Back to Journals » Infection and Drug Resistance » Volume 16
Noninvasive Methods for Detecting Advanced Liver Fibrosis and Cirrhosis in Patients with Chronic Hepatitis B: A Single-Center Retrospective Study
Authors Cheng R, Tan N, Luo H, Kang Q, Xu X
Received 17 June 2023
Accepted for publication 14 September 2023
Published 25 September 2023 Volume 2023:16 Pages 6323—6331
DOI https://doi.org/10.2147/IDR.S426374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ran Cheng,1 Ning Tan,2 Hao Luo,2 Qian Kang,2 Xiaoyuan Xu3
1Department of Infectious Diseases, Peking University Third Hospital, Beijing, People’s Republic of China; 2Department of Infectious Diseases, Peking University First Hospital, Beijing, People’s Republic of China; 3Department of Gastroenterology, Peking University First Hospital, Beijing, People’s Republic of China
Correspondence: Xiaoyuan Xu, Department of Gastroenterology, Peking University First Hospital, 8 Xishiku Street, Beijing, 100034, People’s Republic of China, Tel +86-010-83575787, Fax +86-010-83575787, Email [email protected]
Background and Aims: The performance of noninvasive assessments to rule-in or rule-out fibrosis may improve when combined. We aimed to evaluate the efficiencies of sequential algorithms based on the aspartate aminotransferase-to-platelet ratio index (APRI), the fibrosis index based on four factors (FIB-4), and transient elastography (TE) for the assessment of advanced fibrosis (AF) and cirrhosis.
Methods: This study enrolled 179 CHB subjects who underwent liver biopsy (LB) before antiviral treatment.
Results: AF and cirrhosis were identified in 71 (39.7%) and 28 (15.7%) patients, respectively. Compared with TE alone, sequential FIB-4-TE and APRI-TE algorithms saved a slightly higher number of liver biopsies for the identification of advanced fibrosis (69.3% or 68.2% vs 63.7%, P=0.263 or P=0.372, respectively). For the identification of cirrhosis, sequential FIB-4-TE and APRI-TE algorithms saved a significantly higher number of liver biopsies than TE alone (83.2% or 88.3% vs 69.8%, P=0.003 or P=0.000, respectively). No significant difference was found between the sequential algorithms and TE alone in the diagnostic accuracy for the detection of AF and cirrhosis.
Conclusion: The sequential algorithms could significantly reduce the need for liver biopsy with high accuracy for diagnosis of AF and cirrhosis in CHB patients, which would be optimal especially in resource-limited areas.
Keywords: noninvasive assessments, advanced liver fibrosis, cirrhosis, chronic hepatitis B
Introduction
HBV infection is a public health problem worldwide. Approximately 240 million people are estimated to have persistent HBV infection, and this situation is especially serious in the Asia-Pacific region.1,2 Fibrosis stage is of great significance in the management of patients with HBV infection, not only in evaluating the occasion of antiviral treatment but also in estimating the prognosis.3 Presence of cirrhosis is the most important predictor of HCC in CHB patients.4 This urges early diagnosis of advanced fibrosis and cirrhosis so that prompt antiviral treatment can potentially reverse the fibrosis and reduce the risk of cirrhotic complications.3,5 Liver biopsy has been recognized as the gold standard for the evaluation of liver fibrosis stage.6 However, it has limitations, such as invasiveness, associated risk of complications, and occurrence of intra- and inter-observer variability.7,8 Considering these limitations, extensive resources have been recently dedicated to the development of noninvasive replacements as surrogates for liver biopsy. The use of noninvasive markers is not able to rule in or rule out fibrosis in no more than 30–40% of patients, which is considered unacceptable for many clinicians. Since the first proposal of a combination of transient elastography (TE)9 and FibroTest10 to increase diagnostic accuracy in patients with hepatitis C,11 many algorithms combining either TE and serum biomarkers or several serum biomarkers have been proposed, showing excellent diagnostic performance compared with individual models.12,13 However, similar algorithms that can be applied to CHB patients are very rare. The aim of this study was to evaluate whether sequential algorithms based on the APRI,14 FIB-415 and TE can decrease the rate of liver biopsy and maintain high diagnostic accuracy in detecting HBV-related advanced fibrosis and cirrhosis and to determine which algorithm is superior to others in terms of preventing unnecessary liver biopsy in CHB patients.
Patients and Methods
Patients
From August 2012 to December 2015, 179 consecutive patients who had been diagnosed with chronic HBV infection (HBsAg-positive >6 months) and had undergone a liver biopsy in our hospital were recruited. The inclusion criteria were: 1) age ≥16 years; 2) HBsAg-positive >6 months without having received antiviral treatment before this study; 3) a liver biopsy test; 4) routine laboratory tests and transient elastography performed within 7 days before liver biopsy; and 5) ALT ≤2×upper limit of normal (ULN) (normal range, 7.0–40.0 IU/L) and TBIL<ULN (normal range, 5.1–17.1 μmol/L). The exclusion criteria were: 1) HAV, HCV, HDV, HEV and HIV coinfection; 2) other causes of hepatitis (chronic ethanol consumption (>40 g/day), non-alcoholic steatohepatitis, autoimmune liver disease, other hepatobiliary diseases); 3) decompensated cirrhosis; 4) hepatocellular carcinoma; 5) liver transplantation or previous liver-related surgery; 6) pregnancy; and 7) terminal illness involving the major organs. The study was performed in line with the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Peking University First Hospital, Beijing, China. In addition, written informed consent from the patients or their relatives to participate in the study was obtained, and data were analyzed anonymously.
Liver Histological Analysis
Liver biopsy was performed under the guidance of ultrasound. The specimens were fixed with formalin, embedded in paraffin and stained with hematoxylin and eosin (HE). All liver specimens included at least 11 complete portal tracts.16 Liver fibrosis stage was assessed according to the Scheuer classification.17 The specimens were grouped as S0-S1 (minimal fibrosis), S2-S4 (significant fibrosis), S3-S4 (advanced fibrosis), and S4 (cirrhosis). Histological results were used as the gold standard for the evaluation of noninvasive methods.
Serum Markers and Noninvasive Models
Clinical laboratory parameters were measured within 7 days before liver biopsy. Routine laboratory tests were performed in our hospital laboratory. Serum HBV DNA levels were determined using the COBAS® TaqMan assay (Roche Diagnostics) with a detection limit of 20 IU/mL. Serology markers for HBV, including HBsAg, HBeAg and antibody to HBeAg, were measured by enzyme-linked immunosorbent assay (Abbott Laboratories, Chicago, IL, USA). Both APRI and FIB-4 were calculated based on the published formulae:
APRI=[(AST/ULN)/PLT] × 100.14
FIB-4=(Age ×AST)/(PLT×ALT½).15
The ULN of AST was 40 IU/L in our hospital.
Transient Elastography
Transient elastography was performed with a FibroScan device (Echosens, Paris, France) within 7 days before liver biopsy. A liver stiffness assessment with at least 10 valid measurements at each time, a success rate more than 60% and an interquartile range to median ratio lower than 30% was generally considered reliable.18 LSM is expressed as a median value (kilopascal; kPa).
Statistical Analysis
The data were analyzed using SPSS (version 20.0, IBM, USA) and MedCalc software (version 18.2.1, Ostend, Belgium). Statistically significant difference was set at a two-sided P-value <0.05. Descriptive results with a normal continuous distribution are provided as the mean±standard deviation (SD) and are compared by the independent sample t-test. Nonnormal distributed continuous parameters are expressed as medians (25% quantiles, 75% quantiles) and are compared using the Mann–Whitney U-test. Categorical variables are expressed as frequencies (percentages) and are compared using the χ2 test. Non-parametric Spearman rank correlation tests were applied for correlation of fibrosis stage with APRI, FIB-4, and TE.
The diagnostic performance of each noninvasive method was determined by area under the receiver operating characteristic curves (AUROCs) with a 95% confidence interval (CI). Sensitivity (Sen), Specificity (Spe), positive predictive value (PPV), negative predictive value (NPV) are expressed as percentages. Three sets of cut-offs were calculated as follows: obtain positive likelihood ratio (PLR) above or nearly 10.0 for ruling in diagnosis and negative likelihood ratio (NLR) below or nearly 0.1 for ruling out diagnosis,19,20 or maximizing Youden index (sensitivity+specificity-1). It is recommended that AUROCs should be adjusted according to the prevalence of fibrosis stages using the DANA (difference between advanced and non-advanced fibrosis).21 The adjusted AUROCs (AdjAUROC) were calculated as follows: AdjAUROC= observed AUROC (ObAUROC) + (0.1056) × (2.5-DANA).21 AUROCs comparison was performed by DeLong test.
Results
Patient Characteristics
A total of 179 eligible patients with CHB were selected for study enrollment from a total of 215 patients who underwent liver biopsy. Thirty-six patients were excluded according to the exclusion criteria (Supplementary Figure 1). Table 1 shows the characteristics of the patients recruited into the study. The baseline characteristics of the study population (116 males and 63 females) are shown in Table 1. The median age and body mass index were 34.6 years and 24.2 kg/m2, respectively. Histological fibrosis staging was as follows: S0 in 30, S1 in 33, S2 in 45, S2 in 43, and S4 in 28 patients, respectively.
 |
Table 1 Baseline Characteristics of Patients with HBV Infection |
Correlation of Noninvasive Methods with Fibrosis Stage
There was a significant positive correlation between TE and fibrosis stage (R=0.634, p < 0.001). APRI and FIB-4 were also positively correlated with fibrosis stage (R= 0.292 and 0.351, respectively, both P < 0.001).
Diagnostic Performance for Advanced Fibrosis Assessment and Cirrhosis
The AUROCs of TE were higher than those of APRI (0.884 vs 0.696, P <0.001), and FIB-4 (0.884 vs 0.707, P<0.001) to predict AF (Figure 1A). After DANA standardization, the performance of TE to predict AF was also significantly better than that of FIB-4 and APRI (both P<0.001) (Supplementary Table 1).
 |
Figure 1 ROCs of TE, APRI, and FIB-4 for AF assessment (A) and cirrhosis assessment (B). |
The diagnostic performances of TE, APRI, and FIB-4 for cirrhosis are shown in Figure 1B. Supplementary Table 1 shows the AUROCs and the adjusted AUROCs. The comparison of AUROCs revealed that TE was statistically similar to APRI, and FIB-4 in predicting cirrhosis (0.898 vs 0.820 or 0.818, P = 0.056 or P = 0.109, respectively) (Supplementary Table 1). Table 2 presents the cut-offs of APRI, FIB-4, and TE for the diagnosis of advanced fibrosis and cirrhosis.
 |
Table 2 Cut-off Values for Noninvasive Models in CHB Patients |
Sequential Combinations for Detection of Advanced Fibrosis and Cirrhosis
The sequential combinations were designed to use confirmation and exclusion by the APRI or FIB-4 as an initial screening test. When the value was indeterminate, confirmation and exclusion by TE were performed in sequence. Figures 2 and 3 describes sequential algorithms for detecting AF and cirrhosis, including cut-off values and the related decisional tree.
 |
Figure 2 Sequential FIB-4-TE algorithm (A) and sequential APRI-TE algorithm (B) for detection of AF. |
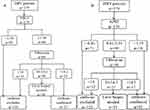 |
Figure 3 Sequential FIB-4-TE algorithm (A) and sequential APRI-TEalgorithm (B) for detection of cirrhosis. |
In detecting AF, the lowest NLR of APRI was 0.3, providing insufficient statistical evidence for ruling out diagnosis. APRI >1.33 included 29 patients with PLR 9.13. For FIB-4, the highest PLR was 4.56, providing insufficient statistical evidence for ruling in diagnosis. FIB-4 < 0.57 excluded 19 patients with NLR 0.16. While TE < 7.6 kPa excluded 57 patients with NLR 0.11, TE > 11.4kPa included 57 patients with PLR 12.93. Therefore, TE freed 104 patients from liver biopsies (Table 2).
Compared with TE alone, sequential FIB-4-TE and APRI-TE algorithms saved a slightly higher number of liver biopsies for the identification of advanced fibrosis (69.3% or 68.2% vs 63.7%, P=0.263 or P=0.372, respectively), but did not improve the PPV (Table 3). No significant differences in misclassification were observed among the TE and two-step approaches (all P > 0.05).
 |
Table 3 Performance of Algorithms for the Diagnosis of Advanced Fibrosis and Cirrhosis |
In detecting cirrhosis, while APRI < 0.41 excluded 71 patients with NLR 0.15, APRI > 1.55 included 18 patients with PLR 10.79. APRI freed 89 patients from liver biopsies. For FIB-4, the highest PLR was 7.19, providing insufficient statistical evidence for ruling in cirrhosis. FIB-4 <1.34 excluded 93 patients with NLR 0.12. TE < 10 kPa excluded 95 patients with NLR 0.11, TE > 14.2kPa included 30 patients with PLR 9.31. TE freed 125 patients from liver biopsies (Table 2).
For the identification of cirrhosis, sequential FIB-4-TE and APRI-TE algorithms correctly saved a significantly higher number of liver biopsies than TE alone (83.2% or 88.3% vs 69.8%, P=0.003 or P=0.000, respectively), at the price of a slight reduction in PPV (Table 3). The diagnostic accuracy of sequential FIB-4-TE is slightly higher than APRI-TE and TE (91.3% vs 89.2% or 89.6%, both P > 0.05).
No significant difference was found between the sequential algorithms and TE alone in the diagnostic accuracy or rate of liver biopsy required for the detection of advanced fibrosis. However, the performances of two-step approaches for predicting cirrhosis are excellent, avoiding more than 80% of liver biopsies. The diagnostic accuracy and the rate of liver biopsy saved showed no statistically significant difference between the sequential FIB-4-TE and APRI-TE algorithms in detecting AF and cirrhosis. More importantly, the two-step approaches can further avoid approximately half of TE scans. Thus, the sequential algorithms may be recommended as an optimal strategy for the assessment of liver cirrhosis.
Discussion
Staging of liver fibrosis has always been considered of great importance for antiviral treatment in patients with CHB.3 In 2015, the World Health Organization recommend the use of APRI and FIB-4 as noninvasive tools to predict significant fibrosis and cirrhosis in resource-limited areas.22 Both FIB-4 and APRI have been validated in patients with HBV infection. In the present study, we compared the performance of the three noninvasive models to predict advanced fibrosis and cirrhosis in a consecutive series of treatment-naive CHB patients with normal and mildly elevated ALT levels. TE was found to have a strong correlation with fibrosis stage, while FIB-4 and APRI had a moderate correlation with the fibrosis stage. TE shows more excellent performance than serum models for detecting severe fibrosis in our study.
Recent studies have reported that combinations of algorithms reached higher diagnostic accuracy in detecting fibrosis than their separate use.23,24 To determine a practical diagnostic cutoff value, a dual cutoff strategy established by likelihood ratio analysis was used.25 Using the FIB-4 or APRI in the initial screening step followed by TE, liver biopsy could be avoided in 69.3% or 68.2% of patients with AF and in 83.2% or 88.3% of patients with cirrhosis, respectively. The performances are similar to those reported in published literature.23,24 According to the results, the diagnostic performance of both stepwise algorithms showed no statistically significant difference in detecting advanced fibrosis and cirrhosis. When applied to advanced fibrosis, both sequential algorithms saved a slightly higher rate of liver biopsy with slight reduction in diagnostic accuracy. Compared to TE alone, the two stepwise algorithms showed excellent diagnostic performance in detecting cirrhosis, notably reducing the need for liver biopsy with high diagnostic accuracy. These findings indicate that APRI and FIB-4, easy to use and inexpensive, may be used for preliminary evaluation, and TE scan could be used for further confirmation.
Moreover, approximately half of TE scans can be avoided when using the stepwise algorithms to detect cirrhosis, which can further reduce the patient’s cost. TE is relatively expensive, and specialized technicians are needed, making TE difficult to perform in low-income countries. In contrast, no extra costs are considered for APRI and FIB-4, as patients with CHB usually undergo routine blood tests during their follow-up. For the purpose of the cost–benefit analysis, the sequential strategy may potentially lead to cost savings.
The limitations of this study should be acknowledged. First, this study was single-centered and retrospective. The number of patients enrolled was small. Inevitably, bias could arise from missing data and selection criteria. Thus, the results should be validated in prospective multicenter studies using larger sample sizes. Second, the current analysis did not present a validation cohort to confirm the validity of the proposed stepwise combination algorithm.
Conclusion
The sequential algorithms could significantly reduce the need for liver biopsy with high accuracy for diagnosis of AF and cirrhosis in CHB patients, which would be optimal especially in resource-limited areas. However, further prospective studies are required before this algorithm can be used in clinics.
Abbreviations
AF, advanced fibrosis; ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet ratio index; AST, aspartate aminotransferase; AUROC, area under the receiver-operating characteristic curve; BMI, body mass index; CHB, chronic hepatitis B; CHC, chronic hepatitis C; CI, confidence interval; DNA, deoxyribonucleic acid; FIB-4, fibrosis index based on the four factors; HAV, Hepatitis A virus; HBV, Hepatitis B virus; HBeAg, hepatitis B envelope antigen; HBsAg, HBV surface antigen; HCV, Hepatitis C virus; HCC, hepatocellular carcinoma; HDV, Hepatitis D virus; HEV, Hepatitis E virus; HIV, human immunodeficiency virus; LSM, liver stiffness measurement; LB, liver biopsy; TBIL, total bilirubin; TE, transient elastography; ULN, upper limit of normal; WHO, World Health Organization.
Acknowledgments
We thank all the physicians, pathologists and technicians whose work contributed to this study.
Funding
The National Science and Technology Major Project for Infectious Diseases (No. 2017ZX10302201, No. 2017ZX10203202); The National Science and Technology Major Special Project for New Drug Development (No.2018ZX09201016); Beijing Municipal Science and Technology Commission of Major Projects (No. D161100002716002, No. D161100002716003, No. D161100003117005).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi:10.1016/S0140-6736(15)61412-X
2. Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. doi:10.1016/j.vaccine.2011.12.116
3. Lampertico P, Agarwal K, Berg T. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:10.1016/j.jhep.2017.03.021
4. Wong VW, Chan SL, Mo F, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28(10):1660–1665. doi:10.1200/JCO.2009.26.2675
5. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi:10.1002/hep.29800
6. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344(7):495–500. doi:10.1056/NEJM200102153440706
7. Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134(6):1670–1681. doi:10.1053/j.gastro.2008.03.001
8. Shackel NA, McCaughan GW. Liver biopsy: is it still relevant? Intern Med J. 2006;36(11):689–691. doi:10.1111/j.1445-5994.2006.01210.x
9. Yoshioka K, Kawabe N, Hashimoto S. Transient elastography: applications and limitations. Hepatol Res. 2008;38(11):1063–1068. doi:10.1111/j.1872-034X.2008.00386.x
10. Halfon P, Munteanu M, Poynard T. FibroTest-ActiTest as a non-invasive marker of liver fibrosis. Gastroenterol Clin Biol. 2008;32(6 Suppl 1):22–39. doi:10.1016/S0399-8320(08)73991-5
11. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–350. doi:10.1053/j.gastro.2004.11.018
12. Leroy V, Hilleret MN, Sturm N, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46(5):775–782. doi:10.1016/j.jhep.2006.12.013
13. Bourliere M, Penaranda G, Renou C, et al. Validation and comparison of indexes for fibrosis and cirrhosis prediction in chronic hepatitis C patients: proposal for a pragmatic approach classification without liver biopsies. J Viral Hepat. 2006;13(10):659–670. doi:10.1111/j.1365-2893.2006.00736.x
14. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526. doi:10.1053/jhep.2003.50346
15. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi:10.1002/hep.21178
16. Guido M, Rugge M. Liver fibrosis: natural history may be affected by the biopsy sample. Gut. 2004;53(12):1878.
17. Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13(3):372–374. doi:10.1016/0168-8278(91)90084-O
18. Kim SU, Jang HW, Cheong JY, et al. The usefulness of liver stiffness measurement using FibroScan in chronic hepatitis C in South Korea: a multicenter, prospective study. J Gastroenterol Hepatol. 2011;26(1):171–178. doi:10.1111/j.1440-1746.2010.06385.x
19. Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006;12(23):3682–3694. doi:10.3748/wjg.v12.i23.3682
20. Guha I, Rosenberg W. Noninvasive assessment of liver fibrosis: serum markers, imaging, and other modalities. Clin Liver Dis. 2008;12(4):883–900, x. doi:10.1016/j.cld.2008.07.010
21. Poynard T, Halfon P, Castera L, et al. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem. 2007;53(9):1615–1622. doi:10.1373/clinchem.2007.085795
22. WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva: World Health Organization Copyright © World Health Organization; 2015.
23. Wong GL, Wong VW, Choi PC, Chan AW, Chan HL. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther. 2010;31(10):1095–1103. doi:10.1111/j.1365-2036.2010.04276.x
24. Boursier J, de Ledinghen V, Zarski JP, et al. A new combination of blood test and fibroscan for accurate non-invasive diagnosis of liver fibrosis stages in chronic hepatitis C. Am J Gastroenterol. 2011;106(7):1255–1263. doi:10.1038/ajg.2011.100
25. Chen YP, Peng J, Hou JL. Non-invasive assessment of liver fibrosis in patients with chronic hepatitis B. Hepatol Int. 2013;7(2):356–368. doi:10.1007/s12072-013-9439-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
