, it combines with the nitrate generated by the oxidation of internally produced NO by the NOS enzymes. Following a nitrate-rich meal, plasma levels of nitrate experience a substantial and sustained rise, with a half-life of 5–6 hours. Plasma nitrite levels also rise after the consumption of nitrate.90 While a significant portion of the nitrate is eliminated by urine, about 25% is actively absorbed by the salivary glands and becomes concentrated up to 20 times in saliva.90,91
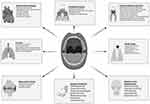 |
Figure 2 Oral microbiome dysbiosis is associated with various local and systemic conditions. |
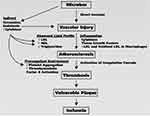 |
Figure 3 Microbiome-related risks and its association with the development of atherosclerosis and ischemia. |
Upon entering the mouth, commensal facultative anaerobic bacteria use nitrate as a substitute electron acceptor instead of oxygen during respiration. This process involves the reduction of salivary nitrate to nitrite via the activity of nitrate reductases.92 These bacteria are necessary for the reduction of human nitrate, indicating a functioning symbiotic interaction, as mammalian cells are unable to metabolize this anion efficiently. Salivary nitrate levels may reach up to 10 mM, whereas nitrite levels can range from 1 to 2 mM after consuming a meal high in nitrate.90 Upon entering the acidic stomach, saliva (about 1–1.5 L per day) undergoes a process where a significant portion of the nitrite present is quickly transformed into nitrous acid (HNO2; pKa ≈ 3.3). This nitrous acid then breaks down into NO and other nitrogen oxides.88,93 Reducing substances including vitamin C and polyphenols—both of which are prevalent in diets—greatly increases the conversion of nitrite to NO.94–96 The significance of oral bacteria in the production of gastric NO is best shown by research with germ-free rats, where the creation of gastric NO is minimal even when exposed to a diet rich in nitrate. Apart from the stomach, there is evidence of a reductive route including nitrate, nitrite, and finally NO in the oral cavity, skin surface, lower gastrointestinal tract, and urine.97,98
Although the scientific community has mostly studied the possible negative impacts of nitrate and nitrite, the well-established antibacterial properties of NO indicate that stomach NO may play a part in the body’s defense mechanisms.99,100 Enteropathogens exhibit remarkable resilience in acidic conditions, however, their survival is significantly reduced when exposed to a mix of acid and nitrite, leading to successful eradication.101–103 DNA, proteins, and cell wall components are among the many targets that reactive NO and others created from acidified nitrite attack harmful bacteria.104–106 Another suggested physiological function of gastric NO is to regulate the flow of blood in the mucosal lining and the production of mucus. Human saliva’s impacts on these two crucial factors of gastric integrity were recently investigated in an in vivo bioassay using rat gastric mucosa. No gas was immediately created and there was an increase in both mucosal blood flow and mucus thickness in a cyclic GMP-dependent manner when the rat stomach mucosa was exposed to saliva high in human nitrite.107 In addition, the introduction of nitrate into drinking water for a duration of one week has comparable outcomes.103,108 The nitrate therapy causes nitrite to build up in the gastrointestinal mucus. When this mucus is removed, the blood flow instantly returns to baseline, showing that the nitrite in the mucus is continuously slowly releasing “NO-like” bioactivity.108 It has also been proposed that salivary nitrite regulates the release of gastrin from the stomach.109
Moreover, NO plays an important role in metabolic and cardiovascular balance and is a multifunctional signaling enzyme. As a powerful endogenous vasodilator, it prevents vascular lesions from forming in atherosclerosis. Nitrite oxidase, which is produced by some of the oral bacteria that cohabit with humans, is essential for the health of endothelial cells and the regulation of arterial blood pressure.87,110 Subsequently, oral dysbiosis might lead to a decline in beneficial bacteria that control nitrate levels in the mouth and a rise in pathogenic bacteria. This finding suggests yet another correlation between CVDs and periodontitis. Diminished NO bioavailability due to defective endothelium-related vasodilation impairment may also play a role in the development of various CVDs, including coronary disease, hypertension, and atherosclerosis.111 NO production, which helps the entero-salivary nitrate cycle, may be responsible for lowering blood pressure in both younger and older persons.112 Indeed, adding nitrates to the diet increases the population of denitrifying organisms like Rothia sp. and Neisseria flavescens. On the other side, species like Prevotella and Veillonella that convert nitrate to ammonium via a dissimilatory pathway see their populations decrease. Lower blood pressure and higher plasma nitrite levels have both been linked to aging. Those who have hypercholesterolemia also benefit greatly from enhanced vascular function.112,113 The total data demonstrates that the degree to which the oral microbiome impacts the NO3-NO2-NO pathway and the related clinical consequences is determined by the presence of particular nitrate-reduction pathways.
Periodontitis may also increase a person’s exposure to microbes and their byproducts, which might enter the bloodstream either directly via inflamed oral tissues or indirectly through the digestive tract. Exposure to these chemicals causes a systemic inflammatory and immune response. Major risk factors for metabolic illnesses include chronic endotoxemia caused by periodontitis.83 Bacterial biomarkers indicative of oral dysbiosis have been linked to subclinical atherosclerosis, existing and probable coronary disease, and the occurrence and recurrence of stroke. Blood pressure patterns were also linked to an individual’s immunological response to periodontal bacteria.85 It is also possible that, as part of this response, antibodies that cross-react with antigens taken from the host (and hence cause atherosclerosis) are produced and stored for a long time.83,114 The term “oralome” describes the sum total of host-microbe interactions in the mouth.115 More than 700 different bacterial species have been found in the oralome, demonstrating its extraordinary richness and diversity.116
Oral diseases including periodontitis and dental caries might originate from a shift in the normally stable oral microbiome. There is a unique group of periodopathogens that may cause PD.117 T. spirochetes, T. denticola, T. forsythia, A. actinomycetemcomitans, F. nucleatum, and P. gingivalis are only few of the anaerobic gram-negative bacteria that proliferate in the subgingival biofilm as the disease develops. Periodontal pockets are created when bacteria invade tissues and create a microenvironment in which microbial and immune system balance is disturbed in both mechanisms.118 Many pro-inflammatory chemicals, including CRP, IL-1 and-6, TNF-alpha, and others, are synthesized locally during the inflammatory response associated with this condition.119 An increase in the number of mediators contributing to the breakdown of periodontal tissues accelerates this process.
Many different pathogen-associated molecular patterns are carried by periodontal pathogens, including peptidoglycan, cytosine phosphate guanine dinucleotide, and LPS. Innate immunity’s inflammatory response is triggered by these patterns and is dependent on the activation of host-cell pattern recognition receptors.120 Many pathogen and damage-associated molecular patterns (PAMPS and DAMPs) are produced by the immune-inflammatory response and the subsequent deterioration of periodontal tissues, which may influence the progression of atherosclerotic CVD.121 One study has compiled data on molecular signatures caused by pathogens and damage. These regularities were shown to represent important molecular pathogenic pathways that link periodontitis and atherosclerosis in the research.122
Toll-like receptors are activated by virulence factors and chemicals generated by periodontal pathogens, triggering an exacerbated innate immune response and ultimately leading to the death of periodontal tissue. By releasing molecular patterns within the circulation or via ectopic bacterial colonization establishment, the mouth cavity may send persistent inflammatory signals to cardiovascular organs. This mechanism then initiates innate immunity inside the vascular milieu, which furthers atherosclerosis development. Foam cell production and LDL changes induced by LPS in macrophages have been detected. Through the activation of PKB and NF-κB signaling pathways, chemotaxis and adherence of monocytes to vascular endothelial cells are promoted. Another mechanism by which LPS promotes the onset of endothelial dysfunction is by inducing the production of angiotensin II and IL-6 in vascular endothelial cells.123 Thus, it is possible that PD and atherosclerosis have a connection via the activation of innate immunity via multiple PAMPs and DAMPs.122 In individuals with CVDs made worse by PD, PAMPs and DAMPs may give prospective targets for therapeutic approaches.
Recommendations and Future Directions
Experimental, clinical, and translational research are all contributing to a deeper understanding of the mechanisms by which PD and CVD are linked. This research has added fresh, intriguing details that might be major factors in the most important events related to cardiovascular illnesses. Oral microbiome dysbiosis leads to activated inflammatory pathways, the presence of PAMPs and DAMPs and their related pattern recognition receptor activation, disruption of the entero-salivary nitrate-nitrite-nitric oxide pathway, impairment of endothelial function, and epigenetic alterations. The final image is quite complex and detailed. Establishing a suitable selection of blood or salivary biomarkers linked with PD that correspond with the key signs related to cardiovascular functioning would be beneficial from a diagnostic standpoint.
Given the strong evidence linking PD to CVDs, it is reasonable to explore the possible role of periodontal treatment in the management or prevention of first- and subsequent-incident CVDs. Most of the follow-up periods in the known studies are shorter than a year, which may restrict the potential to duplicate the results, and the sample sizes are also rather small. Significant adverse cardiovascular events were not reliably represented in most studies because they used surrogate outcomes. Further research is required to consider the potential advantages of reducing inflammatory indicators to minimize cardiovascular risk. Prospective trials with bigger sample numbers and longer follow-up periods are needed to achieve this goal. Several studies have been undertaken to evaluate the effect of periodontal therapy on CVDs, while some trials are still lacking. The results of these trials are encouraging, and they provide evidence that periodontal care may reduce the risk of CVD.40,42–44,124–137
Reducing inflammation around the gums may be an additional, physiologically feasible technique for improving systemic health. Interventional and mechanistic results from recent studies imply that periodontal therapy may have a beneficial effect on cardiovascular health. The effects of periodontal treatment of periodontal bacteria and the decrease in low-grade systemic inflammation are responsible for this benefit. From this vantage point, periodontal medicine, which is now recognized as an emerging field that collaborates with many healthcare professionals within a multidisciplinary/interdisciplinary framework, would be able to effectively tackle systemic disorders connected to PD via the application of appropriate periodontal treatments. Implementing a thorough periodontal care program has the ability to successfully manage the inflammatory disease, and so serve as a preventative treatment or technique of regulating the progression of CVD in people with PD. According to the findings of a recent research,13 this strategy can promote health and encourage the adoption of healthy lifestyles while also mitigating various dental and systemic illnesses of epidemiological relevance. Given the widespread prevalence of oral disorders and the substantial influence of oral health on overall systemic health, it will soon be necessary to provide evidence-based arguments in favor of incorporating oral health care into health promotion initiatives with the goal of improving both oral and systemic health.
Conclusion
Based on the current literature evidence, a remarkable correlation between CVDs and PD exists. This relationship is believed to be mediated by various factors, including inflammation, epigenetics, epithelial dysfunction, oral microbiome dysbiosis, and the efficacy of periodontitis treatment. Numerous studies have demonstrated that chronic inflammation owing to PD can contribute to CVDs development and progression. Inflammatory mediators like IL-6 and CRP are high in individuals with PD and cardiovascular conditions. Epigenetic modifications, like histone acetylation and DNA methylation, have also been implicated within the link between CVD risk and PD. These modifications can affect gene expression patterns related to inflammation and vascular function, potentially contributing to the development of CVDs. Epithelial dysfunction is another crucial factor in this association. Disruption of the oral epithelial barrier due to PD allows for increased oral bacteria and their byproducts translocation within the systemic circulation. This might trigger an immune response leading to systemic inflammation and endothelial dysfunction, key contributors to cardiovascular risk.
Besides, oral microbiome dysbiosis observed in PD individuals further supports this connection. Imbalances in microbial composition can lead to an overgrowth of pathogenic bacteria that produce pro-inflammatory substances. These substances can enter the bloodstream and contribute to systemic inflammation and cardiovascular complications. Lastly, the effectiveness of periodontitis treatment has been shown to impact cardiovascular risk. Studies have demonstrated that successful management of periodontal disease through interventions like scaling and root planing or antimicrobial therapy can improve systemic inflammatory markers and endothelial function, thereby reducing cardiovascular risk. Subsequently, acknowledging PD as a potentially adjustable risk indicator for CVDs etiology and progression was aided by the realization that inflammation plays a pivotal role in the development of these diseases and by the growing body of data connecting PD to biomolecular mechanisms linked to cardiovascular risk.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Santonocito S, Ferlito S, Polizzi A, et al. Therapeutic and metagenomic potential of the biomolecular therapies against periodontitis and the oral microbiome: current evidence and future perspectives. Int J Mol Sci. 2022;23(22):13708. doi:10.3390/ijms232213708
2. Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11(3):30. doi:10.1038/s41368-019-0064-z
3. Trombelli L, Farina R, Silva CO, Tatakis DN. Plaque-induced gingivitis: case definition and diagnostic considerations. J Clin Periodontol. 2018;45(Suppl 20):S44–S67. doi:10.1111/jcpe.12939
4. Murakami S, Mealey BL, Mariotti A, Chapple ILC. Dental plaque-induced gingival conditions. J Periodontol. 2018;89(Suppl 1):S17–S27. doi:10.1002/jper.17-0095
5. Priyamvara A, Dey AK, Bandyopadhyay D, et al. Periodontal inflammation and the risk of cardiovascular disease. Curr Atheroscler Rep. 2020;22(7):28. doi:10.1007/s11883-020-00848-6
6. Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. 2017;44(5):456–462. doi:10.1111/jcpe.12732
7. Chapple ILC. Time to take periodontitis seriously. BMJ. 2014;348:g2645. doi:10.1136/bmj.g2645
8. Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21(7):426–440. doi:10.1038/s41577-020-00488-6
9. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi:10.1016/s0140-6736(18)32279-7
10. Ali S, Misganaw A, Worku A, et al. The burden of cardiovascular diseases in Ethiopia from 1990 to 2017: evidence from the Global Burden of Disease Study. Int Health. 2021;13(4):318–326. doi:10.1093/inthealth/ihaa069
11. Loos BG, Van Dyke TE. The role of inflammation and genetics in periodontal disease. Periodontol 2000. 2020;83(1):26–39. doi:10.1111/prd.12297
12. Botelho J, Mascarenhas P, Viana J, et al. An umbrella review of the evidence linking oral health and systemic noncommunicable diseases. Nat Commun. 2022;13(1):7614. doi:10.1038/s41467-022-35337-8
13. Barranca-Enríquez A, Romo-González T. Your health is in your mouth: a comprehensive view to promote general wellness. Front Oral Health. 2022;3:971223. doi:10.3389/froh.2022.971223
14. Paul O, Arora P, Mayer M, Chatterjee S. Inflammation in periodontal disease: possible link to vascular disease. Front Physiol. 2020;11:609614. doi:10.3389/fphys.2020.609614
15. Bui FQ, Almeida-da-silva CLC, Huynh B, et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42(1):27–35. doi:10.1016/j.bj.2018.12.001
16. Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016;594(8):2061–2073. doi:10.1113/jp270538
17. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–979. doi:10.1056/nejm199704033361401
18. Aoyama N, Suzuki JI, Kobayashi N, et al. Periodontitis deteriorates peripheral arterial disease in Japanese population via enhanced systemic inflammation. Heart Vessels. 2017;32(11):1314–1319. doi:10.1007/s00380-017-1003-6
19. Kure K, Sato H, Aoyama N, Izumi Y. Accelerated inflammation in peripheral artery disease patients with periodontitis. J Periodontal Implant Sci. 2018;48(6):337–346. doi:10.5051/jpis.2018.48.6.337
20. Moura MF, Navarro TP, Silva TA, Cota LOM, Soares Dutra Oliveira AM, Costa FO. Periodontitis and endothelial dysfunction: periodontal clinical parameters and levels of salivary markers Interleukin-1β, tumor necrosis factor-α, matrix metalloproteinase-2, tissue inhibitor of metalloproteinases-2 complex, and nitric oxide. J Periodontol. 2017;88(8):778–787. doi:10.1902/jop.2017.170023
21. Nicolosi LN, Lewin PG, Rudzinski JJ, et al. Relation between periodontal disease and arterial stiffness. J Periodontal Res. 2017;52(1):122–126. doi:10.1111/jre.12376
22. Monteiro AM, Jardini MA, Alves S, et al. Cardiovascular disease parameters in periodontitis. J Periodontol. 2009;80(3):378–388. doi:10.1902/jop.2009.080431
23. D’Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res. 2004;39(4):236–241. doi:10.1111/j.1600-0765.2004.00731.x
24. Zanella SM, Pereira SS, Barbisan JN, et al. Periodontal disease, tooth loss and coronary heart disease assessed by coronary angiography: a cross-sectional observational study. J Periodontal Res. 2016;51(2):221–227. doi:10.1111/jre.12301
25. Dorn JM, Genco RJ, Grossi SG, et al. Periodontal disease and recurrent cardiovascular events in survivors of myocardial infarction (MI): the Western New York Acute MI Study. J Periodontol. 2010;81(4):502–511. doi:10.1902/jop.2009.090499
26. Reichert S, Schulz S, Benten AC, et al. Periodontal conditions and incidence of new cardiovascular events among patients with coronary vascular disease. J Clin Periodontol. 2016;43(11):918–925. doi:10.1111/jcpe.12611
27. Rydén L, Buhlin K, Ekstrand E, et al. Periodontitis increases the risk of a first myocardial infarction: a report from the PAROKRANK Study. Circulation. 2016;133(6):576–583. doi:10.1161/circulationaha.115.020324
28. Renvert S, Ohlsson O, Pettersson T, Persson GR. Periodontitis: a future risk of acute coronary syndrome? A follow-up study over 3 years. J Periodontol. 2010;81(7):992–1000. doi:10.1902/jop.2010.090105
29. Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 2012;125(20):2520–2544. doi:10.1161/CIR.0b013e31825719f3
30. Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Periodontol. 2013;84(4 Suppl):S70–S84. doi:10.1902/jop.2013.134008
31. Sen S, Giamberardino LD, Moss K, et al. Periodontal disease, regular dental care use, and incident ischemic stroke. Stroke. 2018;49(2):355–362. doi:10.1161/strokeaha.117.018990
32. Herrera D, Molina A, Buhlin K, Klinge B. Periodontal diseases and association with atherosclerotic disease. Periodontol 2000. 2020;83(1):66–89. doi:10.1111/prd.12302
33. Sanz M, Marco Del Castillo A, Jepsen S, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. 2020;47(3):268–288. doi:10.1111/jcpe.13189
34. Pirih FQ, Monajemzadeh S, Singh N, et al. Association between metabolic syndrome and periodontitis: the role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontol 2000. 2021;87(1):50–75. doi:10.1111/prd.12379
35. Orlandi M, Muñoz Aguilera E, Marletta D, Petrie A, Suvan J, D’Aiuto F. Impact of the treatment of periodontitis on systemic health and quality of life: a systematic review. J Clin Periodontol. 2022;49(Suppl 24):314–327. doi:10.1111/jcpe.13554
36. Machado V, Botelho J, Escalda C, et al. Serum C-reactive protein and periodontitis: a systematic review and meta-analysis. Front Immunol. 2021;12:706432. doi:10.3389/fimmu.2021.706432
37. Botelho J, Machado V, Hussain SB, et al. Periodontitis and circulating blood cell profiles: a systematic review and meta-analysis. Exp Hematol. 2021;93:1–13. doi:10.1016/j.exphem.2020.10.001
38. Johnston W, Rosier BT, Artacho A, et al. Mechanical biofilm disruption causes microbial and immunological shifts in periodontitis patients. Sci Rep. 2021;11(1):9796. doi:10.1038/s41598-021-89002-z
39. Park SY, Kim SH, Kang SH, et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur Heart J. 2019;40(14):1138–1145. doi:10.1093/eurheartj/ehy836
40. Houcken W, Teeuw WJ, Bizzarro S, et al. Arterial stiffness in periodontitis patients and controls. A case–control and pilot intervention study. J Hum Hypertens. 2016;30(1):24–29. doi:10.1038/jhh.2015.41
41. Graziani F, Gennai S, Marruganti C, et al. Acute-phase response following one-stage full-mouth versus quadrant non-surgical periodontal treatment in subjects with comorbid type 2 diabetes: a randomized clinical trial. J Clin Periodontol. 2023;50(4):487–499. doi:10.1111/jcpe.13760
42. Caúla AL, Lira-Junior R, Tinoco EM, Fischer RG. The effect of periodontal therapy on cardiovascular risk markers: a 6-month randomized clinical trial. J Clin Periodontol. 2014;41(9):875–882. doi:10.1111/jcpe.12290
43. Bresolin AC, Pronsatti MM, Pasqualotto LN, et al. Lipid profiles and inflammatory markers after periodontal treatment in children with congenital heart disease and at risk for atherosclerosis. Vasc Health Risk Manag. 2013;9:703–709. doi:10.2147/vhrm.S52187
44. Bokhari SA, Khan AA, Butt AK, et al. Non-surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J Clin Periodontol. 2012;39(11):1065–1074. doi:10.1111/j.1600-051X.2012.01942.x
45. Teeuw WJ, Slot DE, Susanto H, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. 2014;41(1):70–79. doi:10.1111/jcpe.12171
46. Del Pinto R, Pietropaoli D, Grassi G, et al. Home oral hygiene is associated with blood pressure profiles: results of a nationwide survey in Italian pharmacies. J Clin Periodontol. 2022;49(12):1234–1243. doi:10.1111/jcpe.13720
47. Chang Y, Woo HG, Park J, Lee JS, Song TJ. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: a nationwide population-based cohort study. Eur J Prev Cardiol. 2020;27(17):1835–1845. doi:10.1177/2047487319886018
48. Janket SJ, Lee C, Surakka M, et al. Oral hygiene, mouthwash usage and cardiovascular mortality during 18.8 years of follow-up. Br Dent J. 2023:1–6. doi:10.1038/s41415-023-5507-4
49. Naderi S, Merchant AT. The Association between periodontitis and cardiovascular disease: an update. Curr Atheroscler Rep. 2020;22(10):52. doi:10.1007/s11883-020-00878-0
50. Hamza SA, Asif S, Khurshid Z, Zafar MS, Bokhari SAH. Emerging role of epigenetics in explaining relationship of periodontitis and cardiovascular diseases. Diseases. 2021;9(3):48.
51. Xu S, Pelisek J, Jin ZG. Atherosclerosis Is an Epigenetic Disease. Trends Endocrinol Metab. 2018;29(11):739–742. doi:10.1016/j.tem.2018.04.007
52. Palioto DB, Finoti LS, Kinane DF, Benakanakere M. Epigenetic and inflammatory events in experimental periodontitis following systemic microbial challenge. J Clin Periodontol. 2019;46(8):819–829. doi:10.1111/jcpe.13151
53. Almiñana-Pastor PJ, Boronat-Catalá M, Micó-Martinez P, Bellot-Arcís C, Lopez-Roldan A, Alpiste-Illueca FM. Epigenetics and periodontics: a systematic review. Med Oral Patol Oral Cir Bucal. 2019;24(5):e659–e672. doi:10.4317/medoral.23008
54. Tiensripojamarn N, Lertpimonchai A, Tavedhikul K, et al. Periodontitis is associated with cardiovascular diseases: a 13-year study. J Clin Periodontol. 2021;48(3):348–356. doi:10.1111/jcpe.13418
55. Alfaddagh A, Martin SS, Leucker TM, et al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prev Cardiol. 2020;4:100130. doi:10.1016/j.ajpc.2020.100130
56. Georgakis MK, Gill D, Rannikmae K, et al. Genetically determined levels of circulating cytokines and risk of stroke. Circulation. 2019;139(2):256–268. doi:10.1161/CIRCULATIONAHA.118.035905
57. Bartekova M, Radosinska J, Jelemensky M, Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev. 2018;23(5):733–758. doi:10.1007/s10741-018-9716-x
58. Hoogeveen RC, Ballantyne CM. Residual cardiovascular risk at low LDL: remnants, lipoprotein(a), and inflammation. Clin Chem. 2021;67(1):143–153. doi:10.1093/clinchem/hvaa252
59. Janket SJ, Baird AE, Jones JA, et al. Number of teeth, C-reactive protein, fibrinogen and cardiovascular mortality: a 15-year follow-up study in a Finnish cohort. J Clin Periodontol. 2014;41(2):131–140. doi:10.1111/jcpe.12192
60. Everett BM, MacFadyen JG, Thuren T, Libby P, Glynn RJ, Ridker PM. Inhibition of Interleukin-1β and Reduction in Atherothrombotic Cardiovascular Events in the CANTOS Trial. J Am Coll Cardiol. 2020;76(14):1660–1670. doi:10.1016/j.jacc.2020.08.011
61. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi:10.1056/NEJMoa1707914
62. Bouabdallaoui N, Tardif JC, Waters DD, et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J. 2020;41(42):4092–4099. doi:10.1093/eurheartj/ehaa659
63. Samuel M, Tardif JC, Khairy P, et al. Cost-effectiveness of low-dose colchicine after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J Qual Care Clin Outcomes. 2021;7(5):486–495. doi:10.1093/ehjqcco/qcaa045
64. Dubé MP, Legault MA, Lemaçon A, et al. Pharmacogenomics of the efficacy and safety of colchicine in COLCOT. Circ Genom Precis Med. 2021;14(2):e003183. doi:10.1161/circgen.120.003183
65. Febbraio M, Roy CB, Levin L. Is there a causal link between periodontitis and cardiovascular disease? A concise review of recent findings. Int Dent J. 2022;72(1):37–51. doi:10.1016/j.identj.2021.07.006
66. Hennigs JK, Matuszcak C, Trepel M, Korbelin J. Vascular endothelial cells: heterogeneity and targeting approaches. Cells. 2021;10:2712.
67. Xu K, Yu W, Li Y, et al. Association between tooth loss and hypertension: a systematic review and meta-analysis. J Dent. 2022;123:104178. doi:10.1016/j.jdent.2022.104178
68. Pietropaoli D, Monaco A, D’Aiuto F, et al. Active gingival inflammation is linked to hypertension. J Hypertens. 2020;38(10):2018–2027. doi:10.1097/hjh.0000000000002514
69. Pietropaoli D, Del Pinto R, Ferri C, et al. Poor oral health and blood pressure control among us hypertensive adults. Hypertension. 2018;72(6):1365–1373. doi:10.1161/HYPERTENSIONAHA.118.11528
70. Del Pinto R, Landi L, Grassi G, et al. Hypertension and Periodontitis: a Joint Report by the Italian Society of Hypertension (SIIA) and the Italian Society of Periodontology and Implantology (SIdP). High Blood Press Cardiovasc Prev. 2021;28(5):427–438. doi:10.1007/s40292-021-00466-6
71. Silveira TMD, Silva CFE, Vaucher RA, Angst PDM, Casarin M, Pola NM. Higher frequency of specific periodontopathogens in hypertensive patients. A pilot study. Braz Dent J. 2022;33(5):64–73. doi:10.1590/0103-6440202204914
72. Olsen I, Progulske-Fox A. Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J Oral Microbiol. 2015;7:28788. doi:10.3402/jom.v7.28788
73. Stelzel M, Conrads G, Pankuweit S, et al. Detection of Porphyromonas gingivalis DNA in aortic tissue by PCR. J Periodontol. 2002;73(8):868–870. doi:10.1902/jop.2002.73.8.868
74. Irwandi RA, Chiesa ST, Hajishengallis G, Papayannopoulos V, Deanfield JE, D’Aiuto F. The Roles of Neutrophils Linking Periodontitis and Atherosclerotic Cardiovascular Diseases. Front Immunol. 2022;13:915081. doi:10.3389/fimmu.2022.915081
75. Li Q, Ouyang X, Lin J. The impact of periodontitis on vascular endothelial dysfunction. Front Cell Infect Microbiol. 2022;12:998313. doi:10.3389/fcimb.2022.998313
76. Holtfreter B, Empen K, Glaser S, et al. Periodontitis is associated with endothelial dysfunction in a general population: a cross-sectional study. PLoS One. 2013;8(12):e84603. doi:10.1371/journal.pone.0084603
77. Fujitani T, Aoyama N, Hirata F, Minabe M. Association between periodontitis and vascular endothelial function using noninvasive medical device-A pilot study. Clin Exp Dent Res. 2020;6(5):576–582. doi:10.1002/cre2.312
78. Gerreth P, Maciejczyk M, Zalewska A, Gerreth K, Hojan K. Comprehensive evaluation of the oral health status, salivary gland function, and oxidative stress in the saliva of patients with subacute phase of stroke: a case-control study. J Clin Med. 2020;9(7):2252.
79. Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative stress in oral diseases: understanding its relation with other systemic diseases. Front Physiol. 2017;8:693. doi:10.3389/fphys.2017.00693
80. Xie M, Tang Q, Nie J, et al. BMAL1-downregulation aggravates porphyromonas gingivalis-induced atherosclerosis by encouraging oxidative stress. Circ Res. 2020;126(6):e15–e29. doi:10.1161/CIRCRESAHA.119.315502
81. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3(1):17038. doi:10.1038/nrdp.2017.38
82. Pietiainen M, Liljestrand JM, Kopra E, Pussinen PJ. Mediators between oral dysbiosis and cardiovascular diseases. Eur J Oral Sci. 2018;126(Suppl 1):26–36. doi:10.1111/eos.12423
83. Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002–5017. doi:10.1128/JB.00542-10
84. Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 2000. 2020;83(1):90–106. doi:10.1111/prd.12304
85. Pietropaoli D, Del Pinto R, Ferri C, Ortu E, Monaco A. Definition of hypertension-associated oral pathogens in NHANES. J Periodontol. 2019;90(8):866–876. doi:10.1002/JPER.19-0046
86. Holmlund A, Lampa E, Lind L. Oral health and cardiovascular disease risk in a cohort of periodontitis patients. Atherosclerosis. 2017;262:101–106. doi:10.1016/j.atherosclerosis.2017.05.009
87. Bryan NS, Tribble G, Angelov N. Oral microbiome and nitric oxide: the missing link in the management of blood pressure. Curr Hypertens Rep. 2017;19(4):33. doi:10.1007/s11906-017-0725-2
88. Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35(11):1543–1546. doi:10.1136/gut.35.11.1543
89. Benjamin N, O’Driscoll F, Dougall H, et al. Stomach NO synthesis. Nature. 1994;368(6471):502. doi:10.1038/368502a0
90. Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37(3):395–400. doi:10.1016/j.freeradbiomed.2004.04.027
91. Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976;14(6):545–548. doi:10.1016/s0015-6264(76)80005-3
92. Duncan C, Dougall H, Johnston P, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1(6):546–551. doi:10.1038/nm0695-546
93. Rowland SN, James LJ, O’Donnell E, Bailey SJ. Influence of acute dietary nitrate supplementation timing on nitrate metabolism, central and peripheral blood pressure and exercise tolerance in young men. Eur J Appl Physiol. 2023. doi:10.1007/s00421-023-05369-z
94. Hogwood AC, Anderson KC, Ortiz de Zevallos J, Paterson C, Weltman A, Allen JD. Limited effects of inorganic nitrate supplementation on exercise training responses: a systematic review and meta-analysis. Sports Med Open. 2023;9(1):84. doi:10.1186/s40798-023-00632-1
95. Miller GD, Collins S, Ives J, et al. Efficacy and variability in plasma nitrite levels during long-term supplementation with nitrate containing beetroot juice. J Diet Suppl. 2023;20(6):885–910. doi:10.1080/19390211.2022.2137269
96. Kulbir DS, Devi T, Ghosh S, Chandra Sahoo S, Kumar P. Acid-induced nitrite reduction of nonheme iron(ii)-nitrite: mimicking biological Fe-NiR reactions. Chem Sci. 2023;14(11):2935–2942. doi:10.1039/d2sc06704h
97. Qu XM, Wu ZF, Pang BX, Jin LY, Qin LZ, Wang SL. From nitrate to nitric oxide: the role of salivary glands and oral bacteria. J Dent Res. 2016;95(13):1452–1456. doi:10.1177/0022034516673019
98. Ma L, Hu L, Feng X, Wang S. Nitrate and nitrite in health and disease. Aging Dis. 2018;9(5):938–945. doi:10.14336/ad.2017.1207
99. Lundberg JO, Weitzberg E, Gladwin MT. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi:10.1038/nrd2466
100. Kanner J. Food polyphenols as preventive medicine. Antioxidants. 2023;12(12):2103.
101. Du J, Filipović MR, Wagner BA, Buettner GR. Ascorbate mediates the non-enzymatic reduction of nitrite to nitric oxide. Adv Redox Res. 2023;9. doi:10.1016/j.arres.2023.100079
102. Björne H, Weitzberg E, Lundberg JO. Intragastric generation of antimicrobial nitrogen oxides from saliva--physiological and therapeutic considerations. Free Radic Biol Med. 2006;41(9):1404–1412. doi:10.1016/j.freeradbiomed.2006.07.020
103. Liu H, Huang Y, Huang M, et al. From nitrate to NO: potential effects of nitrate-reducing bacteria on systemic health and disease. Eur J Med Res. 2023;28(1):425. doi:10.1186/s40001-023-01413-y
104. Bright LME, Wu Y, Brisbois EJ, Handa H. Advances in nitric oxide-releasing hydrogels for biomedical applications. Curr Opin Colloid Interface Sci. 2023;66. doi:10.1016/j.cocis.2023.101704
105. Jindal M, Sogi S, Shahi P, Ramesh A, Nautiyal MP, Jindal T. Salivary nitric oxide levels before and after treating caries in children: a comparative study. Int J Clin Pediatr Dent. 2023;16(Suppl 2):133–137. doi:10.5005/jp-journals-10005-2659
106. Park JH, Park CY. Effects of in vitro combination of nitric oxide donors and hypochlorite on acanthamoeba castellanii viability. Transl Vis Sci Technol. 2023;12(9):23. doi:10.1167/tvst.12.9.23
107. Rocha BS. The nitrate-nitrite-nitric oxide pathway on healthy ageing: a review of pre-clinical and clinical data on the impact of dietary nitrate in the elderly. Front Aging. 2021;2:778467. doi:10.3389/fragi.2021.778467
108. Liang TY, Deng RM, Li X, Xu X, Chen G. The role of nitric oxide in peptic ulcer: a narrative review. Med Gas Res. 2021;11(1):42–45. doi:10.4103/2045-9912.310059
109. Bove M, Vieth M, Casselbrant A, Ny L, Lundell L, Ruth M. Acid challenge to the esophageal mucosa: effects on local nitric oxide formation and its relation to epithelial functions. Dig Dis Sci. 2005;50(4):640–648. doi:10.1007/s10620-005-2550-8
110. Kapil V, Weitzberg E, Lundberg JO, Ahluwalia A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide. 2014;38:45–57. doi:10.1016/j.niox.2014.03.162
111. Farah C, Michel LYM, Balligand JL. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol. 2018;15(5):292–316. doi:10.1038/nrcardio.2017.224
112. Vanhatalo A, Blackwell JR, L’Heureux JE, et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med. 2018;124:21–30. doi:10.1016/j.freeradbiomed.2018.05.078
113. Velmurugan S, Gan JM, Rathod KS, et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2016;103(1):25–38. doi:10.3945/ajcn.115.116244
114. Buhlin K, Holmer J, Gustafsson A, et al. Association of periodontitis with persistent, pro-atherogenic antibody responses. J Clin Periodontol. 2015;42(11):1006–1014. doi:10.1111/jcpe.12456
115. Radaic A, Kapila YL. The oralome and its dysbiosis: new insights into oral microbiome-host interactions. Comput Struct Biotechnol J. 2021;19:1335–1360. doi:10.1016/j.csbj.2021.02.010
116. Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. doi:10.1038/s41579-018-0089-x
117. Sedghi L, DiMassa V, Harrington A, Lynch SV, Kapila YL. The oral microbiome: role of key organisms and complex networks in oral health and disease. Periodontol 2000. 2021;87(1):107–131. doi:10.1111/prd.12393
118. Bhuyan R, Bhuyan SK, Mohanty JN, Das S, Juliana N, Juliana IF. Periodontitis and its inflammatory changes linked to various systemic diseases: a review of its underlying mechanisms. Biomedicines. 2022;10(10):2659. doi:10.3390/biomedicines10102659
119. Priyamvara A, Dey AK, Bandyopadhyay D, et al. Periodontal inflammation and the risk of cardiovascular disease. Curr Atherosclerosis Rep. 2020;22:1–6. doi:10.1007/s11883-020-00848-6
120. Kay JG, Kramer JM, Visser MB. Danger signals in oral cavity-related diseases. J Leukoc Biol. 2019;106(1):193–200. doi:10.1002/JLB.4MIR1118-439R
121. Rai V, Agrawal DK. The role of damage- and pathogen-associated molecular patterns in inflammation-mediated vulnerability of atherosclerotic plaques. Can J Physiol Pharmacol. 2017;95(10):1245–1253. doi:10.1139/cjpp-2016-0664
122. Zhu X, Huang H, Zhao L. PAMPs and DAMPs as the bridge between periodontitis and atherosclerosis: the potential therapeutic targets. Front Cell Dev Biol. 2022;10:856118. doi:10.3389/fcell.2022.856118
123. Viafara-Garcia SM, Morantes SJ, Chacon-Quintero Y, Castillo DM, Lafaurie GI, Buitrago DM. Repeated Porphyromonas gingivalis W83 exposure leads to release pro-inflammatory cytokynes and angiotensin II in coronary artery endothelial cells. Sci Rep. 2019;9(1):19379. doi:10.1038/s41598-019-54259-y
124. Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. 2019;40(42):3459–3470. doi:10.1093/eurheartj/ehz646
125. Saffi MAL, Rabelo-Silva ER, Polanczyk CA, et al. Periodontal therapy and endothelial function in coronary artery disease: a randomized controlled trial. Oral Dis. 2018;24(7):1349–1357. doi:10.1111/odi.12909
126. Morozumi T, Yashima A, Gomi K, et al. Increased systemic levels of inflammatory mediators following one-stage full-mouth scaling and root planing. J Periodontal Res. 2018;53(4):536–544. doi:10.1111/jre.12543
127. Jockel-Schneider Y, Bechtold M, Haubitz I, et al. Impact of anti-infective periodontal therapy on parameters of vascular health. J Clin Periodontol. 2018;45(3):354–363. doi:10.1111/jcpe.12849
128. Zhou QB, Xia WH, Ren J, et al. Effect of Intensive periodontal therapy on blood pressure and endothelial microparticles in patients with prehypertension and periodontitis: a randomized controlled trial. J Periodontol. 2017;88(8):711–722. doi:10.1902/jop.2017.160447
129. Arvanitidis E, Bizzarro S, Alvarez Rodriguez E, Loos BG, Nicu EA. Reduced platelet hyper-reactivity and platelet-leukocyte aggregation after periodontal therapy. Thromb J. 2017;15:5. doi:10.1186/s12959-016-0125-x
130. de Souza AB, Okawa RT, Silva CO, Araújo MG. Short-term changes on C-reactive protein (CRP) levels after non-surgical periodontal treatment in systemically healthy individuals. Clin Oral Investig. 2017;21(1):477–484. doi:10.1007/s00784-016-1817-0
131. Torumtay G, Kırzıoğlu FY, Öztürk Tonguç M, Kale B, Calapoğlu M, Orhan H. Effects of periodontal treatment on inflammation and oxidative stress markers in patients with metabolic syndrome. J Periodontal Res. 2016;51(4):489–498. doi:10.1111/jre.12328
132. Hada DS, Garg S, Ramteke GB, Ratre MS. Effect of non-surgical periodontal treatment on clinical and biochemical risk markers of cardiovascular disease: a randomized trial. J Periodontol. 2015;86(11):1201–1211. doi:10.1902/jop.2015.150249
133. Graziani F, Cei S, Orlandi M, et al. Acute-phase response following full-mouth versus quadrant non-surgical periodontal treatment: a randomized clinical trial. J Clin Periodontol. 2015;42(9):843–852. doi:10.1111/jcpe.12451
134. Gupta B, Sawhney A, Patil N, et al. Effect of surgical periodontal therapy on serum C-reactive protein levels using ELISA in both chronic and aggressive periodontitis patient. J Clin Diagn Res. 2015;9(10):Zc01–Zc05. doi:10.7860/jcdr/2015/14680.6558
135. Zhou SY, Duan XQ, Hu R, Ouyang XY. Effect of non-surgical periodontal therapy on serum levels of TNF-a, IL-6 and C-reactive protein in periodontitis subjects with stable coronary heart disease. Chin J Dent Res. 2013;16(2):145–151.
136. Vidal F, Cordovil I, Figueredo CM, Fischer RG. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J Clin Periodontol. 2013;40(7):681–687. doi:10.1111/jcpe.12110
137. López NJ, Quintero A, Casanova PA, Ibieta CI, Baelum V, López R. Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: a controlled clinical trial. J Periodontol. 2012;83(3):267–278. doi:10.1902/jop.2011.110227
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
