Back to Journals » Psychology Research and Behavior Management » Volume 17
A Comparative Analysis of Cognitive Deficits in Rheumatoid Arthritis and Fibromyalgia: Impact of Symptoms Severity and Its Clinical Implications
Authors Galvez-Sánchez CM , Duschek S, Reyes del Paso GA
Received 11 December 2023
Accepted for publication 27 February 2024
Published 28 March 2024 Volume 2024:17 Pages 1399—1415
DOI https://doi.org/10.2147/PRBM.S446798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Mei-Chun Cheung
Carmen M Galvez-Sánchez,1,* Stefan Duschek,2,* Gustavo A Reyes del Paso3,*
1Department of Personality, Evaluation and Psychological Treatment, Faculty of Psychology and Speech Therapy, University of Murcia, Murcia, Spain; 2Department of Psychology, Institute of Psychology, UMIT TIROL - University for Health Sciences Medical Informatics and Technology, Hall in Tirol, Austria; 3Department of Psychology, University of Jaén, Jaén, Spain
*These authors contributed equally to this work
Correspondence: Carmen M Galvez-Sánchez, Department of Personality, Evaluation and Psychological Treatment, Faculty of Psychology and Speech Therapy, University of Murcia, Building 31, Murcia, 30100, Spain, Tel +34 868 88 868 88 7328, Email [email protected]
Purpose: Fibromyalgia syndrome (FMS) and rheumatoid arthritis (RA) are chronic pain disorders, with clearly distinct pathogenetic mechanisms, frequently accompanied by symptoms like depression, fatigue, insomnia and cognitive problems. This study compared performance in various cognitive domains between patients with FMS and RA. The role of clinical symptoms severity in determine the differences in cognitive performance was also investigated.
Patients and Methods: A cross-sectional study was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement. In total, 64 FMS patients, 34 RA patients and 32 healthy controls participated, all women. Using factor analysis, questionnaire scores were combined to yield a symptom severity factor, which was used as a control variable in the group comparisons.
Results: Without controlling for symptom severity, both patient groups performed worse than controls in all the cognitive domains assessed (visuospatial memory; verbal memory; strategic planning and self-regulation; processing speed, attention and cognitive flexibility; and planning and organizational abilities); overall deficits were greater in FMS than in RA patients. FMS patients reported more severe clinical symptoms (current pain intensity, total pain, state anxiety, depression, fatigue and insomnia) than RA patients. After controlling for symptom severity, a large proportion of the cognitive test parameters no longer differed between FMS and RA patients.
Conclusion: The study confirmed significant impairments in attention, memory, and higher cognitive functions in both FMS and RA. The greater deficits seen in FMS patients may at least partly be explained by more severe pain and secondary symptoms. Cognitive screening may facilitate the development of personalized treatment plans to optimize the quality of life of FMS and RA patients.
Plain Language Summary: The investigation substantiated noteworthy impairments in attention, memory, and executive functions among individuals diagnosed with Fibromyalgia Syndrome (FMS) and Rheumatoid Arthritis (RA).The heightened cognitive deficits observed in FMS patients compared to those with RA could be attributed in part to the heightened severity of pain and secondary symptoms characteristic of FMS.Semantic clustering, by leveraging cognitive resources optimally, may serve as a compensatory mechanism for memory deficits and thus warrants inclusion in interventions aimed at assisting patients in coping with cognitive impairments.Incorporating cognitive deficit screenings into routine diagnostic protocols for FMS and RA is recommended, as it may facilitate the development of personalized treatment strategies aimed at enhancing the overall quality of life for affected individuals.
Keywords: fibromyalgia syndrome, rheumatoid arthritis, cognitive impairments, depression, anxiety, fatigue, insomnia
Introduction
Fibromyalgia syndrome (FMS) is a severe condition of chronic widespread pain that affects 2–4% of the general population and has a higher prevalence in women than men.1 Accompanying symptoms include depression, anxiety, fatigue, insomnia, and cognitive problems.1,2 Cognitive impairments may include attention and memory deficits, mental slowness, language-related difficulties, and reduced organization and planning abilities.2–7 Based on patients´ and clinicians’ reports, cognitive deficits have a considerable negative impact on psychosocial functioning and quality of life and are therefore considered among the most serious symptoms of the disorder.8–10
Rheumatoid arthritis (RA) is a persistent autoimmune disorder associated with painful, stiff, and swollen joints.11 Its prevalence is estimated at 0.5–1% and it is more common in women and older adults.12 Cognitive impairments were also reported in patients suffering from RA.13–19 Even though fewer data are available than for FMS, current evidence suggest problems in attention, memory, and verbal, visuospatial, and executive functions in patients affected by RA (see Meade et al20 for an overview).
Research directly comparing cognitive performance between patients with FMS and RA is still scarce. Some studies suggested deficits of similar magnitude in both patient groups. For example, impairments in selective and sustained attention3 and working memory21 did not differ between FMS and RA patients. However, further studies revealed greater cognitive impairments in FMS. For example, longer reaction times and more errors in FMS than RA patients were seen on the Attentional Network Test, which allows for a comprehensive assessment of attentional functions.16 Moreover, FMS patients exhibited lower performance than those with RA in tasks assessing spatial orientation, spatial memory,22 and executive functions.23 Another study revealed poorer logical memory, phonemic verbal fluency, and verbal reasoning in FMS than RA patients.15 However, the RA patients performed better in semantic verbal fluency and figural fluency tasks.22,23 The findings presented herein underscore the imperative of incorporating cognitive assessments into the evaluation of both aforementioned conditions to enhance their optimal management. Presently, there is a growing demand within clinical practice for more nuanced interventions grounded in a transdiagnostic and holistic framework. In response to this exigency, there is a requisite for the evaluation of cognitive impairments in individuals with chronic pain, aiming to formulate and implement interventions rooted in neuropsychological training. This approach is intended to ameliorate cognitive performance and mitigate its consequential impact on health-related quality of life.
In some studies of FMS patients, the magnitude of cognitive impairment was associated with clinical pain severity.6,24–26 In addition, deficits correlated with symptoms of depression, anxiety, fatigue, and insomnia.5,24,27–30 Similar associations were reported for patients with RA.31,32 Both pain and emotional symptoms tend to be stronger in FMS than RA.3,16,23 Therefore, it seems plausible that these factors at least partly account for the greater severity of cognitive impairments observed in FMS than RA patients.
The aim of the present study was to compare performance among FMS patients, RA patients, and healthy individuals in various cognitive domains, namely visuospatial and verbal memory, attention, processing speed, cognitive flexibility, self-regulation, and planning and organizational abilities. The following hypotheses were tested: (1) While both patient groups will show poorer performance on cognitive tests than healthy individuals, the performance reduction will be greater in FMS than RA patients. (2) Patients with FMS will exhibit more severe pain and symptoms of depression, anxiety, fatigue, and sleep problems than those with RA. (3) Differences in cognitive test performance between FMS patients and RA patients will at least partly disappear after controlling for pain severity and levels of secondary symptoms in the statistical analysis.
Materials and Methods
The study -performed between 2019 and 2020- recruited women with FMS, via the Fibromyalgia Association of Jaén (Spain), who met the 1990 and 2010 American College of Rheumatology (ACR) criteria;1,33 women with RA, via the Rheumatoid Arthritis Association of Jaén (Spain), who met the 1987 American Rheumatology Association (ARA) criteria;34 and healthy women (healthy controls, HC) recruited through neighborhoods associations and friends of the patient’s associations. Considering the much higher prevalence of FMS in women than in men,1,33 the study was focused on women. Patients were previously diagnosed by their own rheumatologist. None of the patients met the criteria for both FMS and RA. The exclusion criteria for all study groups were aged < 18 or > 65 years, the presence of metabolic abnormalities, neurological disorders, drug abuse, and severe somatic (eg, cancer) or psychiatric (eg, psychotic) diseases. HC were additionally required to be free from acute or chronic pain of any kind.
Cognitive Performance Assessment
The following cognitive tests were applied:
The Rey-Osterrieth Complex Figure Test (ROCF) (Rey et al.35 Spanish version by Peña-Casanova36) was used to assess visuospatial memory. In this task, an abstract figure comprising 18 different parts is presented and has to be copied on a sheet of paper. After 30 minutes have elapsed, the participant is then asked to reproduce the figure from memory. The total number of correctly copied and reproduced parts, and the time needed to copy and reproduce the figure, index performance.
The Verbal Learning Test (TAVEC)37 was applied to quantify verbal memory function. A list of 16 words (“Monday shopping list”, List A) is read to the participant five times; the participant then has to reproduce as many words as possible directly after each trial (immediate free recall). After this, another list is read once (“Tuesday shopping list”, List B), and has to be immediately reproduced (interference control). Straight after List B, participants are asked to reproduce List A once again (short-term recall). Following a 20-min break, the words comprising List A have to be reproduced again (long-term recall). Thereafter, a list of 44 words is read to the participant, which comprises all words from List A, some words from List B, and distractor words not included on either list. The participant has to decide whether or not each of these words belong to List A (recognition). In this study, performance parameters for the TAVEC included the numbers of correctly reproduced words from List A (immediate free recall) and List B (interference control), and from List A after 20 min (long-term recall). Correct responses, false positives, omission errors, and a discrimination score (DS; computed according to the formula DS =1- [(FP + OE)/44] × 100) were used to quantify recognition performance. In addition, the tendency to use serial and semantic strategies in the immediate, short-term, and long-term recall conditions was assessed. Serial strategies refer to the reproduction of words in the order in which they were initially presented, while in semantic strategies words are reproduced according to semantic categories (eg, fruits, clothes or tools). The use of the strategies was assessed according to the number of words reproduced in the order of presentation (serial strategies), or within the same semantic category (semantic strategies).
The Revised Strategy Application Test (R-SAT) was employed as a measure of strategic planning and self-regulation.38 The R-SAT includes three simple activities, ie, figure tracing, sentence copying, and object numbering. Activities are presented as two different stacks, each comprising 120 items. Items differ in terms of size (large, small) and time requirements (brief, medium, long). A large item scores 0 points and a small one scores 100 points; participants are instructed to obtain as many points as possible. Moreover, items in which a face is displayed have to be avoided. The items are intermixed; however, the number of brief items decreases progressively within both stacks. As the execution time of the task is restricted to 10 min, the most efficient strategy is to complete brief rather than long items. Thus, the predisposition to complete items in the presented sequence has to be overcome. To evaluate prospective memory and self-regulation, participants are asked to place a “control mark” in a table with 10 fields every minute after beginning the test. They have to estimate the time elapsed on their own; they are not allowed to use a watch and there are no external prompts. Performance is indexed by the number of correct responses (ie, the proportion of short items [%]), the number of mistakes (ie, face items), and the number of control marks. After completion of the task, participants are asked to state the strategy that, in their opinion, was optimal to obtain the maximum number of points. Awareness of the strategy (coded as 1 or 0) was used as an additional test parameter.
The Trail Making Test (TMT) was used to evaluate processing speed, attention, and cognitive flexibility. The TMT version of Delis et al39 (Spanish adaption by Ibor40) was applied instead of the standard form, which allows for a more comprehensive performance assessment (because it includes assessments of visual scanning and motor speed). The TMT, in which visual targets (numbers, letters) are presented on sheets of paper, includes the following task conditions: (1) visual scanning (cross out all number 3 s on a page with different numbers), (2) number sequence (connect the numbers 1–16 in sequential order), (3) letter sequence (connect the letters A to P in alphabetic order), (4) switching (connect numbers and letters in alternating order, ie, 1, A, 2, B, etc.), and (5) motor speed (trace a predefined path). In addition to the execution times for each condition, the following types of mistakes were recorded in this study: (1) sequence (connection of a correct item with an incorrect one), (2) set loss (connection of items of different categories) and (3) time out (exceeding the time limit of 150 s for conditions 1 to 3 and 240 s for condition 4).
The Zoo Map Test (ZMT) from the Behavioral Assessment of the Dysexecutive Syndrome battery was applied to measure planning and organizational abilities (Wilson et al.41 Spanish adaptation by Vargas et al42). In this test, the participant has to plan a route to visit 6 of 12 possible locations in a zoo. The ZMT has two parts, ie, a more demanding open situation, in which little information is provided that would help to generate an appropriate plan (version 1), and a situation that involves simply following a concrete, externally imposed strategy (version 2). The execution time and the number of correct responses for versions 1 and version 2 were taken as performance indices, in addition to the total number of correct responses.
Clinical Assessments
The patients´ clinical history and demographic data were obtained via a semi-structured interview. The Structured Clinical Interview for Axis I Disorders of the Diagnostic and Statistical Manual for Mental Disorders (SCID, First et al43) was used to diagnose possible mental disorders. In addition, the following self-report questionnaires were administered:
McGill Pain Questionnaire (MPQ) (Melzack;44 Spanish version by Lázaro et al45). This 73-item instrument allows for quantification of the sensorial, emotional, and cognitive components of the pain experience. The MPQ Total Pain score (range: 0–167) and Current Pain Intensity score (visual analog scale, range: 0–5) were calculated in this study. A Cronbach´s α value of 0.74 was reported for the Total Pain score.45
State-Trait Anxiety Inventory (STAI) (Spanish version by Spielberger et al46). This instrument enables quantification of current and habitual anxiety levels (20 items each) using 4-point Likert scales (score range: 0–60). The Cronbach´s α values are 0.93 and 0.87 for the State Anxiety and Trait Anxiety scales, respectively.46
Beck Depression Inventory (BDI) (Beck et al.47 Spanish adaptation by Vázquez & Sanz48). This 21-item scale was applied to assess the severity of symptoms of depression (4-point Likert scale, score range: 0–63). The Cronbach´s α of the instrument is 0.95.48
Fatigue Severity Scale (FSS) (Krupp et al.49 Spanish version by Bulbena et al50). This scale measures fatigue based on nine items (7-point Likert scales; score range: 9–63). A Cronbach´s α of 0.88 was reported.50
Oviedo Quality of Sleep Questionnaire (COS, Bobes et al51). The Insomnia subscale of the COS, comprising nine items (5-point Likert scales; score range: 9–45), was used in the study; its Cronbach´s α is 0.88.51
Procedure
A cross-sectional study was conducted based on the STROBE Statement: guidelines for reporting observational studies, specifically, the STROBE checklist: cross-sectional studies guidelines52 was used in order to enhance the quality and transparency of the current research (see Table S1). To reduce possible biases participants were blinded by a code for both, authors who collected the data and those who conducted the statistical analyses. The study was conducted in two separate sessions, performed on the same day and supervised by the same experimenter. During the first session, the patients´ clinical history and sociodemographic and medication use data were recorded, and the SCID interviews were conducted. Thereafter, participants completed the questionnaires. During the second session, the following cognitive assessment instruments were completed: ROCF, ZMT, reproduction part of the ROCF, R-SAT, the first part of the TAVEC, TMT, and recognition task of the TAVEC. The tests were presented in this order to avoid interference between the different cognitive domains, especially between visual and verbal memory. Between each test, participants had a break of 10 minutes. The study protocol was approved by the Ethics Committee of the University of Jaén and all participants provided written informed consent. The study was performed in accordance with the current legislation and meets the principles of the Helsinki Declaration.
Statistical Analysis
In order to determine the optimal sample size based on expected effect sizes, the G*Power 3.1.7 program was used.53 Previous comparisons between FMS patients and healthy individuals on neuropsychological variables showed medium to large effect sizes, with Cohen´s d in the range 0.52–1.10.2,4 Assuming an effect size of 0.60, an alpha level of 0.05 and a Beta error of 20% as a basis, a sample size of 21 participants per group appeared optimal. The Kolmogorov–Smirnov and Levene tests showed no deviation from normality or homogeneity in the measured variables. Comparisons between groups on clinical and demographic variables were performed using independent-samples F-tests or χ2 tests. Group differences in cognitive test parameters were analyzed using multivariate analysis of variance (MANOVA) and covariance (MANCOVA). As previous studies revealed differences in cognitive performance between FMS patients and healthy women on the tests applied herein2,4,16 and to obtain more specific information, separate MANOVAs were computed comparing FMS patients and RA patients, RA patients and HC, and FMS patients and HC. To control for the effects of years of education, linear regression analyses were performed with years of education as a predictor and cognitive test parameters as dependent variables. Prior to the MANOVA, cognitive parameters were replaced by the unstandardized residuals resulting from the regression analyses, which are independent of years of education.
The scales representing clinical factors (MPQ Total Pain, MPQ Current Pain Intensity, State Anxiety, Trait Anxiety, FSS, COS) were closely associated with each other (see Table 1). To avoid collinearity and limit the number of control variables in the analysis of group differences in cognitive performance, factor analysis was performed on the questionnaire scores. For that analysis, the criterion for significance was an eigenvalue > 1; only one latent factor (referred to as “symptom severity” in the following) was identified, which explained 58.73% of the variance. This variable (ie, the factor values) was used as a covariate in the MANCOVAs.
 |
Table 1 Pearson’s Product Moment Correlation Coefficients Between Questionnaire Scores in the Total Sample (N=130) |
Effect sizes of the group comparisons are reported as adjusted eta squared (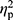 ). Statistical significance was set at p < 0.05. Statistical analyses were performed with the IBM SPSS Statistics (v.22).
). Statistical significance was set at p < 0.05. Statistical analyses were performed with the IBM SPSS Statistics (v.22).
Results
In total, 64 women with FMS, 34 women with RA and 32 healthy women were recruited. Table 2 provides the demographic and clinical data of the sample. While the groups did not differ in age or Body Mass Index (BMI), educational level (indexed by years of education) was lower in patients with RA than HC.
 |
Table 2 Sociodemographic Data, Clinical Variables and Questionnaire Scores of RA Patients, FMS Patients and HC (M±SD or Number of Participants and %); Statistics of the Group Comparisons |
Clinical Data
Patients with FMS had higher values on all clinical questionnaires and were more frequently diagnosed with depression and anxiety disorders than patients with RA and HC (see Table 2). Moreover, RA patients reported greater clinical pain and more anxiety disorders than HC. FMS patients were using medications of all kinds more frequently than HC, and were using antidepressants and anxiolytics more frequently than RA patients. Use of analgesics and opiates was more frequent in RA patients than HC.
Multivariate Group Comparisons
The MANOVAs revealed multivariate group effects on the cognitive test parameters in the comparisons between FMS and RA patients (F(35,62)=2.20, p=0.003, 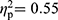 ), between RA patients and HC (F(35,30)=2.83, p=0.002,
), between RA patients and HC (F(35,30)=2.83, p=0.002, 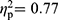 ), and between FMS patients and HC (F(35,60)=3.98, p<0.001,
), and between FMS patients and HC (F(35,60)=3.98, p<0.001, 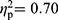 ). The same applies to the MANCOVAs controlling for symptom severity (FMS vs RA patients, F(35,61)=1.62, p=0.049,
). The same applies to the MANCOVAs controlling for symptom severity (FMS vs RA patients, F(35,61)=1.62, p=0.049, 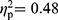 ; RA patients vs HC, F(35,29)=2.99, p=0.002,
; RA patients vs HC, F(35,29)=2.99, p=0.002, 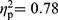 ; FMS patients vs HC, F(35,59)=1.93, p=0.013,
; FMS patients vs HC, F(35,59)=1.93, p=0.013, 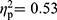 ). Symptom severity (covariate) showed a significant multivariate effect in the comparison between patients with FMS and HC (F(35,59)=2.50, p=0.001,
). Symptom severity (covariate) showed a significant multivariate effect in the comparison between patients with FMS and HC (F(35,59)=2.50, p=0.001, 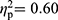 ), but not in the comparisons between patients with RA and HC (F(35,29)=1.69, p=0.076,
), but not in the comparisons between patients with RA and HC (F(35,29)=1.69, p=0.076, 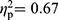 ), and patients with FMS and RA (F(35,61)=1.27, p=0.21,
), and patients with FMS and RA (F(35,61)=1.27, p=0.21, 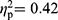 ).
).
Univariate Group Comparisons
Table 3 presents the results of univariate group comparisons without and with control for symptom severity, and the univariate effects of symptom severity (covariate). While the mean ± SD values represent the original test scores, the statistics of the group comparisons are based on residuals derived from the regression analyses.
Group Differences Without Control for Symptom Severity
The analysis without control for symptom severity revealed lower visual memory performance on the ROCF in FMS than RA patients, indexed by a longer figure reproduction time and fewer correct marks. FMS patients also exhibited a longer copy time than those with RA. Poorer verbal memory performance of FMS than RA patients was suggested by the results of the TAVEC recognition task, where FMS patients had fewer correct responses, more omission errors and false positives, and a lower discrimination score. Moreover, in the free recall tasks, the serial (immediate recall) and semantic (short- and long-term recall) strategies were used less frequently by RA than FMS patients. Regarding planning and self-regulation abilities, FMS patients had fewer correct responses (ie, a lower proportion of short items), fewer control marks, and more mistakes (face items) on the R-SAT than RA patients. With respect to processing speed, attention, and cognitive flexibility, FMS patients had a longer execution time in TMT conditions 2 (number sequence), 3 (letter sequence), 4 (switching), and 5 (motor speed), and made more mistakes (of all types: sequence, set loss, and time out mistakes) than RA patients. Moreover, a longer execution time and fewer correct responses for version 2 of the ZMT, and fewer correct responses overall on the test, suggested poorer planning abilities in FMS than RA patients. RA patients exhibited poorer verbal memory performance than HC, indexed by lower TAVEC immediate (list A) and short-term recall scores. With respect to planning and self-regulation, RA patients showed less awareness of the strategy for the R-SAT than HC. The comparison between FMS patients and HC suggested poorer visual memory performance in FMS patients, indicated by fewer correct marks in both conditions of the ROCF. Copy time was also longer In FMS patients. Poorer verbal memory in FMS patients than HC was suggested by lower TAVEC immediate recall (List A), interference control (List B), and short- and long-term recall scores. In the recognition task, FMS patients had fewer correct responses, more omission errors and false positives, and a lower discrimination score than HC. Additionally, the serial (immediate recall) and semantic (short- and long-term recall) strategies were less prevalent in FMS patients than HC. Fewer control marks and correct responses (ie, a lower proportion of short items), more mistakes (face items), and less awareness of the strategy for the R-SAT suggested poorer planning and self-regulation in FMS patients than HC. Concerning processing speed, attention, and cognitive flexibility, FMS patients exhibited a longer execution time for TMT conditions 2 (number sequence), 3 (letter sequence), 4 (switching), and 5 (motor speed), and more sequence, set loss, and time out mistakes, than HC. Finally, longer execution times and fewer correct responses on versions 1 and 2 of the ZMT indicated poorer planning abilities in FMS than HC.
Group Differences After Controlling for Symptom Severity
According to the analysis controlling for symptom severity, FMS patients showed a longer copy time on the visual memory test (ROCF) than RA patients. In the verbal memory domain, FMS patients exhibited a lower discrimination score (TAVEC recognition task) than RA patients. Additionally, the use of semantic strategy memory (short- and long-term recall) was more prevalent among FMS than RA patients. In contrast, RA patients more frequently used the serial strategy (short-term recall) more frequently. Concerning processing speed, attention, and cognitive flexibility (TMT), FMS patients had a longer execution time in condition 4 (switching) and made more time out mistakes than RA patients. RA patients, as compared to HC, had lower scores for short-term recall on the verbal memory test (TAVEC). Moreover, RA patients exhibited less awareness of the strategy for the R-SAT than HC, suggesting poorer planning ability. The comparison between FMS patients and HC in terms of verbal memory (TAVEC) revealed less frequent use of the serial strategy (short- and long-term recall) in the former group. With respect to processing speed, attention, and cognitive flexibility (TMT), FMS patients had a longer execution time in condition 4 (switching) and more time out mistakes than HC.
Covariate Effect of Symptom Severity
In the univariate analyses comparing FMS and RA patients, the covariate symptom severity reached significance for the serial strategy (short-term recall) of the verbal memory test (TAVEC). In the comparison between RA patients and HC, the effect of symptom severity was significant for execution time and correct marks in the reproduction condition of the visual memory test (ROCF), as well as for time out mistakes in the processing speed, attention, and cognitive flexibility test (TMT), awareness of the strategy in the planning and self-regulation test (R-SAT) and the total score of the planning task (ZMT). In the comparison between FMS patients and HC, a significant covariate effect was seen for execution time and correct marks in the reproduction condition of the visual memory test (ROCF), the serial strategy (short- and long-term recall) in the verbal memory test (TAVEC), sequence mistakes in the processing speed, attention and cognitive flexibility test (TMT), correct responses and awareness of the strategy in the planning and self-regulation test (R-SAT) and the total number of correct responses on the planning task (ZMT). Symptom severity was inversely associated with correct responses in all tests, the use of the serial strategy in the TAVEC, and awareness of the strategy in the R-SAT; moreover, it was positively associated with reproduction time in the ROCF.
Discussion
This study compared performance in various cognitive domains between patients with FMS, patients with RA and HC. While both patient groups performed worse than HC in the domains of visuospatial and verbal memory, attention, processing speed, cognitive flexibility, planning, and self-regulation, the overall deficits were greater in FMS than AR patients. Moreover, the comparison between patients with FMS and AR revealed higher performance in all domains in those with RA. A considerable proportion of the group comparisons lost significance when symptom severity was statistically controlled for, thus implicating clinical symptoms in the group differences in cognitive performance.
The analysis that did not control for clinical symptom severity revealed poorer visual memory performance in FMS patients than HC, reflected in a more accurate reproduction of the abstract figure of the ROCF in the latter group. The less accurate and slower figure copying of the FMS patients may be attributable to perceptual problems and reduced motor speed rather than memory deficits. Poorer verbal memory performance in FMS patients than HC was reflected in lower TAVEC scores for the immediate, short- and long-term free recall conditions. Fewer correct responses, more omission errors and false alarms, and a lower discrimination score on this test suggest impaired verbal recognition. Furthermore, the results for the TMT suggested a slower processing speed, and poorer attention and cognitive flexibility, in FMS patients than HC. In addition to longer execution times in the number sequence, letter sequence, switching and motor speed conditions, FMS patients made more sequence, set loss, and time out mistakes. Planning and self-regulation abilities were assessed using the R-SAT. Poorer performance in these domains in FMS patients than HC was indicated by fewer correct responses and more mistakes on this test, as well as less awareness of the strategy required to successfully complete the task. In addition, lower values for the control mark parameter suggest poorer prospective memory. Reduced planning and organizational abilities in FMS patients were confirmed by longer execution times and fewer correct responses on both versions of the ZMT. The present observations are in accordance with previous findings of impairments in figural and verbal memory3,4,54,55 basic and higher attentional processes3,16,25,56 as well as executive functions and planning skills6,7,27,57–59 in FMS.
According to the group comparisons not controlling for symptom severity, RA patients showed poorer verbal memory performance than HC, indexed by lower TAVEC scores for immediate and short-term free recall. Moreover, less awareness of the strategy required for the R-SAT points toward poorer planning abilities. These findings are in line with earlier reports of impairments of attention,16,17 memory,18 and executive functions13,14,19 in RA.
In the direct comparison between FMS and RA patients not controlling for symptom severity, numerous test scores suggested lower performance in those with FMS. Less accurate and slower reproduction of the abstract ROCF figure reflected poorer figural memory in FMS, and poorer verbal memory in FMS patients was suggested by fewer correct responses, more omission errors and false positives, and a lower discrimination score in the TAVEC recognition task. Moreover, a slower processing speed, and poorer attention and cognitive flexibility, in FMS patients were evidenced by a longer execution time in the TMT conditions of number sequence, letter sequence, switching and motor speed, in addition to the more frequent sequence, set loss, and time out mistakes. Fewer correct responses, more mistakes and fewer control marks suggested poorer planning abilities and prospective memory in FMS than RA patients. The conclusion regarding planning was confirmed by the longer execution time for version 2 (externally imposed strategy) and fewer correct responses for both versions of the ZMT in FMS patients. These findings are only partially consistent with previous studies. For example, greater impairments in FMS than RA patients were seen previously in basic and higher attentional functions, visual orientation, spatial and memory, verbal fluency, and executive functions.15,16,22,23 However, similar deficits in both patient groups were reported for selective and sustained attention, working memory, language, and visuospatial processes.3,21,23 Differences in sample composition, cognitive tests, and control of other variables may account for the divergent findings.
This study evaluated the possible role of symptom severity in the differences among FMS patients, RA patients and HC. FMS patients reported more severe clinical pain and depression symptoms, higher levels of state and trait anxiety, and greater fatigue and insomnia than those with RA. Strong correlations were seen among the questionnaire scores representing these symptoms, and factor analysis revealed that a single latent factor explained 59% of the variance in the scores. Therefore, the questionnaire scores were combined, and the factor values resulting from this analysis were used as a covariate in the group comparisons. While differences among all three groups remained in the multivariate analysis, in univariate comparisons a large proportion of the group differences were no longer significant.
The comparison between FMS patients and HC controlling only for symptom severity revealed a longer execution time in the switching condition of the TMT, and more time out mistakes on this test of attention and cognitive flexibility in the former group. Regarding the differences between AR and HC, only those for short-term free recall on the TAVEC and awareness of the strategy for the R-SAT remained significant. Poorer performance in FMS than RA patients was reflected in a longer copy time for the ROCF figure, lower discrimination score in the recognition condition of the TAVEC, and more time out mistakes and a longer execution time in the switching TMT condition. According to these findings, differences among the groups may relate to differences in clinical symptom severity. This is also reflected in the significant covariate effect of symptom severity in the univariate analysis. This covariate was significant in the comparison between FMS patients and HC in terms of the speed and accuracy with which the ROCF figure was reproduced, the number of correct responses and awareness of the strategy required for the R-SAT, number of sequence mistakes on the TMT, and the total number of correct responses on the ZMT. Regarding the FMS and RA patients, symptom severity was a significant covariate in the comparisons of the speed and accuracy with which the ROCF figure was reproduced, awareness of the strategy required for the R-SAT, number of time out mistakes on the TMT, and the total number of correct responses on the ZMT.
The covariate effects suggest poorer cognitive performance in patients with greater symptom severity. This corroborates previous findings of positive correlations between clinical symptoms and cognitive impairments in patients with FMS and RA. In FMS patients, pain severity was inversely associated with measures of attention,4,6,25,54,56 memory,4,26,54 and executive functions.4,7,58 Although negative correlations of depression symptoms, anxiety, and fatigue with cognitive performance have also been reported,6,7,26,56 pain severity was the factor most closely associated with performance in most studies (see Muñoz Ladrón de Guevara et al7 for an overview). Similar findings were obtained in patients with RA, where clinical pain was inversely associated with measures of processing speed, memory, and executive functions.31,32 Some studies also documented negative correlations of depression symptoms,32,60 anxiety,61 and fatigue31 with cognitive performance in RA.
Controlling for symptom severity was particularly relevant to the comparison between patients with FMS and HC, where the group difference disappeared for a substantial proportion of the performance measures. This may help explain the mixed results of previous comparisons between these groups.3,16,21–23 In a study reporting poorer attentional performance in FMS than RA patients on the Attentional Network Test, the group difference remained significant after controlling for pain severity.16 However, in studies reporting more problems with logical visuospatial memory, visual orientation, verbal fluency, and executive functions in FMS than RA patients, clinical symptoms were not controlled for.15,22,23 Therefore, it may be that the greater cognitive impairments seen in FMS than RA patients are at least partly due to more severe pain and secondary symptoms in the former group.
As a secondary result, this study suggested differences in the use of strategies of memory recall on the TAVEC between FMS patients and the other groups. The analyses without and with control for symptom severity revealed less frequent use of serial and semantic strategies in FMS patients than RA patients and HC during immediate, short-term, and long-term free recall of words. Studies of healthy individuals reported that the use of semantic strategies may improve memory performance.62 These strategies also proved beneficial for overcoming memory deficits in various patient groups, including those with schizophrenia,63 geriatric depression,64 and prefrontal lesions.65 Semantic clustering may compensate for memory problems through optimal use of the available cognitive resources.66 As such, the findings may also be relevant for the optimization of psychological treatments for FMS67–72 where the inclusion of semantic-based memory strategies in treatment programs may help patients cope with cognitive impairments.
The study has several limitations. First, information regarding the possible influence of psychotropic and pain medication on cognitive performance in FMS and RA patients was not obtained. This issue could have been investigated by comparing patient subgroups distinguished according to the use of specific drugs or combinations thereof. However, the sample size was insufficient to form such subgroups, although previous studies did not suggest substantial effects of medication on cognition in FMS patients.4,7,25,26 Secondly, no information on disease activity and remissive treatment of RA is provided, and performance may be lower during high disease activity. However, no patient (for both study groups) was evaluated during a period of clinical crisis, as indexed by manifested symptoms. The third, limitation pertains to the analysis of the effects of clinical symptoms on cognitive performance. On the basis of factor analysis, a single symptom severity factor was used as a covariate in the group comparisons. However, this impeded the analysis of the effects of particular symptoms, like pain or secondary symptoms of FMS and RA. Not all psychological factors potentially impacting cognition could be considered in the analysis. For example, in FMS negative and positive affect, self-esteem and alexithymia were associated with cognitive impairments.16 Fourth, as only women were included in the sample, the generalizability of the results to male patients with FMS and RA is certainly limited. Fifth, both FMS and RA diagnosis were performed by different rheumatologists, which may has introduced bias in the selection of the patients. Finally, given the inherent limitations of clinical studies and access to clinical samples in small-medium populations, the recruitment of participants was no randomly performed.
Conclusion
Accordingly to our hypotheses, we can conclude that (1) both patient groups showed poorer cognitive performance than healthy individuals, but deficits were overall greater in FMS than RA patients (hypothesis 1); (2) patients with FMS exhibited more severe pain and symptoms of fatigue, depression, anxiety, and sleep problems than those with RA (hypothesis 2); (3) differences in cognitive test performance between FMS patients and RA patients partly disappeared after controlling for clinical severity (hypothesis 3), suggesting the relevance of the clinical state in determining cognitive functioning. The clinical relevance of cognitive problems and their influence in patients´ functional status and daily life is beyond question. +Based on the present results, it is recommended that screening for cognitive deficits be part of routine diagnostics for FMS and RA, which may help to guide the design of personalized interventions to optimize cognitive performance of patients with FMS and RA.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge all participants in this study.
Funding
This work was supported by a grant from the Spanish Ministry of Science, Innovation and Universities [PID2022-139731OB-I00] and a grant from the Regional Ministry of University, Research and Innovation in the field of I+D+i de la Junta de Andalucía [ProyExcel_00374].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. doi:10.1002/acr.20140
2. Galvez-Sánchez CM, Muñoz Ladrón de Guevara C, Montoro CI, Fernández-Serrano MJ, Duschek S, Reyes DEl Paso GA. Cognitive deficits in fibromyalgia syndrome are associated with pain responses to low intensity pressure stimulation. PLoS One. 2018;13(8):e0201488. doi:10.1371/journal.pone.0201488
3. Dick B, Eccleston C, Crombez G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 2002;47(6):639–644. doi:10.1002/art.10800
4. Galvez-Sánchez CM, Reyes DEl Paso GA, Duschek S. Cognitive impairments in fibromyalgia syndrome: associations with positive and negative affect, alexithymia, pain catastrophizing and self-esteem. Front Psychol. 2018;9:377. doi:10.3389/fpsyg.2018.00377
5. Gelonch O, Garolera M, Valls J, Rosselló L, Pifarré J. Cognitive complaints in women with fibromyalgia: are they due to depression or to objective cognitive dysfunction? J Clin Exp Neuropsychol. 2017;39(10):1013–1025. doi:10.1080/13803395.2017.1301391
6. Muñoz Ladrón de Guevara C, Fernández-Serrano MJ, DEl Paso GA R, Duschek S. Executive function impairments in fibromyalgia syndrome: relevance of clinical variables and body mass index. PLoS One. 2018;13(4):e0196329. doi:10.1371/journal.pone.0196329
7. Montoro CI, Duschek S, Muñoz Ladrón de Guevara C, Fernández-Serrano MJ, Reyes DEl Paso GA. Aberrant cerebral blood flow responses during cognition: implications for the understanding of cognitive deficits in fibromyalgia. Neuropsychology. 2015;29(2):173–182. doi:10.1037/neu0000138
8. Arnold LM, Crofford LJ, Mease PJ, et al. Patient perspectives on the impact of fibromyalgia. Patient Educ Couns. 2008;73(1):114–120. doi:10.1016/j.pec.2008.06.005
9. Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi:10.1186/1471-2474-8-27
10. Williams DA, Clauw DJ, Glass JM. Perceived cognitive dysfunction in fibromyalgia syndrome. J Musculoskelet Pain. 2011;19:66–75. doi:10.3109/10582452.2011.558989
11. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi:10.1016/S0140-6736(10)60826-4
12. Jeffery RC. Clinical features of rheumatoid arthritis. Medicine. 2010;38(4):167–171. doi:10.1016/j.mpmed.2009.12.004
13. Bartolini M, Candela M, Brugni M, et al. Are behaviour and motor performances of rheumatoid arthritis patients influenced by subclinical cognitive impairments? A clinical and neuroimaging study. Clin Exp Rheumatol. 2002;20(4):491–497.
14. Chaurasia N, Singh A, Singh IL, Singh T, Tiwari T. Cognitive dysfunction in patients of rheumatoid arthritis. J Family Med Prim Care. 2020;9(5):2219–2225. doi:10.4103/jfmpc.jfmpc_307_20
15. de Melo LF, Da-Silva SL. Neuropsychological assessment of cognitive disorders in patients with fibromyalgia, rheumatoid arthritis, and systemic lupus erythematosus. Rev Bras Reumatol. 2012;52(2):181–188.
16. Galvez-Sánchez CM, de la Coba P, Colmenero JM, Reyes DEl Paso GA, Duschek S. Attentional function in fibromyalgia and rheumatoid arthritis. PLoS One. 2021;16(1):e0246128. doi:10.1371/journal.pone.0246128
17. Vitturi BK, Nascimento BAC, Alves BR, de Campos FSC, Torigoe DY. Cognitive impairment in patients with rheumatoid arthritis. J Clin Neurosci. 2019;69:81–87. doi:10.1016/j.jocn.2019.08.027
18. Katchamart W, Narongroeknawin P, Phutthinart N, Srinonprasert V, Muangpaisan W, Chaiamnauy S. Disease activity is associated with cognitive impairment in patients with rheumatoid arthritis. Clin Rheumatol. 2019;38(7):1851–1856. doi:10.1007/s10067-019-04488-3
19. Oláh C, Kardos Z, Andrejkovics M, et al. Assessment of cognitive function in female rheumatoid arthritis patients: associations with cerebrovascular pathology, depression and anxiety. Rheumatol Int. 2020;40(4):529–540. doi:10.1007/s00296-019-04449-8
20. Meade T, Manolios N, Cumming SR, Conaghan PG, Katz P. Cognitive impairment in rheumatoid arthritis: a systematic review. Arthritis Care Res. 2018;70(1):39–52. doi:10.1002/acr.23243
21. Akdogan S, Ayhan FF, Yildirim S, Borman P. Impact of fatigue on cognitive functioning among premenopausal women with fibromyalgia syndrome and rheumatoid arthritis: the controlled study. J Musculoskelet Pain. 2013;21(2):135–146. doi:10.3109/10582452.2013.806977
22. Roldán-Tapia L, Cánovas-López R, Cimadevilla J, Valverde M. Déficit mnésicos y perceptivos en la fibromialgia y la artritis reumatoide. Reumatol Clin. 2007;3(3):101–109. doi:10.1016/S1699-258X(07)73676-8
23. Bilgici A, Terzi M, Guz H, Kuru O. Comparison of the cognitive performance between healthy controls, rheumatoid arthritis and fibromyalgia patients without depression. J Clin Anal. 2014;5:214–219. doi:10.4328/JCAM.1181
24. Duschek S, Werner NS, Winkelmann A, Wankner S. Implicit memory function in fibromyalgia syndrome. Behav Med. 2013;39(1):11–16. doi:10.1080/08964289.2012.708684
25. DEl Paso GA R, Pulgar A, Duschek S, Garrido S. Cognitive impairment in fibromyalgia syndrome: the impact of cardiovascular regulation, pain, emotional disorders and medication. Eur J Pain. 2012;16(3):421–429. doi:10.1002/j.1532-2149.2011.00032.x
26. Munguía-Izquierdo D, Legaz-Arrese A, Moliner-Urdiales D, Reverter-Masia J. Neuropsychological performance in patients with fibromyalgia syndrome: relation to pain and anxiety. Psicothema. 2008;20:427–431.
27. Bertolucci PH, de Oliveira FF. Cognitive impairment in fibromyalgia. Curr Pain Headache Rep. 2013;17(7):344. doi:10.1007/s11916-013-0344-9
28. Gelonch O, Garolera M, Valls J, Rosselló L, Pifarré J. Executive function in fibromyalgia: comparing subjective and objective measures. Compr Psychiatry. 2016;66:113–122. doi:10.1016/j.comppsych.2016.01.002
29. Hassett AL, Simonelli LE, Radvanski DC, Buyske S, Savage SV, Sigal LH. The relationship between affect balance style and clinical outcomes in fibromyalgia. Arthritis Rheum. 2008;59(6):833–840. doi:10.1002/art.23708
30. Teodoro T, Edwards MJ, Isaacs JD. A unifying theory for cognitive abnormalities in functional neurological disorders, fibromyalgia and chronic fatigue syndrome: systematic review. J Neurol Neurosurg Psychiatry. 2018;89(12):1308–1319. doi:10.1136/jnnp-2017-317823
31. Abeare CA, Cohen JL, Axelrod BN, Leisen JC, Mosley-Williams A, Lumley MA. Pain, executive functioning, and affect in patients with rheumatoid arthritis. Clin J Pain. 2010;26(8):683–689. doi:10.1097/AJP.0b013e3181ed1762
32. Brown SC, Glass JM, Park DC. The relationship of pain and depression to cognitive function in rheumatoid arthritis patients. Pain. 2002;96(3):279–284. doi:10.1016/S0304-3959(01)00457-2
33. Wolfe F, Smythe HA, Yunus MB, et al. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–172. doi:10.1002/art.1780330203
34. Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi:10.1002/art.1780310302
35. Rey A. L’examen Clinique En Psychologie. Paris: Presses Universitaires de France; 1964.
36. Peña-Casanova J, Gramunt-Fombuena N, Quiñones-Ubeda S, et al. Spanish Multicenter Normative Studies (NEURONORMA Project): norms for the Rey-Osterrieth complex figure (copy and memory), and free and cued selective reminding test. Arch Clin Neuropsychol. 2009;24(4):371–393. doi:10.1093/arclin/acp041
37. Benedet MJ, Alejandre MA, Pamos A Test de Aprendizaje Verbal España-Complutense: manual. Madrid: TEA Ediciones;1998.
38. Birnboim S. Strategy application test: discriminate validity studies. Can J Occup Ther. 2004;71(1):47–55. doi:10.1177/000841740407100109
39. Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS). New York: The Psychological Corporation; 2001.
40. Ibor JJ. The Trail Making Tests A+B. Schizophr Res. 2005;78:147–156. doi:10.1016/j.schres.2005.06.004
41. Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ. Behavioural Assessment of the Dysexecutive Syndrome. London: Thames Valley Test Company; 1996.
42. Vargas ML, Sanz JC, Marín JJ. Behavioral assessment of the dysexecutive syndrome battery (BADS) in schizophrenia: a pilot study in the Spanish population. Cogn Behav Neurol. 2009;22(2):95–100. doi:10.1097/WNN.0b013e318192cd08
43. First M, Spitzer RL, Gibbon M, Williams JBW. Entrevista Clínica Estructurada para los trastornos del eje I del DSM-IV: SCIDI. Versión Clínica. Paris: Masson; 1999.
44. Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi:10.1016/0304-3959(75)90044-5
45. Lázaro C, Bosch F, Torrubia R, Baños JE. The development of a Spanish Questionnaire for assessing pain: preliminary data concerning reliability and validity. Eur J Psychol Assess. 1994;10:145–151.
46. Spielberger CD, Gorsuch RL, Lushene RE. Manual del Cuestionario de Ansiedad Estado/Rasgo (STAI). Madrid: TEA Ediciones; 1982.
47. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi:10.1001/archpsyc.1961.01710120031004
48. Vázquez C, Sanz J. Fiabilidad y validez de la versión española del Inventario para la Depresión de Beck de 1978 en pacientes con trastornos psicológicos. Clin Salud. 1999;1:59–81.
49. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi:10.1001/archneur.1989.00520460115022
50. Bulbena A, Berrios GE, Fernández de Larrinoa P. Medición clínica en psiquiatría y psicología. Paris: Masson; 2000.
51. Bobes J, González MP, Sáinz PA, Bascarán MT, Iglesias C, Fernández J. M. Propiedades psicométricas del cuestionario Oviedo de Sueño. Psicothema. 2000;1:107–112.
52. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi:10.1016/j.jclinepi.2007.11.008
53. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi:10.3758/BRM.41.4.1149
54. Park DC, Glass JM, Minear M, Crofford LJ. Cognitive function in fibromyalgia patients. Arthritis Rheum. 2001;44(9):2125–2133. doi:10.1002/1529-0131(200109)44:9<2125::AID-ART365>3.0.CO;2-1
55. Cherry BJ, Zettel-Watson L, Shimizu R, Roberson I, Rutledge DN, Jones CJ. Cognitive performance in women aged 50 years and older with and without fibromyalgia. J Gerontol B Psychol Sci Soc Sci. 2014;69(2):199–208. doi:10.1093/geronb/gbs122
56. DEl Paso GA R, Montoro CI, Duschek S. Reaction time, cerebral blood flow, and heart rate responses in fibromyalgia: evidence of alterations in attentional control. J Clin Exp Neuropsychol. 2015;37(4):414–428. doi:10.1080/13803395.2015.1023265
57. Verdejo-García A, López-Torrecillas F, Calandre EP, Delgado-Rodríguez A, Bechara A. Executive function and decision-making in women with fibromyalgia. Arch Clin Neuropsychol. 2009;24(1):113–122. doi:10.1093/arclin/acp014
58. Walteros C, Sánchez-Navarro JP, Muñoz MA, Martínez-Selva JM, Chialvo D, Montoya P. Altered associative learning and emotional decision making in fibromyalgia. J Psychosom Res. 2011;70(3):294–301. doi:10.1016/j.jpsychores.2010.07.013
59. Glass JM, Park DC, Minear M, Crofford LJ. Memory beliefs and function in fibromyalgia patients. J Psychosom Res. 2005;58:263–269. doi:10.1016/j.jpsychores.2004.09.004
60. Kozora E, Laudenslager M, Lemieux A, West SG. Inflammatory and hormonal measures predict neuropsychological functioning in systemic lupus erythematosus and rheumatoid arthritis patients. J Int Neuropsychol Soc. 2001;7(6):745–754. doi:10.1017/s1355617701766106
61. Tomasević-Todorovic S, Bosković K, Filipović D, Naumović N. Assessment of memory in patients with rheumatoid arthritis. Vojnosanit Pregl. 2011;68(6):481–488. doi:10.2298/vsp1106481t
62. Brunet HE, Kramer JH, Lupas GJ, Foley JM. Strategy use and verbal memory in older adults: the role of intellectual functioning and the preferential impact of semantic clustering. Clin Neuropsychol. 2020;34(1):204–216. doi:10.1080/13854046.2019.1590640
63. Guimond S, Béland S, Lepage M. Strategy for Semantic Association Memory (SESAME) training: effects on brain functioning in schizophrenia. Psychiatry Res Neuroimaging. 2018;271:50–58. doi:10.1016/j.jocn.2019.08.027
64. Morimoto SS, Gunning FM, Kanellopoulos D, et al. Semantic organizational strategy predicts verbal memory and remission rate of geriatric depression. Int J Geriatr Psychiatry. 2012;27(5):506–512. doi:10.3109/10582452.2013.806977
65. Miotto EC, Savage CR, Evans JJ, et al. Semantic strategy training increases memory performance and brain activity in patients with prefrontal cortex lesions. Clin Neurol Neurosurg. 2013;115(3):309–316. doi:10.1016/j.clineuro.2012.05.024
66. Ford J, Zheng B, Hurtado B, et al. Strategy or symptom: semantic clustering and risk of Alzheimer’s disease-related impairment. J Clin Exp Neuropsychol. 2020;42(8):849–856. doi:10.1080/13803395.2020.1819964
67. Davydov DM, Galvez-Sánchez CM, Montoro CI, de Guevara CML, Reyes DEl Paso GA. Personalized behavior management as a replacement for medications for pain control and mood regulation. Sci Rep. 2021;11(1):20297. doi:10.1038/s41598-021-99803-x
68. Shin SY, Julian L, Katz P. The relationship between cognitive function and physical function in rheumatoid arthritis. J Rheumatol. 2013;40(3):236–243. doi:10.3899/jrheum.120871
69. Bruehl S. Personalized pain medicine: pipe dream or reality? Anesthesiology. 2015;122(5):967–978. doi:10.1097/ALN.0000000000000638
70. Braš M, Dorđević V, Milunović V, Brajković L, Miličić D, Konopka L. Person-centered medicine versus personalized medicine: is it just a sophism? A view from chronic pain management. Psychiatry Danub. 2011;23(3):246–250.
71. Cutolo M, Kitas GD, van Riel PL. Burden of disease in treated rheumatoid arthritis patients: going beyond the joint. Semin Arthritis Rheum. 2014;43(4):479–488. doi:10.1016/j.semarthrit.2013.08.004
72. Ibraheem W, Mckenzie S, Wilcox-Omubo V, et al. Pathophysiology and clinical implications of cognitive dysfunction in fibromyalgia. Cureus. 2021;13(10):e19123. doi:10.7759/cureus.19123
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Short Sleep Duration as a Risk Factor for Depression, Anxiety and Fatigue in Patients with Leukemia
Huan Y, Mujun X, Xin L, Ping Z, Limei F, Aming L, Xinquan L
Neuropsychiatric Disease and Treatment 2022, 18:1573-1582
Published Date: 29 July 2022
RETRACTED ARTICLE: Psychotherapy and Follow-Up in Health Care Workers After the COVID-19 Epidemic: A Single Center’s Experience
Chen H, Ma Q, Du B, Huang Y, Zhu SG, Li SL, Geng DQ, Xu XS
Psychology Research and Behavior Management 2022, 15:2245-2258
Published Date: 18 August 2022
Subjective Sleep Disruption and Mood Disorders are Associated with the Risk of Chronic Pain in Patients with Obstructive Sleep Apnea
Liu L, Li X, Xue P, Wu M, Zeng S, Dai Y, Zhou J
Nature and Science of Sleep 2022, 14:2023-2032
Published Date: 7 November 2022
Mind, Body and Machine: Preliminary Study to Explore Predictors of Treatment Response After a Sleep Robot Intervention for Adults with Insomnia
Støre SJ, Tillfors M, Wästlund E, Angelhoff C, Andersson G, Norell A
Nature and Science of Sleep 2023, 15:567-577
Published Date: 13 July 2023
Anxiety and Insomnia Mediate the Association of Fear of Infection and Fatigue: A Cross-Sectional Survey of Nurses Deployed to a COVID-19 Epicenter in China
Liu Z, Zhang H, Wang N, Feng Y, Liu J, Wu L, Liu Z, Liu X, Liang L, Liu J, Wu Q, Liu C
Journal of Multidisciplinary Healthcare 2023, 16:2439-2448
Published Date: 24 August 2023

