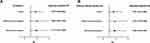Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Association Between Metabolic Dysfunction-Associated Fatty Liver Disease and the Risk of Cirrhosis in Patients with Chronic Hepatitis B—A Retrospective Cohort Study
Authors Wang QX, Xue J , Shi MJ, Xie YB, Xiao HM, Li S, Lin M, Chi XL
Received 7 April 2022
Accepted for publication 19 July 2022
Published 2 August 2022 Volume 2022:15 Pages 2311—2322
DOI https://doi.org/10.2147/DMSO.S369824
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Qing-Xia Wang,1 Jiao Xue,1 Mei-Jie Shi,2 Yu-Bao Xie,2 Huan-Ming Xiao,2 Sheng Li,2 Ming Lin,2 Xiao-Ling Chi2
1The Second School of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 2Department of Hepatology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, People’s Republic of China
Correspondence: Xiao-Ling Chi, Department of Hepatology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, People’s Republic of China, Tel +86+39318398, Fax +86-020-81867705, Email [email protected]
Background: Metabolic dysfunction-associated fatty liver disease (MAFLD) is a novel proposed concept that is being recognized worldwide. Both chronic hepatitis B (CHB) and MAFLD have been independently attributed to an increased risk of disease development to cirrhosis. However, it is still unclear whether MAFLD is associated with an increased risk of cirrhosis in CHB patients.
Aim: This study aimed to analyze the impact of MAFLD on the risk of cirrhosis in CHB patients.
Methods: In this retrospective cohort study, consecutive CHB patients with or without MAFLD were enrolled from January 1st, 2007, to May 1st, 2020, in Guangdong Provincial Hospital of Chinese Medicine. Inverse probability treatment weighting (IPTW) was performed to balance the covariates across groups. The weighted Kaplan–Meier analysis and Cox regression analysis were used to compare both groups for the risk of cirrhosis.
Results: A total of 1223 CHB patients were included in this study during the median follow-up of 5.25 years; of these patients, 355 were CHB-MAFLD patients. After IPTW, the weighted Kaplan–Meier analysis showed that the weighted cumulative incidence of cirrhosis was significantly higher in patients with MAFLD than that in patients without MAFLD (12.6% versus 7.1%, P=0.015). In the weighted multivariate Cox analysis, coexisting MAFLD was related to an increased risk of cirrhosis [adjusted weighted hazard ratio (HR) 1.790; P =0.020]. Age (> 40 years, adjusted weighted HR, 1.950; P=0.015), diabetes mellitus (adjusted weighted HR, 1.883; P=0.041), non-antiviral treatment (adjusted weighted HR, 2.037; P=0.013), and baseline serum HBV DNA levels (> 2.4 log10 IU/mL, adjusted weighted HR, 1.756; P=0.045) were significant risk factors for cirrhosis.
Conclusion: We found that MAFLD was associated with a higher risk of cirrhosis in CHB patients.
Keywords: chronic hepatitis B, metabolic dysfunction-associated fatty liver disease, hepatic steatosis, cirrhosis
Introduction
Chronic hepatitis B (CHB) has become a global health problem, and almost 240 million to 350 million individuals have chronic hepatitis B virus (HBV) infection globally.1 Although the long-term prognosis for CHB patients has been improved by using antiviral treatment, chronic HBV infection is still the most common primary etiologies for cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC).2,3 The WHO’s 2015 Global Health Estimates (GHE) dataset showed that hepatitis B infection accounted for 27.8% to 69.2% of all deaths due to cirrhosis and other chronic liver diseases in the Asia-Pacific region, and this value was 63% in China.4
Metabolic dysfunction-associated fatty liver disease (MAFLD) has been proposed recently and is defined as hepatic steatosis (HS) combined with obesity, diabetes, and/or evidence of other metabolic abnormalities.5–7 The prevalence of MAFLD is approximately 30%, as the proportion of overweight, metabolic syndrome, diabetes, and dyslipidemia are gradually growing.8–12 The interaction between CHB and MAFLD has become a focus of recent research. A recent study revealed that MAFLD was a significant risk factor for fibrosis and moderate-to-severe liver activity in HBeAg-negative CHB patients.13 Previous studies found that hepatic steatosis and metabolic syndrome were independently associated with fibrosis progression and hepatic malignancies in CHB patients.14–16 Nevertheless, other researchers observed a significant correlation between hepatic steatosis and a lower risk of cirrhosis and HCC in CHB patients.17,18 Hence, the role of MAFLD in the prognosis of CHB to cirrhosis or the development of HCC is unclear. Whether the co-existence of MAFLD contributes to the risk of cirrhosis in CHB patients has never been reported.
Therefore, this current retrospective cohort study aimed to evaluate the influence of radiologically-confirmed MAFLD on the risk of cirrhosis in CHB patients using inverse probability treatment weighting (IPTW) to rigorously adjust for possible confounding factors.
Patients and Methods
Study Design and Patient Population
This retrospective cohort study enrolled consecutive CHB patients with or without MAFLD from January 1st, 2007 to May 1st, 2020 in Guangdong Provincial Hospital of Chinese Medicine. The inclusion criteria were as follows: (1) older than 18 years old; (2) evidence of serum HBsAg positivity for at least 6 months;19 and (3) undergoing periodic abdominal image examination, such as ultrasonography, magnetic resonance (MR), or computed tomography (CT). The exclusion criteria were as follows: (1) history of malignant disease, including HCC; (2) a follow-up time of fewer than 6 months; (3) diagnosed with cirrhosis within 6 months from the index date; and (4) missing more than 10% of relevant clinical information.
This study was approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (approval number: YE2021-200-01). Written informed consent was waived due to the retrospective nature of our study. All data was anonymized to maintain confidentiality.
Exposure Definition
The exposure of interest was MAFLD, and the comparator group was CHB patients without MAFLD. We defined CHB-MAFLD patients as subjects who were newly diagnosed with MAFLD during the follow-up period or those who already had MAFLD. According to the international expert consensus statement,6 MAFLD diagnoses were established radiologically by evidence of HS combined with overweight (BMI ≥ 23 kg/m2), diabetes mellitus (DM), or a combination of at least two of the following metabolic risk factors: (1) blood pressure ≥ 130/85 mmHg or receiving drug treatment for hypertension; (2) plasma triglycerides ≥ 1.7 mmol/L or receiving lipid-lowering drugs treatment; (3) plasma high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/L in men or < 1.3 mmol/L in women or lipid-lowering drug treatment; (4) prediabetes defined as fasting blood glucose 5.6–6.9 mmol/L; (5) waist circumference ≥ 90 cm in men or ≥ 80 cm in women; (6) homeostatic model assessment of insulin resistance (HOMA-IR) ≥ 2.5; or (7) plasma C-reactive protein level > 2 mg/L. Data for the last three criteria were unavailable.
Imaging diagnoses of HS were established as follows: an increased liver parenchyma echogenicity and decreased definition of liver structures, such as vessel walls, based on an ultrasound; hepatic attenuation divided by splenic attenuation ≤ 1.0, based on CT; and proton-density fat fraction values ≥ 5%, based on MR.20
End-Point
The end-point of this retrospective study was the occurrence of cirrhosis. According to the 2018 clinical practice Chinese guidelines, cirrhosis diagnoses were established radiologically with a nodularity of the liver surface, coarse echo pattern of the liver parenchyma, splenomegaly, hepatomegaly, ascites, or thrombus of the portal vein using ultrasonography, CT, or MR.21
Data Collection
Clinical and Laboratory Data
Clinical and laboratory data were obtained through a retrospective review of outpatient case notes and inpatient case notes. Baseline clinical data were collected at the index date, and these data included age, sex, alcohol intake habit, body mass index (BMI), duration of antiviral treatment, past medical history (diabetes mellitus and hypertension), HBV DNA levels, hepatitis B e antigen (HBeAg), and blood chemistry parameters. The following blood chemistry parameters were recorded: alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, total bilirubin, alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), uric acid (UA), fasting glucose, triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
The calculation of BMI was based on height and weight. The definition of overweight was BMI ≥ 23 kg/m2, according to the demarcation points for determining overweight in Asian populations.22
Imaging Data
Imaging data such as abdominal ultrasonography, CT and MR of the enrolled patients were obtained from medical records. Our researchers recorded relevant clinical information every 6 months.
Follow-Up
The index date was defined as the time of the patient’s first visit to the department of hepatology, Guangdong Provincial Hospital of Chinese Medicine. We defined the follow-up duration as the period between the index date and the date of cirrhosis diagnosis or the last visit (May 1st, 2020). The enrolled patients were assessed every 6 months during the follow-up time.
Statistical Analysis
Continuous variables are expressed as a median and interquartile range (IQR) and were compared by a non-parametric test (Mann–Whitney). The categorical parameters of the two groups were analyzed by the chi-squared test.
IPTW was applied to minimize the influence of potential confounding variables associated with CHB. The calculation of propensity scores was performed by fitting a logistic regression model. Variables with P<0.05 via univariable analysis remained in the model, and these variables included age, sex, albumin, ALT, GGT, HBV DNA, and HBeAg. Each patient was weighted by the inverse probability (1/propensity score for CHB patients with MAFLD and 1/ [1−propensity score] for CHB patients without MAFLD). A stabilized IPTW was calculated by truncation at the 5th and 95th percentiles.23 After IPTW, the balance of the measured variables between groups was re-evaluated. Then, the cumulative incidence of cirrhosis was compared using the weighted Kaplan-Meier analysis. The differences between the two groups were assessed using the Log rank test. The weighted hazard ratio (weighted HR; CHB-MAFLD vs CHB) and 95% confidence interval between the two groups were calculated using a weighted Cox proportional hazards regression model.
Results were considered statistically significant when P<0.05 on two sides. Statistical analyses were performed using SPSS, version 25.0 (IBM SPSS Inc., Armonk, New York, USA), and R Statistical Software, version 4.0.3 (R Foundation for Statistical Computing, 2020).
Results
Baseline Characteristics of the Study Population
A total of 1223 CHB patients were enrolled in this study, including 355 patients with MAFLD and 868 patients without MAFLD (Figure 1). Of these CHB-MAFLD patients, 85.64% presented with BMI ≥ 23 kg/m2, 11.83% presented with BMI < 23 kg/m2 plus metabolic dysfunction, and 2.53% presented with BMI < 23 kg/m2 plus DM (Figure 2A). In the CHB-MAFLD group, 82.53% of patients had at least one metabolic risk factor (Figure 2B). The median follow-up for the entire cohort was 5.25 years (IQR 2.91–7.25 years). Table 1 shows the baseline characteristics of enrolled patients before and after IPTW.
 |
Table 1 Baseline Characteristics of Patients Before and After IPTW |
 |
Figure 1 Flowchart of the study cohorts. |
Before IPTW, patients in the CHB-MAFLD group were significantly older and had a higher BMI. This group also had substantially higher proportions of males and patients that consumed alcohol than those in the CHB group. In addition, patients in the CHB-MAFLD group had significantly more metabolic risk factors, including higher levels of serum glucose, TGs, and HDL-C, than those in the CHB group (all P<0.001). Patients in the CHB-MAFLD group had lower HBV DNA levels and a higher proportion of HBeAg-positive patients at baseline (P<0.001 and P=0.015, respectively).
The parameters of age, sex, albumin levels, ALT levels, GGT levels, HBV DNA levels, and HBeAg levels were similar in the two groups after IPTW (Table 1). A total of 2154 CHB patients were included in the IPTW analysis; there were 1078 patients with MAFLD and 1076 patients without MAFLD (Figure 1). The median duration of follow-up was not significantly different between the CHB-MAFLD group and the CHB group (5.41 vs 5.33 years, P = 0.501).
Cirrhosis Risk Assessment Between the CHB-MAFLD and CHB Groups After IPTW
During a median follow-up time of 5.42 years (IQR 3.08–7.33 years), the weighted cumulative incidence of cirrhosis in the entire cohort was 9.8% after IPTW. Figure 3 shows the weighted cumulative incidence of cirrhosis per year in the two groups. The weighted Kaplan–Meier analysis (Figure 4) indicated that the cumulative incidence of cirrhosis in the CHB-MAFLD group was significantly higher than that in the CHB group (12.6% and 7.1%, respectively, P=0.015).
 |
Figure 3 Cumulative incidence of cirrhosis per year after IPTW. The different weighted cumulative incidence of cirrhosis per year between the CHB-MAFLD group and the CHB group after IPTW. |
We further performed a cirrhosis risk assessment that was stratified by antiviral treatment status. Similar outcomes were observed in patients with and without antiviral treatment. Untreated CHB-MAFLD patients had a significantly higher cumulative incidence of cirrhosis (14.8% vs 8.2%, P=0.009) than untreated CHB patients (Figure 5A). Among the patients with antiviral treatment, the weighted cumulative incidence of cirrhosis was also significantly higher in the CHB-MAFLD group compared to the CHB group (10.9% vs 6.3%, P=0.012, Figure 5B).
Weighted Univariate and Multivariate Cox Analysis of Risk Factors for Cirrhosis in CHB Patients After IPTW
The weighted univariate Cox regression analysis identified age, antiviral treatment status (untreated), DM, coexisting MAFLD, baseline serum GGT, and HBV DNA levels as significant risk factors associated with cirrhosis (Table 2). Coexisting MAFLD was still associated with a higher cirrhosis risk after adjusting for alcohol use and other variables in the weighted multivariate Cox proportional hazards regression analysis [adjusted weighted hazard ratio (HR) 1.790; 95% confidence interval (95% CI), 1.095–2.926; P=0.020]. Moreover, age (>40 years, adjusted weighted HR, 1.950; 95% CI, 1.141–3.332; P=0.015), antiviral treatment status (untreated, adjusted weighted HR, 2.037; 95% CI, 1.164–3.567; P=0.013), DM (adjusted weighted HR, 1.883; 95% CI, 1.026–3.456; P=0.041), baseline serum GGT levels (adjusted weighted HR, 1.004; 95% CI, 1.001–1.007; P=0.005) and HBV DNA levels (>2.4 log10 IU/mL, adjusted weighted HR, 1.756; 95% CI, 1.013–3.042; P=0.045) were revealed to be independent risk factors of cirrhosis in CHB patients.
 |
Table 2 Weighted Univariate and Multivariate Cox Analysis of Risk Factors Related to Cirrhosis After IPTW |
Then, we conducted the weighted Cox analysis according to whether antiviral treatment was carried out. As shown in Figure 6A, the association between MAFLD and an increased risk of cirrhosis remained for both untreated patients (adjusted weighted HR, 1.988; 95% CI, 1.029–3.879; P=0.041) and treated patients (adjusted weighted HR, 1.917; 95% CI, 1.021–3.600; P=0.043). In order to exclude the influence of alcohol consumption on the results, we re-analyzed the data after excluding patients who consumed alcohol. Figure 6B indicated that MAFLD was still a risk factor for cirrhosis (adjusted weighted HR, 1.647; 95% CI, 1.041–2.964; P=0.034), regardless of whether the patient underwent antiviral therapy or did not undergo antiviral therapy (without antiviral treatment, adjusted weighted HR, 1.873; 95% CI, 1.016–3.858; P=0.045; with antiviral treatment, adjusted weighted HR, 1.849; 95% CI, 1.018–3.594; P=0.044).
Discussion
In the present study, a total of 1223 CHB patients were included. Among them, 355 patients had MAFLD, and these patients had a median follow-up time of 5.25 years. We found that the weighted cumulative incidence of cirrhosis in the CHB-MAFLD group was significantly higher than that in the only-CHB group after further IPTW analysis. Concurrent radiologically-confirmed MAFLD was associated with an increased risk of cirrhosis in CHB patients. These results were consistent in patients with or without antiviral therapy. To the best of our knowledge, this is the first study to report the correlation between MAFLD and the risk of cirrhosis in CHB patients.
We found that most CHB-MAFLD patients (85.64%) were presented with BMI ≥ 23 kg/m2. A recent Korean study suggested that a higher BMI was positively associated with the risk of fibrosis progression in patients with nonalcoholic fatty liver disease (NAFLD).24 In addition, the majority (82.53%) of patients in the CHB-MAFLD group had at least one metabolic dysfunction characteristic, and low HDL-C levels and prediabetes were the most common characteristic. Wong et al observed that the risk of advanced fibrosis associated with metabolic comorbidities was significantly increased in nonalcoholic steatohepatitis patients.25 Therefore, CHB-MAFLD individuals might require metabolic‐specific interventions and should maintain a normal healthy weight.
Patients in the CHB-MAFLD group had a higher proportion of HBeAg-positive patients but lower HBV DNA levels compared to patients in the CHB group in our study. The reason for this result may be related to metabolic dysfunction. A Chinese study indicated that patients with metabolic syndrome had HBeAg seroclearance later than those with normal metabolic features. Similar findings were also observed in patients with one or two metabolic risk factors.26 The lower HBV DNA levels in CHB-MAFLD patients might be associated with delayed HBeAg seroclearance.
Although previous studies have validated that NAFLD was associated with cirrhosis, the role of NAFLD in the prognosis of CHB to cirrhosis is controversial.27 Whether MAFLD influences the risk of cirrhosis in CHB patients remains unclear. In our study, the weighted Kaplan–Meier analysis indicated that CHB patients with MAFLD had a statistically higher cumulative incidence of cirrhosis than CHB patients without MAFLD (12.6% and 7.1%, respectively, P=0.015). This association remained consistent in patients with or without antiviral treatment, further confirming the impact of MAFLD on the risk of cirrhosis occurrence in CHB patients. A study carried out by Seto et al in Hong Kong also demonstrated a similar result. They found that the prevalence of severe fibrosis was higher in patients with severe HS than in patients without HS (21.4% and 11.9%, respectively; P<0.001).27 However, a retrospective cohort study performed by Natarajan et al demonstrated that the 5-year cumulative incidence rate of cirrhosis was not significantly different in patients with normal ALT with or without evidence of HS (4.0 per 1000 patients vs 4.1 per 1000 patients, P=0.19). The reasons for this difference might be due to differences in the baseline characteristics in the HS group and non-HS group in this study.28
Our study found that concurrent MAFLD was a risk factor for cirrhosis in CHB patients (adjusted weighted HR, 1.79; 95% CI, 1.10–2.93). Consistent with our present study, a Chinese study found that MAFLD increased the risk of significant fibrosis (F≥2) in treatment-naïve CHB patients [odds ratio (OR) 1.84; 95% CI, 1.19–2.87].13 Similar results were found in some previous studies on the association between HS and the risk of HBV-related cirrhosis. A cohort of Malaysian CHB patients who received transient elastography indicated that persistent HS was a significant risk factor for advanced fibrosis (OR, 1.95; 95% CI, 1.25–3.06).29 Another study reported that the risk of cirrhosis was significantly difference between patients with and without severe HS (OR, 2.38; 95% CI, 1.23–4.60).15 In summary, concurrent MAFLD increases the risk of cirrhosis in CHB patients. This suggests that these patients should receive more attention and better management of MAFLD. However, other researchers present inconsistent opinions. Li et al reported that fatty liver disease was significantly associated with a lower risk of cirrhosis in antiviral-treated CHB patients (HR, 0.19; 95% CI, 0.12–0.33).18 This discrepancy might be due to the heterogeneity of the patient populations. Li et al concentrated on fatty liver patients from communities, while our study focused on MAFLD patients at a tertiary hospital. A recent study showed that MAFLD better identifies patients with significant hepatic fibrosis than NAFLD.30 How MAFLD affects the liver is intricate, and the specific mechanisms of action remain to be explored and confirmed in further experimental studies.
Age, DM, no antiviral treatment, the baseline serum GGT levels, and HBV DNA levels were also identified as significant risk factors for cirrhosis in CHB patients. However, the weighted HR value of baseline serum GGT levels was close to 1, indicating a negligible effect on the risk of cirrhosis. Our outcomes also support prior reports. A multicenter study found that age and antiviral treatment status/duration significantly correlated with an increased cirrhosis risk.31 Recent studies have shown that DM and HBV DNA levels were significantly correlated with advanced fibrosis.32,33 The above findings suggest that CHB patients with or without MAFLD should receive antiviral treatment as early as possible while continuing to monitor changes in HBV DNA and blood glucose levels to prevent the progression to cirrhosis.
The strength of this study is that, as far as we know, our study is the first to evaluate the impact of MAFLD on the risk of liver cirrhosis in CHB patients. MAFLD is a new and recently introduced concept, and its prevalence will continue to increase in the coming decades.34 The elucidation of the association between MAFLD and cirrhosis risk among CHB patients is of clinical importance.
However, the present study has a few limitations. First, our study was a retrospective study, which may lead to bias and confounding factors. To minimize confounding factors between the CHB and CHB-MAFLD groups, we used IPTW to balance the baseline characteristics related to CHB. We further carried out a subgroup analysis to determine the consistency of the results. Second, we did not use liver biopsy for diagnosing HS and cirrhosis. Our study cohort included HBV-infected patients undergoing non-invasive radiology (including ultrasound, MR, and CT for diagnosis). Although liver biopsy is the gold standard for the diagnosis these conditions, non-invasive diagnostic tools are safer, better tolerated, easily accepted by patients, and largely repeatable as needed. Third, our cohort did not account for the potential contribution of insulin resistance to the outcomes. There is a close relationship between insulin resistance and advanced fibrosis.35 However, insulin measurements are not part of the routine examination of CHB patients, and thus, this data was absent.
Conclusion
MAFLD is common in CHB patients. The presence of radiologically-proven MAFLD is an independent risk factor for cirrhosis in CHB patients, and it increases the risk of cirrhosis nearly two-fold. These findings are consistent in both patients who underwent antiviral therapy and those who did not. These findings suggest that CHB-MAFLD patients should be given extra clinical attention, and MAFLD should be treated aggressively. The role of MAFLD in the natural course of cirrhosis and therapeutic response in CHB patients needs to be further confirmed in prospective longitudinal clinical studies with larger patient populations.
Abbreviations
CHB, chronic hepatitis B; MAFLD, metabolic dysfunction-associated fatty liver disease; HBV, hepatitis B virus; HS, hepatic steatosis; NAFLD, nonalcoholic fatty liver disease; HCC, hepatocellular carcinoma; DM, diabetes mellitus; GHE, Global Health Estimates; IPTW, inverse probability treatment weighting; HR, hazard ratio; OR, odds ratio; CI, confidence interval; IQR, interquartile range; MR, magnetic resonance; CT, computed tomography; BMI, body mass index; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; UA, uric acid; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HBeAg, hepatitis B e antigen; HOMA-IR, homeostatic model assessment of insulin resistance.
Data Sharing Statement
The datasets supporting the conclusions of the current study are available at Guangdong Provincial Hospital of Chinese Medicine, which are available from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Ethics Approval and Informed Consent
All procedures in this retrospective study were performed following the ethical standards of the institutional and national research committee and were in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Approval for these investigations was obtained from the Ethics Committee of the Guangdong Provincial Hospital of Chinese Medicine (approval number: YE2021-200-01). This study is a retrospective, observational, non-interventional study that only analyzed data from medical records from previous clinical treatment. Therefore, the Ethics Committee of the Guangdong Provincial Hospital of Chinese Medicine agreed to waive informed consent. All data was anonymized and maintained with confidentiality.
Consent for Publication
All authors agree to publish this manuscript.
Funding
The present study was funded by the Thirteen Five-Year Plan for Major and Special Program of the National Science and Technology of China (2018ZX10725506-003 and 2018ZX10725505-004); the biological resources project collaborated by Guangdong Provincial Hospital of Chinese Medicine and Shanghai chip National Engineering Center (YN2016XP03), the Science and Technology research project of Traditional Chinese Medicine of Guangdong Provincial Hospital of Chinese Medicine(YN2021DB07), and the Clinical research projects of Guangdong Provincial Hospital of Chinese Medicine (YN10101903).
Disclosure
All authors declare that they have no conflicts of interest.
References
1. Maclachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5(5):a021410. doi:10.1101/cshperspect.a021410
2. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491. doi:10.1053/j.gastro.2018.08.065
3. Liu K, Choi J, Le A, Yip TC, Wong GL. Tenofovir disoproxil fumarate reduces hepatocellular carcinoma, decompensation and death in chronic hepatitis B patients with cirrhosis. Aliment Pharmacol Ther. 2019;50(9):1037–1048. doi:10.1111/apt.15499
4. Sarin SK, Kumar M, Eslam M, et al. Liver diseases in the Asia-Pacific region: a lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. 2020;5(2):167–228.
5. Eslam M, Sanyal AJ, Mafld: GJ, Consensus-Driven Proposed A. Nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):e1991. doi:10.1053/j.gastro.2019.11.312
6. Eslam M, Newsome PN, Anstee QM, Targher G, George J. A new definition for metabolic associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
7. Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020;40(6):1254–1261. doi:10.1111/liv.14478
8. Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41(6):1290–1293. doi:10.1111/liv.14828
9. Lin S, Huang J, Wang M, Kumar R, Zhu Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver International. 2020;40(9):2082–2089. doi:10.1111/liv.14548
10. Huang J, Xue W, Wang M, MAFLD CriteriaMay YW. Overlook a subtype of patient with steatohepatitis and significant fibrosis. Diabetes Metab Syndr Obes. 2021;14:3417–3425. doi:10.2147/DMSO.S316096
11. Liu Z, Suo C, Shi O, et al. The health impact of MAFLD, a Novel Disease Cluster of NAFLD, is amplified by the integrated effect of fatty liver disease-related genetic variants. Clin Gastroenterol Hepatol. 2022;20(4):e855–e875. doi:10.1016/j.cgh.2020.12.033
12. Li H, Guo M, An Z, et al. Prevalence and risk factors of metabolic associated fatty liver disease in Xinxiang, China. Int J Environ Res Public Health. 2020;17:6.
13. Chen X, Zhou J, Wu L, Zhu X, Deng H. MAFLD is associated with the risk of liver fibrosis and inflammatory activity in HBeAg-negative CHB patients. Diabetes Metab Syndr Obes. 2022;15:673–683. doi:10.2147/DMSO.S351492
14. Yun BL, Ha Y, Chon YE, Mi NK, Hwang SG. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2018;25(1):52–64.
15. Mak L, Hui R, Fung J, et al. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol. 2020;73(4):800–806. doi:10.1016/j.jhep.2020.05.040
16. Mak LY, Seto WK, Hui RW, et al. Fibrosis evolution in chronic hepatitis B e antigen-negative patients across a 10-year interval. J Viral Hepat. 2019;26(7):818–827. doi:10.1111/jvh.13095
17. Mak LY, Hui RW, Fung J, et al. Reduced hepatic steatosis is associated with higher risk of hepatocellular carcinoma in chronic hepatitis B infection. Hepatol Int. 2021;15(4):901–911. doi:10.1007/s12072-021-10218-2
18. Li J, Yang HI, Yeh ML, Le MH, Nguyen MH. Association between fatty liver and cirrhosis, hepatocellular carcinoma, and HBsAg seroclearance in chronic hepatitis B. J Infect Dis. 2020;224(2):294–302. doi:10.1093/infdis/jiaa739
19. Chinese Society of Infectious Diseases, Chinese Medical Association. Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (2019 version). J Chin Hepatol. 2019;35(12):2648–2669.
20. Fan JG, Wei L, Zhuang H. National workshop on fatty liver and alcoholic liver disease, Chinese Society of Hepatology, Chinese Medical Association; fatty liver expert committee, Chinese medical doctor association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018. J Prac Hepatol. 2018;26(3):195–203.
21. Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis. J Chin Hepatol. 2019;35(11):846–865.
22. Tan K, Consultation WE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163.
23. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi:10.1093/aje/kwn164
24. Kim Y, Chang Y, Cho YK, Jiin A, Hocheol S, Seungho R. Obesity and weight gain are associated with progression of fibrosis in patients with non-alcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(3):e542. doi:10.1016/j.cgh.2018.07.006
25. Wong RJ, Tran T, Kaufman H, Niles J, Gish R. Increasing metabolic co-morbidities are associated with higher risk of advanced fibrosis in nonalcoholic steatohepatitis. PLoS One. 2019;14(8):e0220612. doi:10.1371/journal.pone.0220612
26. Hsiang J, Wong G, Chan H, et al. Metabolic syndrome delays HBeAg seroclearance in Chinese patients with hepatitis B. Aliment Pharmacol Ther. 2014;40(6):716–726. doi:10.1111/apt.12874
27. Seto WK, Hui RW, Mak LY, et al. Association between hepatic steatosis, measured by controlled attenuation parameter, and fibrosis burden in chronic hepatitis B. Clin Gastroenterol Hepatol. 2018;16(4):e572. doi:10.1016/j.cgh.2017.09.044
28. Natarajan Y, Kramer J, Yu X. Risk of cirrhosis and hepatocellular cancer in patients with NAFLD and normal liver enzymes. Hepatology. 2020;72(4):1242–1252. doi:10.1002/hep.31157
29. Wong S, Chan W, Mohamed R. Fatty liver is associated with advanced fibrosis but does not predict adverse outcomes in patients with chronic hepatitis B. J Viral Hepat. 2020;27(12):1297–1305. doi:10.1111/jvh.13361
30. Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40(12):3018–3030. doi:10.1111/liv.14675
31. Le AK, Yang HI, Yeh ML, Jin M, Nguyen MH. Development and validation of a risk score for liver cirrhosis prediction in untreated and treated chronic hepatitis B. J Infect Dis. 2020;223(1):139–146. doi:10.1093/infdis/jiaa330
32. Charatcharoenwitthaya P, Pongpaibul A, Kaosombatwattana U, et al. The prevalence of steatohepatitis in chronic hepatitis B patients and its impact on disease severity and treatment response. Liver Int. 2017;37(4):542–551. doi:10.1111/liv.13271
33. Mak L, Lee C, Cheung KS, Wong D, Seto W. Association of adipokines with hepatic steatosis and fibrosis in chronic hepatitis B patients on long‐term nucleoside analogue. Liver Int. 2019;39(7):1217–1225. doi:10.1111/liv.14104
34. Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(9):2672–2682. doi:10.1002/hep.30251
35. Clarembeau F, Bale G, Lanthier N. Cirrhosis and insulin resistance: current knowledge, pathophysiological mechanisms, complications and potential treatments. Clin Sci. 2020;134(16):2117–2135. doi:10.1042/CS20200022
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.