Back to Journals » Journal of Inflammation Research » Volume 15
A Novel Gene Signature Associated with Inflammatory Responses and Immune Status Assists in Prognosis and Intervention for Patients with HCC
Authors Lu G, Du R , Feng B, Wang J , Zhang F, Pei J, Wang Y , Shang Y
Received 23 September 2022
Accepted for publication 5 December 2022
Published 13 December 2022 Volume 2022:15 Pages 6729—6743
DOI https://doi.org/10.2147/JIR.S390113
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Guofang Lu,1,2,* Rui Du,3,* Bin Feng,1,4 Jianlin Wang,3 Fengrui Zhang,5 Jianming Pei,2 Yuanyong Wang,6,* Yulong Shang1
1State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, People’s Republic of China; 2Department of Physiology and Pathophysiology, National Key Discipline of Cell Biology, Fourth Military Medical University, Xi’an, People’s Republic of China; 3Institute for Biomedical Sciences of Pain, Tangdu Hospital, Fourth Military Medical University, Xi’an, People’s Republic of China; 4Department of Radiation Oncology, Xijing Hospital, Fourth Military Medical University, Xi’an, People’s Republic of China; 5CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, People’s Republic of China; 6Department of Thoracic Surgery, Tangdu Hospital, Fourth Military Medical University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yulong Shang; Jianming Pei, Tel +86 29 84771506, Fax +86 29 82539041, Email [email protected]; [email protected]
Background: Tumor growth depends on tumor cells and the tumor microenvironment, which are regulated by inflammation and immune responses. However, the roles of inflammation and immune status in hepatocellular carcinoma (HCC) remain unclear. The aim of this study was to evaluate the prognostic value of an inflammatory response- related gene signature associated with immune status, which may provide insight into new treatment options for HCC patients.
Materials and Methods: Differentially expressed genes associated with inflammation were obtained from The Cancer Genome Atlas (TCGA), the Gene Expression Omnibus, and the Molecular Signatures Database. An inflammation-associated prognostic gene signature was constructed and validated using TCGA and the International Cancer Genome Consortium datasets, respectively, using LASSO Cox regression analysis. Log-rank was performed to compare the overall survival of low- and high-risk score cohorts. Immune cell infiltration and immune-related functions were analyzed using single-sample gene enrichment analysis. The structures of the drugs identified by the prognostic model were predicted using PubChem. The drugs sensitivity of bleomycin, simvastatin and zoledronate detected by CCK8 colorimetric assay. The mRNA levels of 7 genes in HCC after drug treatment analyzed via qRT-PCR.
Results: Inflammation-associated genes, including ITGA5, MEP1A, P2RX4, RIPK2, SLC7A1 and SRI, were identified and found to be associated with the prognosis of HCC. We further found that the high-risk patients experienced poor prognosis, which was observed to be an independent and significant risk factor for prognosis. Moreover, we observed elevated expression levels in multiple immune cell types and immune function. Lastly, we validated that bleomycin, simvastatin and zoledronate could regulate these genes in HCC.
Conclusion: The inflammatory-response-associated gene signature could predict the prognosis and the immunological status of HCC patients. Additionally, bleomycin, simvastatin and zoledronate may represent potential drug candidates that could inhibit these genes. This may constitute a new approach for the treatment of HCC.
Keywords: inflammation-associated gene, prognostic gene signature, immune infiltration, immune checkpoint, hepatocellular carcinoma, drug structure
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancer types in humans, killing millions of people worldwide each year; in some African and Asian countries, HCC has become a major cause of cancer death.1 The incidence of HCC in China accounts for about one-half of the world’s total, and most patients are already in the late stage of the disease when they are clinically diagnosed, resulting in a 5-year overall survival (OS) rate of only about 14.1%.2 Although a small number of patients can receive radical surgery, the recurrence rate is as high as 70% five years after intervention. Despite the great strides in diagnosis and treatment, HCC remains a highly lethal tumor due to metastasis and recurrence.
In recent years, several studies have examined the link between inflammation and cancer. In 1863, the German pathologist Rudolph Virchow proposed that tumors originated from sites of chronic inflammation, and that tissue damage and subsequent inflammation were important causes of tumor formation.3 Current research shows that, under normal conditions, inflammation is usually self-limited, but abnormalities in any of the intermediate links can lead to damage to cellular DNA and impaired cell proliferation, ultimately leading to tumorigenesis.4,5 Previous studies in our laboratory have demonstrated how pro-inflammatory signaling pathways promote gastric cancer cell proliferation and metastasis.6,7 Bu et al also found a relationship between chronic intestinal inflammation and colon cancer.8 Moreover, chronic liver injury can lead to chronic hepatitis and liver cell death, further promoting hepatic injury, cirrhosis and HCC, suggesting that a chronic inflammatory state may be required for the development and progression of HCC.9 Tumor growth depends not only on the genetic changes of malignant tumor cells, but also on alterations of the tumor microenvironment (TME) including stroma, blood vessels, and tumor-infiltrating cells. Immunity and inflammation constitute the two core elements of the TME.10,11 Innate and adaptive immunity play important roles in tumorigenesis, progression and metastasis.12 Inflammation not only promotes an immune response, but can also lead to immunosuppression.13 However, the key inflammatory mechanisms leading to the development of HCC are still poorly understood. Therefore, characterizing the inflammatory mechanisms involved in HCC and targeting inflammation may become important for the treatment of HCC.
The combination of Atezolizumab with Bevacizumab (Atezo-Bev) is currently the first-choice first-line treatment, as it confers a superior survival benefit compared to Sorafenib.14,15 Whereas the clinical success of chemotherapy with HCC patients is limited. Previous studies have also described the development of various immune checkpoint inhibitors, such as anti-programmed cell death 1 (PD-1) and programmed cell death-ligand 1 (PD-L1), which have now entered clinical practice. Immunotherapy targets immune cells to enhance the anti-tumor immune response and improve the objective response rate to the treatment.16 Anti-PD-1 therapy in particular has proven successful for the treatment of HCC.17 Indeed, the effective rate of anti-PD-1 alone in the treatment of HCC is 15%.18 These study reminder us that immune checkpoint blockade immunotherapy and related combination therapies are the trends in the treatment of advanced hepatocellular carcinoma.
In the current study, we identified inflammation-associated differentially expressed genes using The Cancer Genome Atlas (TCGA), the Gene Expression Omnibus (GEO), and the Molecular Signatures Database. We then constructed and validated an inflammation-associated prognostic gene signature in TCGA and International Cancer Genome Consortium (ICGC) datasets. Furthermore, we analyzed the relationship between the inflammation-associated prognostic gene signature and the tumor immune microenvironment, including immune cell infiltration, immune-related functions, immune infiltration subtypes and immune checkpoint molecules. We also predicted three drugs using the prognostic gene signature and verified the expression levels of seven genes using reverse transcription-PCR. The results highlighted the combined role of inflammatory responses and immune status in prognosis and identified a new approach for the clinical management of HCC.
Materials and Methods
Data Preparation
RNA sequencing data and clinical characteristics from HCC patients were downloaded from TCGA (http://www.cancer.gov/tcga), GEO (http://www.ncbi.nlm.nih.gov/geo/) and ICGC (https://icgc.org/). The inflammation-associated genes were found in the Molecular Signatures Database. All data used in this study are freely available to the public. The “limma” R package was used in TCGA and ICGC datasets to identify differently expressed genes (DEGs) in HCC tissue and adjacent non-tumor tissue using a false discovery rate <0.05 and a fold change (FC) >2. In the GSE87630 database, DEGs in HCC and adjacent non-tumor tissue were identified using GEO2R with log2|FC| ≥ 0.5 and adjusted P-value ≤ 0.05. All DEGs were analyzed GraphPad Prism 8 software.
Identification and Validation of the Inflammation-Associated Prognostic Gene Signature
The prognostic significance of inflammatory response-associated genes was analyzed using Univariate Cox regression analysis. The prognostic gene signature was built using LASSO-penalized Cox analysis.19 A tenfold cross-validation was used to evaluate the penalty parameter (λ) of the prognostic gene signature. To determine the risk scores, we analyzed the levels of seven inflammation-associated genes and the matching regression coefficient. The score was defined as: esum (expression of each gene × corresponding coefficient). According to the median risk score of the cohort, the patients were divided into low- and high-risk groups. To examine gene expression levels in these two groups, t-Distributed Stochastic Neighbor Embedding (t-SNE) and Principal Component Analysis (PCA) were carried out using the “Rtsne” and “ggplot2” R packages. The OS analysis of the low- and high-risk cohorts were performed using “survminer”. The predictive performance of the signature was assessed using the time-dependent ROC curve analysis. Additionally, multivariate and univariate Cox regression analyses were carried out to assess the prognostic value of the gene signature for HCC patients. All data were made using R software (Version 3.6.1).
Tumor Immune Status Analysis
The levels of immune cell infiltration and stromal cells were measured using immune and stromal scores by cell-type identification by estimating relative subsets of RNA transcript (CIBERSORT) web portal (https://cibersort.stanford.edu/). Spearman correlation was used to analyze the relationship between the different risk scores and the stromal or immune scores. The relationship between the different risk scores and the subtype of immune infiltration was analyzed using a two-way ANOVA.
Predicted of Drug Structure
We predicted 121 potential molecular drugs targeting the ITGA5, MEP1A, P2RX4, RIPK2, SERPINE1, SLC7A1 and SRI genes from TCGA. We identified three potential target drugs through the correlation analysis and published article review. The two-dimensional chemical structure images of bleomycin, simvastatin and zoledronate were obtained from PubChem (pubchem.ncbi.nlm.nih.gov). The 121 molecular drugs are listed in Supplementary Table 1.
Cell Line
Human HL7701 cell, HCC cell Huh-1 and Hep3b were obtained from China Infrastructure of Cell Line Resources and Stem Cell Bank, Chinese Academy of Sciences. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) at 37°C in a 5% CO2 incubator. The medium was supplemented with 10% FBS (South America origin; IC-1905; BioCytoSci, TX, USA), and 1% penicillin- streptomycin.
RNA Extraction and Quantitative Reverse Transcription PCR (qRT-PCR)
Total RNA from Huh-1 and Hep3b cells and normal liver cells were extracted using TRIzol (Invitrogen, USA) according to the manufacturer’s instructions as described previously.20 Reverse transcription was performed using the Advantage RT-for-PCR Kit (Takara Bio). The RT-PCR was conducted using SYBR Premix Pro Taq HS qPCR kit (Accurate Biotechnology, Hunan, China) on a CFX96™ Real-Time PCR Detection system (Bio-Rad Laboratories, USA). The primer sequences are listed in Supplementary Table 2. The 2−ΔΔCt method was used to analyze the relative expression levels.
Statistical Analysis
The Wilcoxon test was used to analyze the DEGs in tumor and adjacent tissues. Categorical data were analyzed using the χ2 test. Mann–Whitney’s U-test was used to compare the ssGSEA scores of immune cells or immunological pathways in different cohorts. The Log rank test was to analyze the differences in OS. Multivariate and univariate analysis was carried out to comprehensive analyze the prognostic risk factors of HCC based on the clinical baseline factors. The R software (version 3.6.1) and other tools, including “survminer”, “corrplot”, “venn”, “ggplot2”, “pheatmap”, “igraph”, and “ggpubr” were used to generate the graphs.
Results
Identification and Construction a Prognostic Gene Signature Using TCGA Cohorts
To evaluate the role of inflammation-associated genes in HCC, we first identified DEGs from TCGA and GSE87630 in HCC (the clinicopathological characteristics of the patients were presented in Table 1), and 200 inflammatory response- related genes were identified from the Molecular Signatures Database (Supplementary Table 3). Next, we obtained 49 inflammation-associated DEGs that were shared between the three databases (Figure 1A). We then evaluated functional enrichment in these 49 genes using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation. As shown in Figure S1A and B, the datasets were enriched with four functional categories: GO biological process (BP), GO molecular function (MF), GO cellular component (CC), and KEGG pathway (KEGG).
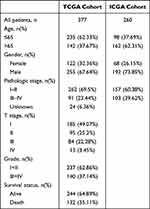 |
Table 1 Clinicopathological Characteristics in HCC from TCGA and ICGC Database |
Using univariate Cox analysis, 12 of the aforementioned genes were associated with OS in patients with HCC (Figure 1B). The hazard ratio for the OS of the patients with high 12 inflammation-related DEGs is shown in Figure 1C. The analysis demonstrated that high gene expression of the following genes was an independent risk factor for HCC: HRH1 [1.623 (1.089–2.419), p-value = 0.017], ADORA2B [1.517 (1.146–2.008), p-value = 0.004] and P2RX4 [1.455 (1.142–1.855, p-value = 0.002]. Furthermore, we analyzed the interaction between these 12 inflammation-related genes using STRING and found that 11 genes exhibited strong positive significant correlation each other (Figure 1D).
The prognostic gene signature of 12 genes was assessed in TCGA (training) cohort using the penalized LASSO Cox PH regression (GLMnet R Package) in order to investigate the linear relationship among different genes. The graph for all combinations of λ values is shown in Figure S2A and B. The results demonstrated that the λ values at seven-gene maker was the optimal value. The risk score was calculated as follows: expression level of ITGA5 * 0.067+expression level of MEP1A * 0.093+expression level of P2RX4 * 0.141+expression level of RIPK2 * 0.163+expression level of SERPINE1 * 0.040+expression level of SLC7A1 * 0.121+expression level of SRI * 0.164. The patients were divided into a high- and a low-risk group according to the median value of the groups. The correlation between gene expression and clinicopathological characteristics of HCC in the TCGA and ICGC cohorts were presented in Table 1. To compare the prognostic gene signature between the high- and low-risk groups, a scatter plot was generated. The results revealed that high-risk patients with HCC had an increased likelihood of dying earlier compared to those in the low-risk groups (Figure 2A). Moreover, we analyzed the two risk groups using PCA and t-SNE, and the data points were found to be clearly separated in the PCA and t-SNE analysis (Figure 2C). To evaluate the effect of these genes on HCC prognosis, we then examined the relationship between their expression levels and OS in TCGA cohort (Figure S3). In addition, Kaplan-Meier survival analysis indicated that high expression of these 7 genes (ITGA5, MEP1A, P2RX4, RIPK2, SERPINE1, SLC7A1 and SRI) was associated with poor prognosis in HCC patients (Figure 2E). Moreover, we used a time-dependent ROC curve and AUC to assess the prognostic performance of the gene signature. For the prognostic gene signature, the AUCs at 1, 2 and 3 years were 0.673, 0.627 or 0.607, respectively (Figure 2F).
Validation of the Seven-Gene Gene Signature in HCC Using the ICGC Database
The robustness of the association between the expression of the 7 genes and prognosis in HCC was further validated in another cohort (ICGC cohort) (Figure 2B, D, G–H). The ICGC datasets were also divided into a high- and a low-risk group based on the median of the dataset. The result of the scatter plot suggested that high-risk patients in HCC had an increased likelihood of dying earlier, compared with the low-risk group (Figure 2B). The results of PCA and t-SNE are shown in Figure 2D, in which unsupervised clustering accurately separated the samples according to the expression levels. There was a similar trend for OS in HCC from ICGC datasets, as high expression of all seven genes was associated with shorter OS (Figure 2G). For the seven-gene time-dependent ROC analysis, the AUCs at 1, 2 or 3 years were 0.667, 0.656 and 0.681, respectively (Figure 2H).
Prognostic Value of the Seven-Gene Gene Signature in HCC in TCGA and ICGC Cohorts
We performed univariate and multivariate Cox regression analysis of parameters potentially associated with OS in HCC in order to determine whether the risk score could be an independent predictive indicator. First, univariate Cox regression analysis was carried out on age, gender, grade (only TCGA cohort) and stage. The results demonstrated that these factors are positive prognostic factors informing HCC patient OS. In addition, the hazard ratio of the risk scores in TCGA cohort was 3.237 (95% CI = 2.035–5.183, p < 0.001) (Figure 3A). Subsequently, HCC stage was evaluated using multivariate Cox regression analysis, and the results suggested that this parameter could be an independent predictive factor for OS (HR = 2.721, 95% CI = 1.653–4.364, p < 0.001) (Figure 3B). Moreover, we also validated the role of relevant factors for OS in ICGC cohorts. The results also identified stage as an independent prognostic indicator using univariate (4.481, 95% CI = 2.086–9.588, p < 0.001) and multivariate (3.599, 95% CI = 1.455–6.902) Cox regression analysis (Figure 3C–D). These results identified the prognostic value of tumor stage phase for HCC patient OS.
Next, we analyzed the association between age, sex, grade and stage and OS for HCC patients. Results demonstrated that patients aged ≤ 65 years had significantly higher risk scores than patients aged > 65 years (p = 0.019; Figure 3E1). There was no difference in risk score between age groups (> 65 or ≤ 65, p = 0.68) (Figure 3E2) or sex (female or male) in TCGA (p = 0.067) and ICGC (p = 0.21) datasets, respectively (Figure 3F1 and F2). However, a significant difference in risk score was observed between patients with stage-I/II tumors and patients with stage-III/IV tumors, both in TCGA (p = 0.0051) and ICGC datasets (p = 0.00026) (Figure 3H1 and H2). Additionally, the difference in risk score in grade-1/2 vs grade-3/4 patients was significant in TCGA dataset (p = 1.5e-05) (Figure 3G). Altogether, this suggested that tumor stage and high risk scores in patients with stage-III/IV tumors may be a prognostic factor for HCC patients.
Immune Status and Tumor Immune Microenvironment of Different Risk Groups from TCGA Datasets
Enrichment scores from ssGSEA represent the infiltration levels of 16 immune cell types and 13 immunological functions from TCGA and ICGC cohorts, which was used to assess the relationship between 2 risk groups (Figure 2A) and immune status in HCC. The expression scores of classic dendritic cells (aDCs), CD8+ T-cells (ICGC only), inflammatory dendritic cell (iDCs), macrophages, mast cells (TCGA only), neutrophils, plasaytid dendritic cells (pDCs), T follicular helper cells (Tfh cells), Th1-cells, Th2-cells, Tumor infiltrating Lymphocytes (TILs) and Tregs in the high-risk group were significantly higher than those in the low-risk group, in both TCGA and ICGC datasets (Figure 4A and B). For immune-related functions, the enrichment scores for APC co-inhibition, APC co-stimulation, Chemokine receptor (CCR), Checkpoint, Human Leukocyte Antigen (HLA), Major Histocompatibility Complex (MHC) class-1, Parainflammation, T-cell co-inhibition, T-cell co-stimulation and Type II IFN response in the high-risk were greater than those in the low-risk group (Figure 4C and D). The data suggested immunological activation.
Subsequently, as immune infiltration level is associated with OS and prognosis in tumors, we investigated the link between immune infiltrates and risk scores. Immune cell infiltration is commonly seen in many types of tumors, including HCC, and its prognostic value has been reported.21 Here, we analyzed four types of immune infiltrate, including C1 (wound healing), C2 (IFN-γ dominant), C3 (inflammatory) and C4 (lymphocyte-depleted) in HCC from TCGA database. The findings revealed that a high-risk score was linked to C1, whereas a reduced risk score was linked to C4 (Figure 4E). Moreover, using TCGA data, we analyzed the correlation between the RNA stemness score (RNAss), which is premised on mRNA expression, the DNA stemness score (DNAss), which is premised on DNA methylation pattern, can both be used to test tumor stemness.22 We also analyzed the immune and stromal score, which could affect the status of tumor immune microenvironment and the risk score. The results suggested that the risk score negatively correlated with DNAss (r = −0.23, p = 1.2e-05), indicating that a high-risk score might be associated with reduced stemness. In addition, the risk score positively correlated with the immune score (r = 0.25, p = 1.5e-06), suggesting that a high-risk score might promote the stemness property in TAMs. However, there were no significant correlations between RNAss (r = 0.06, p = 0.25) and stromal score (r = 0.082, p = 0.12) with risk score (Figure 4F).
The PD-1/PD-L1 and PD-2/PD-L2 axis play a pivotal role in the progression of tumors by regulating immune surveillance. In the current study, we investigated the expression of the immune checkpoint molecules PD-L1/2, B7-1 (known as CD80) and B7-2 (known as CD86) in the high- and low-risk groups. The results showed that the expression levels of these four checkpoint molecules in the high-risk group were significantly increased compared with those in low-risk group (p < 0.05) (Figure 5A–D). And expression of these 4 check molecules had a positive correlation with risk scores, (p < 0.01) (Figure 5E–H). Thus, the risk factor may be associated with immune cell infiltration, immune-related functions and status of tumor immune microenvironment.
Prediction of the Effects of Targeted Drugs on the Seven Genes in HCC
Molecular targeted drugs are the first choice for systemic treatment of HCC.23 We identified that seven inflammation-related DEGs could serve critical role in inflammatory and immune microenvironment in HCC. Thus, we next sought to identify the potential key tumor targeted drugs that could inhibit the expression of these seven genes using public datasets. First, we predicted 121 potential molecular drugs targeting the ITGA5, MEP1A, P2RX4, SERPINE1, SLC7A1 and SRI genes in TCGA (Table S2), then identified the top three drugs as: Bleomycin, simvastatin and zoledronate (p < 0.01). We then carefully read and studied existing research, to verify the effectiveness of these drugs in HCC. Figure 6A presents detailed information on these three drugs, including their structure, molecular weight, effects and references. In addition, two-dimensional chemical structure images of all three drugs were generated using PubChem (Figure 6B). Moreover, we verified the drug sensitivity of Bleomycin, Simvastatin and Zoledronate drugs in HCC Huh-1 and Hep3b cells in vitro analysis (Figure S4).
Lastly, the mRNA expression levels of all 7 genes in Huh-1 and Hep3b cells and in HL7702, a normal liver cell line, were verified using RT–PCR. The expression levels of ITGA5, MEP1A, P2RX4, RIPK2, SLC7A1 and SRI in the Huh-1 and Hep3b cells cell line were significantly higher than in the HL7702 cell line (p < 0.05) (Figure 7). Moreover, the downregulated expression of ITGA5, SERPINE1 and P2RX4 were observed after Bleomycin treatment, the downregulated level of ITGA5, SERPINE1, SRI and SLC7A1 were determined by Simvastatin treatment, and the downregulated expression of ITGA5, SERPINE1, RIPK2 and MEP1A were observed after Zoledronate (Figure S5).
Discussion
HCC is the fifth most prevalent cancer worldwide and the third most common cause of cancer mortality.24 Most patients with HCC are in either sub-Saharan Africa or in Eastern Asia. In China, HCC accounts for more than 50% of global cases (age-standardized incidence rate: men, 35.2/100,000; women, 13.3/100,000).1 Although the key mechanisms leading to the development of HCC are still unclear, one possible explanation for hepatocarcinogenesis is cirrhosis induced by inflammatory responses, as hepatitis causes alterations of the microenvironment including altered cytokine secretion from activated stellate cells and inflammatory signaling from infiltrating immune cells, which can influence the progression of HCC.1,25 Moreover, recent studies have shown that several types of tumors come from infected and chronically stimulated inflammatory sites, and the tumor microenvironment, which is composed of inflammatory cells, is an important factor leading to tumorigenesis. In other terms, long-term chronic inflammation increases the risk of developing tumors and promotes tumorigenesis.26,27 In addition, the combination of inflammatory responses and immune status plays an important role in HCC. Previous studies have reported that inflammation-associated genes risk signature can be used to predict patient prognosis and guide treatment for esophageal squamous cell carcinoma, lung adenocarcinoma and urothelial bladder cancer.28–31 Other hand, Sia et al determined that inflammation response- related gene signature could be correlated with the immune exhausted.32 Many cancers are characterized by simultaneous immunosuppression and inflammation, their regulation is integrally linked, which can induce a dysfunctional immune state unable to eliminate disease. One possibility limit excessive immunopathology while maintaining some level of immunological control.33 In the current study, we first constructed and validated a prognostic gene signature consisting of inflammation-associated DEGs, namely ITGA5, MEP1A, P2RX4, RIPK2, SERP1NE1, SLC7A1 and SRI, which could be used as an independent risk factor to predict the prognosis of HCC patients.
Integrins are transmembrane proteins that regulate communication between cells and the extracellular matrix. Integrin α5 (ITGA5), a member of the integrin family, primarily interacts with the integrin β1 subunit to form an α5β1 heterodimer that recognizes its specific ligand fibronectin.34 Increasing evidence demonstrates that ITGA5 is strongly associated with poor prognosis, and its expression level is significantly associated with tumor purity and infiltration levels of different immune cells in gastrointestinal and HCC tumors.35,36 MEP1A belongs to the metzincin superfamily and can cleave a wide variety of substrates, including membrane proteins, cytokines and protein kinases.37 A previous study has suggested that MEP1A promotes HCC cell proliferation, migration, and invasion by regulating cytoskeletal events and induces epithelial-to-mesenchymal transition in HCC cells.38 P2RX2 is an ion channel purinoreceptor for adenosine triphosphate associated with cellular stress and inflammation and has been reported as a potential diagnostic and prognostic marker.39 The amino acid transporter SLC7A1 is significantly associated with the prognosis of HCC patients40 and impaired T cell differentiation through the modulation of mTORC1 signaling.41 SRI is a soluble resistance-related calcium-binding protein. A previous study has demonstrated that the interaction between ANXA7 and SRI regulates epithelial-to-mesenchymal transition, migration, invasion, and proliferation in HCC cells.42
Our results determined that these genes, alone or in combination, may be independent risk factors for the progression of HCC. Tumors are complex environments, composed of transformed cells as well as stromal and immune infiltrates. In this study, we found that the scores of aDCs, iDCs, macrophages, neutrophils, pDCs, Tfh cells, Th1-cells, Th2-cells, TILs and Treg in the high-risk group was significantly higher than those of the low-risk group. In addition, APCs, T cells, HLA and type-II IFN responses were activated. The results suggested that immune cell infiltration and activated immunological functions in the high-risk group could result in poor prognosis.
Immunotherapy targets immune cells in order to enhance the anti-tumor immune response. The rapid development of immunotherapy has opened up new avenues for tumor treatment and has achieved outstanding results in the treatment of HCC. The successive approval of single-drug and combination therapy has also brought more options for patients with advanced HCC.15,43 Checkpoint inhibitors, such as, durvalumab (MEDI4736) and atezolizumab (MPDL3280A) directed at the PD-1/PD-L1 axis are now approved for the treatment of multiple cancers, including HCC.44–46 Previous study has determined that Anti-PD-L1, Anti- B7-1 (CD80) and B7-2 (CD86) checkpoint has been an innovative immunotherapeutic strategy.47,48 In our study, we also found that expression levels of the PD-L1/2, B7-1 (CD80) and B7-2 (CD86) checkpoint molecules in the high-risk group were significantly increased compared with those in the low-risk group. This suggests that inhibition of these four immune checkpoint molecules can suppress the expression level of 7 gene signature, further inhibition of HCC progression.
Previous study by Lee et al reported longer progression-free survival following combination treatment with atezolizumab and bevacizumab, compared with atezolizumab alone, in patients with unresectable HCC not previously treated with systemic therapy.49 Another study also determined that lenvatinib plus pembrolizumab treatment resulted in effective antitumor activity in HCC.43 Thus, combined immunotherapy may exert a therapeutic effect in HCC. Although immunotherapy has limited value for patients with late-stage HCC, patients with early and mid-stage HCC can benefit from combined immunotherapy. Using publicly available datasets, we identified that bleomycin,50 simvastatin51 and zoledronate52 were potential key tumor-targeting drugs that can suppress the expression of seven genes in HCC. Bleomycins are a family of compounds produced by Streptomyces verticillus that have potent tumor-killing properties and thus occupy an important place in cancer chemotherapy.53 Bleomycin-induced upregulation of BCLXL/L1 and MDM2 suggests that the ratio of pro-apoptotic to anti-apoptotic proteins regulates the responsiveness of HCC cells to chemotherapy; that is, treatment sensitivity or drug resistance.54 Simvastatin is a cholesterol-reducing drug that has been reported to inhibit the activity of hydroxymethylglutaryl coenzyme A reductase and the HIF-1α/PPAR-γ/PKM2 axis by suppressing PKM2-mediated glycolysis, resulting in decreased proliferation and increased apoptosis in HCC cells and re-sensitizing these cells to sorafenib. Additionally, sorafenib and simvastatin co-treatment can improve sorafenib resistance in HCC.55,56 Zoledronate is a bisphosphonate reported to be effective for the treatment of bone metastasis in patients with various cancers, including HCC. One possible mechanism is that zoledronate delays both the development and severity of pain associated with bone metastasis in patients with HCC and has a direct effect on HCC cells.57 Our findings suggest that bleomycin, simvastatin and zoledronate can inhibit the expression levels of the genes identified using the inflammation-associated prognostic gene signature and may represent therapeutic options for HCC patients.
Conclusion
In summary, the results of this study indicate that the inflammation-related gene signature could predict OS for HCC patients. The analysis of the relationship between the inflammation-associated prognostic gene signature and the tumor immune microenvironment, including immune cell infiltration, immune-related functions, immune infiltration subtypes and immune checkpoint molecules, provides new insight for HCC immunotherapy. Moreover, bleomycin, simvastatin and zoledronate could regulate the expression levels of these genes. These findings may help identify a new strategy for the clinical treatment of HCC.
Study Approval
All datasets in the present study were downloaded from public databases, including TCGA, GEO and ICGC. These public databases allowed researchers to download and analyze public datasets for scientific purposes. The current research follows the TCGA, GEO and ICGC data access policies and publication guidelines. Users can download relevant data for free, our study is based on open-source data, there are no ethical issues and other conflicts of interest. Inclusion of the Human cell lines in the study were approved by Xijing Hospital review board.
Author Contributions
Guofang Lu, Yuanyong Wang, Rui Du and Yulong Shang conceived, designed and carried out the study and the relevant data analysis and interpretation. Bin Feng, Jianglin Wang and Fengrui Zhang carried out the study and the relevant data analysis and interpretation. Guofang Lu, Yuanyong Wang and Rui Du wrote the manuscript. Jianming Pei and Yulong Shang reviewed and revised the article. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to Journal of Inflammation Research the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The study was funded by the National Natural Science Foundation of China (nos. 82173241, 81871914 and 81773072), Youth Project, Lingyun Program of Fourth Military Medical University (2019cyjhsyl) and Supported by Fourth Military Medical University Doctoral Science Foundation (2021D04lgf & 2021D08wyy).
Disclosure
The authors have declared no competing interest in this work.
References
1. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi:10.1053/j.gastro.2007.04.061
2. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (Concord-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi:10.1016/S0140-6736(17)33326-3
3. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
4. Boege Y, Malehmir M, Healy ME, et al. A dual role of caspase-8 in triggering and sensing proliferation-associated DNA damage, a key determinant of liver cancer development. Cancer Cell. 2017;32(3):342–359. doi:10.1016/j.ccell.2017.08.010
5. Donne R, Saroul-Ainama M, Cordier P, et al. Replication stress triggered by nucleotide pool imbalance drives DNA damage and cGAS-STING pathway activation in NAFLD. Dev Cell. 2022;57(14):1728–1741. doi:10.1016/j.devcel.2022.06.003
6. Lu G, Tian S, Sun Y, et al. NEK9, a novel effector of IL-6/STAT3, regulates metastasis of gastric cancer by targeting ARHGEF2 phosphorylation. Theranostics. 2021;11(5):2460–2474. doi:10.7150/thno.53169
7. Li T, Guo H, Zhao X, et al. Gastric cancer cell proliferation and survival is enabled by a cyclophilin B/STAT3/miR-520d-5p signaling feedback loop. Cancer Res. 2017;77(5):1227–1240. doi:10.1158/0008-5472.CAN-16-0357
8. Bu P, Wang L, Chen KY, et al. A miR-34a-numb feedforward loop triggered by inflammation regulates asymmetric stem cell division in intestine and colon cancer. Cell Stem Cell. 2016;18(2):189–202. doi:10.1016/j.stem.2016.01.006
9. Shinkawa H, Nakai T, Tamori A, et al. Hepatocellular carcinoma (HCC) recurring 10 years after clearance of hepatitis B surface antigen and 20 years after resection of hepatitis B virus-related HCC. Int J Clin Oncol. 2008;13(6):562–566. doi:10.1007/s10147-008-0785-z
10. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
11. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi:10.1158/2159-8290.CD-21-1059
12. Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. doi:10.1038/s41392-021-00658-5
13. Sperandio RC, Pestana RC, Miyamura BV, Kaseb AO. Hepatocellular carcinoma immunotherapy. Annu Rev Med. 2022;73:267–278. doi:10.1146/annurev-med-042220-021121
14. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
15. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
16. Zongyi Y, Xiaowu L. Immunotherapy for hepatocellular carcinoma. Cancer Lett. 2020;470:8–17. doi:10.1016/j.canlet.2019.12.002
17. Ruiz DGM, Bresnahan E, Molina-Sanchez P, et al. beta-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9(8):1124–1141. doi:10.1158/2159-8290.CD-19-0074
18. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, Phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
19. Simon N, Friedman J, Hastie T, Tibshirani R. Regularization paths for cox’s proportional hazards model via coordinate descent. J Stat Softw. 2011;39(5):1–13. doi:10.18637/jss.v039.i05
20. Wang Y, Wang Z, Shao C, et al. Melatonin may suppress lung adenocarcinoma progression via regulation of the circular noncoding RNA hsa_circ_0017109/miR-135b-3p/TOX3 axis. J Pineal Res. 2022;73(2):e12813. doi:10.1111/jpi.12813
21. Liu Y, Zhang X, Zhang J, Tan J, Li J, Song Z. Development and validation of a combined ferroptosis and immune prognostic classifier for hepatocellular carcinoma. Front Cell Dev Biol. 2020;8:596679. doi:10.3389/fcell.2020.596679
22. Malta TM, Sokolov A, Gentles AJ, et al. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. 2018;173(2):338–354. doi:10.1016/j.cell.2018.03.034
23. Cheng X, Chen JZ, Guo YB. 肝癌分子靶向药对免疫系统的调节作用 [Regulatory effect of molecular targeted drugs on the immune system for liver cancer]. Zhonghua Gan Zang Bing Za Zhi. Chinese. 2021;29(10):1031–1034. doi:10.3760/cma.j.cn501113-20191006-00363
24. Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–543. doi:10.1016/S1470-2045(01)00486-7
25. Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18(12):2650–2666. doi:10.1016/j.cgh.2019.07.060
26. Karki R, Kanneganti T. Diverging inflammasome signals in tumorigenesis and potential targeting. Nature Rev Cancer. 2019;19(4):197–214. doi:10.1038/s41568-019-0123-y
27. de Bono JS, Guo C, Gurel B, et al. Prostate carcinogenesis: inflammatory storms. Nature Rev Cancer. 2020;20(8):455–469. doi:10.1038/s41568-020-0267-9
28. Masson-Lecomte A, López De Maturana E, Goddard ME, et al. Inflammatory-related genetic variants in non–muscle-invasive bladder cancer prognosis: a multimarker bayesian assessment. Cancer Epidemiol Biomark Prevent. 2016;25(7):1144–1150. doi:10.1158/1055-9965.EPI-15-0894
29. Zhai W, Duan F, Chen S, et al. A novel inflammatory-related gene signature based model for risk stratification and prognosis prediction in lung adenocarcinoma. Front Genet. 2022;12:798131.
30. Zhao Y, Schetter AJ, Yang GB, et al. microRNA and inflammatory gene expression as prognostic marker for overall survival in esophageal squamous cell carcinoma. Int J Cancer. 2013;132(12):2901–2909. doi:10.1002/ijc.27954
31. Wang Y, Yang Y, Zhao Z, et al. A new nomogram model for prognosis of hepatocellular carcinoma based on novel gene signature that regulates cross-talk between immune and tumor cells. Bmc Cancer. 2022;22(1):1–8.
32. Sia D, Jiao Y, Martinez-Quetglas I, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. 2017;153(3):812–826. doi:10.1053/j.gastro.2017.06.007
33. Snell LM, McGaha TL, Brooks DG. Type I interferon in chronic virus infection and cancer. Trends Immunol. 2017;38(8):542–557. doi:10.1016/j.it.2017.05.005
34. Morgan MR, Byron A, Humphries MJ, Bass MD. Giving off mixed signals-Distinct functions of α5 β1 and αv β3 integrins in regulating cell behaviour. Iubmb Life. 2009;61(7):731–738. doi:10.1002/iub.200
35. Ren J, Yang Y, Li C, et al. A novel prognostic model of early-stage lung adenocarcinoma integrating methylation and immune biomarkers. Front Genet. 2021;11. doi:10.3389/fgene.2020.634634
36. Lei Y, Yan W, Lin Z, Liu J, Tian D, Han P. Comprehensive analysis of partial epithelial mesenchymal transition‐related genes in hepatocellular carcinoma. J Cell Mol Med. 2021;25(1):448–462. doi:10.1111/jcmm.16099
37. Sterchi EE, Stocker W, Bond JS. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Aspects Med. 2008;29(5):309–328. doi:10.1016/j.mam.2008.08.002
38. OuYang H, Xu J, Luo J, et al. MEP1A contributes to tumor progression and predicts poor clinical outcome in human hepatocellular carcinoma. Hepatology. 2016;63(4):1227–1239. doi:10.1002/hep.28397
39. Asif A, Khalid M, Manzoor S, Ahmad H, Rehman AU. Role of purinergic receptors in hepatobiliary carcinoma in Pakistani population: an approach towards proinflammatory role of P2X4 and P2X7 receptors. Purinerg Signal. 2019;15(3):367–374. doi:10.1007/s11302-019-09675-0
40. Mo Z, Cao Z, Luo S, Chen Y, Zhang S. Novel molecular subtypes associated with 5mC methylation and their role in hepatocellular carcinoma immunotherapy. Front Mol Biosci. 2020;7. doi:10.3389/fmolb.2020.562441
41. Huang H, Zhou P, Wei J, et al. In vivo CRISPR screening reveals nutrient signaling processes underpinning CD8+ T cell fate decisions. Cell. 2021;184(5):1245–1261. doi:10.1016/j.cell.2021.02.021
42. Ling F, Zhang H, Sun Y, et al. AnnexinA7 promotes epithelial-mesenchymal transition by interacting with Sorcin and contributes to aggressiveness in hepatocellular carcinoma. Cell Death Dis. 2021;12(11):1018. doi:10.1038/s41419-021-04287-2
43. Finn RS, Ikeda M, Zhu AX, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
44. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi:10.1056/NEJMoa1503093
45. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi:10.1038/nature13904
46. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi:10.1093/annonc/mdv383
47. Donne R, Lujambio A. The liver cancer immune microenvironment: therapeutic implications for hepatocellular carcinoma. Hepatology. 2022. doi:10.1002/hep.32740
48. Chen R, Ganesan A, Okoye I, et al. Targeting B7‐1 in immunotherapy. Med Res Rev. 2020;40(2):654–682. doi:10.1002/med.21632
49. Lee MS, Ryoo B, Hsu C, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–820. doi:10.1016/S1470-2045(20)30156-X
50. Iitaka Y, Nakamura H, Nakatani T, et al. Chemistry of bleomycin. XX. The X-ray structure determination of P-3A Cu(II)-complex a biosynthetic intermediate of bleomycin. J Antibiot. 1978;31(10):1070–1072. doi:10.7164/antibiotics.31.1070
51. Cejka J, Kratochvil B, Cisarova I, Jegorov A. Simvastatin. Acta Crystallogr C. 2003;59(Pt 8):o428–o430. doi:10.1107/S0108270103012514
52. Sarkar A. Cukrowski I. Tris(dicyclohexylammonium) hydrogen [1-hydroxy-2-(1H -imidazol-1-yl)-1-phosphonatoethane]phosphonate ethanol monosolvate monohydrate. Acta Crystallogr Sect E Struct Rep Online. 2011;67(11):o2980. doi:10.1107/S1600536811042206
53. Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65(2):81–94. doi:10.1007/BF02034932
54. Seitz SJ, Schleithoff ES, Koch A, et al. Chemotherapy-induced apoptosis in hepatocellular carcinoma involves the p53 family and is mediated via the extrinsic and the intrinsic pathway. Int J Cancer. 2010;126(9):2049–2066. doi:10.1002/ijc.24861
55. Pose E, Trebicka J, Mookerjee RP, Angeli P, Gines P. Statins: old drugs as new therapy for liver diseases? J Hepatol. 2019;70(1):194–202. doi:10.1016/j.jhep.2018.07.019
56. Feng J, Dai W, Mao Y, et al. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J Exp Clin Canc Res. 2020;39(1). doi:10.1186/s13046-020-1528-x
57. Honda Y, Aikata H, Honda F, et al. Clinical outcome and prognostic factors in hepatocellular carcinoma patients with bone metastases medicated with zoledronic acid. Hepatol Res. 2017;47(10):1053–1060. doi:10.1111/hepr.12844
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







