Back to Journals » Vascular Health and Risk Management » Volume 18
Various Obesity Indices and Arterial Function Evaluated with CAVI – Is Waist Circumference Adequate to Define Metabolic Syndrome?
Authors Nagayama D , Sugiura T, Choi SY , Shirai K
Received 12 June 2022
Accepted for publication 24 August 2022
Published 12 September 2022 Volume 2022:18 Pages 721—733
DOI https://doi.org/10.2147/VHRM.S378288
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Harry Struijker-Boudier
Daiji Nagayama,1,2 Tomonori Sugiura,3 Su-Yeon Choi,4 Kohji Shirai5
1Department of Internal Medicine, Nagayama Clinic, Tochigi, Japan; 2Center of Diabetes, Endocrinology and Metabolism, Toho University, Sakura Medical Center, Chiba, Japan; 3Department of Cardiology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan; 4Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Republic of Korea; 5Department of Internal Medicine, Mihama Hospital, Chiba, Japan
Correspondence: Daiji Nagayama, Nagayama Clinic, 2-12-22, Tenjin-Cho, Oyama-City, Tochigi, 323-0032, Japan, Tel/Fax +81-285-22-0219, Email [email protected]
Abstract: Obesity has been known to relate to various diseases and metabolic disorders. Since the implication of body shape has been mentioned, obesity can be divided into visceral obesity and subcutaneous obesity. The former is considered the upstream pathophysiology of metabolic syndrome (MetS), and has been emphasized worldwide for the prevention of cardiovascular diseases in the last quarter century. However, some prospective studies have shown that cardiovascular mortality and morbidity are not necessarily higher in patients with MetS compared to those without. Recently, cardio-ankle vascular index (CAVI) has been established as an indicator of arteriosclerosis. This parameter is independent of blood pressure at the measuring time, and reflects systemic arterial stiffness from the aortic origin to the ankle. However, since CAVI is not necessarily high in MetS patients, attempts have been made to clarify this unexpected phenomenon. In several studies, CAVI was found to correlate negatively with body mass index (BMI), and also with waist circumference (WC) which is a widely used representative visceral obesity index. On the other hand, a body shape index (ABSI) is also a visceral obesity index designed to be minimally associated with BMI, and is calculated by dividing WC by an allometric regression of weight and height. Replacing high WC with high ABSI in MetS diagnosis promoted the identification of MetS patients with increased CAVI in cross-sectional studies on Japanese and Korean populations. Additionally, the incidence of MetS diagnosed using high ABSI was associated with significant increase in CAVI after 1 year of observation. Enhanced predictive ability for renal function decline by replacing WC with ABSI in MetS diagnosis was also observed in a longitudinal study in Japanese urban residents. These findings suggest that MetS diagnosis using high ABSI instead of high WC as a visceral obesity index needs to be reconsidered. However, further research is desirable on Caucasian, whose body shape differs slightly from that of Asians.
Keywords: CAVI, metabolic syndrome, waist circumference, a body shape index, renal function decline
Plain Language Summary
- Cardio-ankle vascular index (CAVI) is a candidate arterial stiffness parameter.
- CAVI is not necessarily high in individuals with metabolic syndrome (MetS).
- A body shape index (ABSI) more accurately reflects increased CAVI than waist circumference (WC).
- MetS diagnosis using high ABSI instead of high WC is valid to identify individuals at risk of increased CAVI and renal function decline.
Waist Circumference is Regarded as a Visceral Obesity Index to Define Metabolic Syndrome
Obesity defined by body mass index (BMI) has been recognized as a leading cause of increased mortality through various metabolic disorders.1 However, since BMI does not reflect body composition or fat distribution, various body adiposity indices have been proposed to assess body shape.2 Obesity can be divided into visceral obesity and subcutaneous obesity, with the former known to be closely associated with metabolic disorders such as impaired glucose tolerance, metabolic dyslipidemia (hypertriglyceridemia and hypo-HDL cholesterolemia) and elevated blood pressure (BP).3,4 These metabolic disorders combined with waist circumference (WC), an indicator of visceral fat accumulation, are used to diagnose metabolic syndrome (MetS). The diagnostic criteria for MetS have been issued separately by several different societies, each with their own criteria for each component dealing with the different cutoffs.5,6 On the other hand, all diagnostic criteria for MetS commonly adopt WC as a visceral obesity index, which does not reflect body size and shape.
Several epidemiological studies have demonstrated that MetS increases the risk of not only cardiovascular disease (CVD), but also morbidity and mortality.7,8 Nevertheless, the validity of MetS criteria remains controversial. Reaven9 reported the fact that there are many non-MetS patients who are clearly at higher CVD risks than MetS patients. Furthermore, it has been claimed that MetS does not necessarily predict CVD risk above and beyond its individual components.10,11
Cardio-Ankle Vascular Index (CAVI), a Systemic Arterial Stiffness Parameter
Systemic arterial stiffness reflects vascular aging and reduced elasticity of the artery, and is used as a predictor of CVDs.12 Pulse wave velocity (PWV) has been used as a gold standard to assess vascular function worldwide. However, PWV is essentially affected by blood pressure (BP) at the measuring time,13 and thus, may underestimate the degree of vascular dysfunction caused by CVD risks other than hypertension. To overcome this problem, cardio-ankle vascular index (CAVI) has been established. CAVI, which reflects the stiffness of the arterial tree from the aortic origin to the ankle without dependency on BP, is now almost established as an index of arteriosclerosis.14 This arterial stiffness parameter has been reported to be associated positively with a number of CVD risk factors,15–17 severity of CVD18 and future CVD events.19 Additionally, appropriate therapeutic interventions to reduce CAVI are expected to contribute to prevent future CVD events.20
Recently, Spronck et al21 have disputed that CAVI is inherently dependent on BP at the measuring time, and proposed CAVI0 as a variant form of CAVI that theoretically excludes dependency on BP. However, it was noted that CAVI0 may underestimate arterial stiffness in individuals with high diastolic pressure due to its modification.22 Moreover, we confirmed the superior predictability of CAVI compared to PWV and CAVI0 for renal function decline in a longitudinal study of Japanese urban residents.23
Despite the establishment of superior predictability for CVD of CAVI, current MetS criteria is not necessarily associated with increased CAVI.24–26 This fact is consistent with the aforementioned findings that MetS is inadequate for the identification of CVD risks. Against these backgrounds, this review aimed to examine why CAVI is not high in patients with MetS. Furthermore, the suitable visceral obesity index alternative to WC for MetS diagnosis was validated.
The Relationship Between Various Obesity Indices and CAVI
We previously investigated the relationship of CAVI with BMI in Japanese urban residents receiving health screening.27 As shown in Figure 1, CAVI adjusted for age, systolic BP and HDL-cholesterol was negatively associated with BMI in both sexes. This linear inverse relationship was observed from a BMI of less than 18.5 kg/m2 to more than 28 kg/m2. The similar results were also obtained in other Japanese workers28 and Korean population.29 Furthermore, the similar relationships with BMI were observed also in other arterial stiffness parameters such as brachial-ankle PWV (baPWV)30 and flow mediated dilation.31 These findings are consistent with the “obesity paradox”, meaning that obesity sometimes contributes to improved prognosis in CVD patients. Simultaneously, adipose tissue mass reflected in BMI may be a protective factor for systemic arterial stiffening. Accordingly, the suitable visceral obesity index as a surrogate marker of visceral fat accumulation that exerts vascular toxicity should be independent of BMI.
 |
Figure 1 Relationship between adjusted cardio-ankle vascular index and BMI in (A) male and (B) female subjects. 23,257 Japanese urban residents (median age 47 years) who participated in a public health screening program. CAVI was adjusted by age, SBP, and HDL-C. Data are presented as mean ± standard deviation. A significant linear trend [F = 53.732, p < 0.001 in (A) and F = 61.386, p < 0.001 in (B)] calculated using ANOVA was observed for the whole BMI distribution. Reproduced from Nagayama D, Imamura H, Sato Y, et al. Inverse relationship of cardio-ankle vascular index with BMI in healthy Japanese subjects: a cross-sectional study. Vasc Health Risk Manag. 2016;13:1–9.27 Abbreviations: CAVI, cardio-ankle vascular index; BMI, body mass index; SBP, systolic blood pressure; HDL-C, high-density-lipoprotein cholesterol; ANOVA, analysis of variance. |
Next, the correlations between BMI and the following body adiposity indices were examined in 62,514 Japanese urban residents.2 We adopted the indices whose usefulness has been reported in several major medical journals and which can be calculated from height, weight and WC.
BMI = Weight (kg)/Height2 (m)
A body shape index32 = WC (m) × Height (m)5/6 × Weight (kg)2/3
Waist-to-height ratio33 = WC (m)/Height (m)
WC/BMI ratio34 = WC (m)/BMI (kg/m2)
Conicity index35 = WC (m)/ [0.109 × √ {Weight (kg)/ Height (m)}]
Resultantly, Waist-to-height ratio (WHtR), WC/BMI ratio, Conicity index and WC correlated strongly with BMI, whereas only ABSI hardly correlated with BMI as shown in Table 1. In addition, Sugiura et al28 reported that WC was negatively correlated with CAVI as well as BMI in Japanese workers (Table 2), indicating the inadequacy of WC as a visceral obesity index.
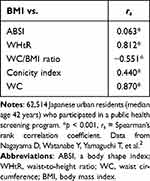 |
Table 1 Correlation Between BMI and Body Adiposity Indices |
 |
Table 2 Results of Univariate Regression Analysis Showing Relationships Among Obesity-Related Indices, Cardio-Ankle Vascular Index, and Carotid Intima-Media Thickness in All Participants (n = 7750) |
In 1983, the distribution of body fat was assessed by computed tomography (CT) in Japan,6 and the contribution of visceral fat accumulation to the development of metabolic disorders was mentioned.36 The association of visceral fat area with CAVI in Japanese workers has been already reported (Table 2).28 Furthermore, Choi et al37 reported that not only visceral fat area, but also epicardial fat mass obtained using CT were related positively with CAVI in Korean population. These results suggest that CAVI reflects the severity of systemic arterial stiffening associated with visceral/ectopic fat accumulation. Simultaneously, considering the inverse relationship of CAVI with BMI and WC, the vasoprotective effect of subcutaneous adipose tissue was speculated. Anyway, those results might indicate that CAVI could distinguish benign (subcutaneous type) obesity and malignant (visceral type) obesity. In addition, it is speculated that the reason why CAVI did not show higher values in MetS patients might be due to the current MetS definition using WC as an indicator of visceral fat accumulation.
The Relationship of ABSI with CAVI and Renal Function
CT is considered the gold standard in the assessment of body fat compartments and visceral fat. However, CT is expensive, exposed radiation and lacked versatility, so there is a need for a simple and non-invasive visceral obesity index.
We therefore validated a visceral obesity index more suitable than WC for MetS diagnosis. As shown in Tables 3 and 4, the contribution of each body adiposity index to high CAVI or renal impairment was investigated in Japanese urban residents.2 High CAVI was arbitrarily defined as equal to or higher than 9.0, corresponding substantially to the cutoff for the presence of coronary artery disease.18,20 In addition, renal impairment was defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2, corresponding to GFR category 3a or worse.38 The receiver operating characteristic analyses were used to evaluate the discriminatory power of each body adiposity indices for high CAVI or renal impairment.
 |
Table 3 Discriminatory Powers of Body Adiposity Indices for High CAVI (≥ 9.0) |
 |
Table 4 Discriminatory Powers of Body Adiposity Indices for eGFR < 60 Ml/Min/1.73m2 |
Resultantly, ABSI showed the highest discriminatory power for high CAVI in both sexes (Area under curve: 0.728 in men, 0.734 in women), indicating the superiority of ABSI to reflect vascular toxicity induced by visceral obesity (Table 3). For contribution to renal impairment, all body adiposity indices were of similar strength, except for the WC/BMI ratio in both sexes (Table 4). The ABSI cutoff value for high CAVI estimated by Youden’s J Index was 0.079 (sensitivity 0.683, specificity 0.658) in men, and 0.081 (sensitivity 0.651, specificity 0.700) in women. Besides, the ABSI cutoff value for renal impairment was calculated to be 0.080 in both sexes (male: sensitivity 0.497, specificity 0.698; female: sensitivity 0.625, specificity 0.541). Based on these findings, an ABSI cutoff value of 0.080 for the definition of MetS in the following studies.
To further confirm the differential contribution to CAVI by BMI, WC and ABSI, we examined the relationship of CAVI adjusted by gender, age and systolic BP with stratified tertiles of each body adiposity index as shown in Figure 2. Resultantly, BMI and WC were negatively correlated with adjusted CAVI (Figure 2A and B), whereas ABSI was positively correlated (Figure 2C).
 |
Figure 2 Comparison of metabolic disorder severity and adjusted CAVI stratified by tertiles of each adiposity index. 62,514 Japanese urban residents (median age 42 years) who participated in a public health screening program. Relationship of adjusted CAVI with tertiles of (A) BMI, (B) WC and (C) ABSI. CAVI was adjusted by gender, age, and SBP. Data are presented as mean ± standard error. *p < 0.001, ANOVA followed by post-hoc Bonferroni method. Data from Nagayama D, Watanabe Y, Yamaguchi T, et al.2 Abbreviations: BMI, body mass index; WC, waist circumference; ABSI, a body shape index; SBP, systolic blood pressure; CAVI, cardio-ankle vascular index. |
It is already known that ABSI not only reflects systemic arterial stiffening, but also achieves efficient risk stratification of all-cause mortality regardless of obesity determined by BMI.39 However, cutoffs and gender differences for the relationship of ABSI with cardiometabolic disorders may differ between ethnic groups, and further validation is required.
The Significance of MetS Defined Using ABSI Instead of WC
An ABSI cutoff value of 0.080 for atherosclerotic disease seems appropriate in Japanese, as mentioned above. Details of 3 representative MetS criteria (Japanese, IDF, and NCEP-ATPIII) are shown in Table 5. All current criteria adopt WC as a visceral obesity index, although the cutoff values differ between criteria. It was proposed to change the criterion of visceral obesity from high WC to high ABSI (ABSI ≥ 0.080) in both sexes for all 3 criteria. The validity of this proposal for predicting increased CAVI and renal function decline was then examined by cross-sectional and longitudinal studies.
 |
Table 5 Proposal to Replace High WC by High ABSI as the Criterion of Visceral Obesity in the Diagnosis of MetS |
Cross-Sectional Studies on CAVI in Japanese and Korean Population
The differences in age-adjusted CAVI between MetS and non-MetS Japanese urban residents diagnosed by various MetS criteria using WC (WC-MetS) or ABSI (ABSI-MetS) are shown in Figure 3.5 Age-adjusted CAVI did not differ significantly between subjects diagnosed with Japanese WC-MetS or NCEP-ATPIII WC-MetS compared to those without the diagnosis (Figure 3A and C). In contrast, age-adjusted CAVI was clearly higher in subjects diagnosed with Japanese ABSI-MetS or NCEP-ATPIII ABSI-MetS compared to those without the diagnosis (Figure 3D and F) in both sexes. For IDF criteria only, IDF-MetS subjects had higher age-adjusted CAVI than non-IDF-MetS subjects when diagnosed using either WC or ABSI (Figure 3B and E).
 |
Figure 3 Comparison of age-adjusted CAVI in MetS (+) vs MetS (-) diagnosed by various MetS criteria using WC or ABSI. MetS was diagnosed by (A) Japanese, (B) IDF and (C) NCEP-ATPIII criteria using WC, and also diagnosed by (D) Japanese, (E) IDF and (F) NCEP-ATPIII criteria using ABSI instead of WC. Data are presented as mean ± standard deviation and analyzed by One way analysis of covariance with the covariate set to age followed by Bonferroni multiple comparison tests. Adapted from Nagayama D, Fujishiro K, Tsuda S, et al. Enhanced prediction of renal function decline by replacing waist circumference with “A Body Shape Index (ABSI)” in diagnosing metabolic syndrome: a retrospective cohort study in Japan. Int J Obes (Lond). 2022;46(3):564–573.5 Abbreviations: CAVI, cardio-ankle vascular index; WC-MetS, conventional metabolic syndrome (MetS) diagnosed using waist circumference (WC); ABSI-MetS, MetS diagnosed using a body shape index (ABSI) instead of WC; Japanese, criteria developed by the Japanese Committee for the Diagnostic Criteria of MetS; IDF, International Diabetes Federation; NCEP-ATPIII; National Cholesterol Education Program Adult Treatment Panel III. |
Sugiura et al40 also emphasized that ABSI could serve to identify individuals with MetS components and increased CAVI, in 10,182 middle-aged Japanese workers in Toyota Motor Corporation (Toyota, Japan) (Figure 4). This report then revealed that the cutoff for ABSI corresponding to high VFA (≥ 100 cm2) was 0.078, nevertheless ABSI was associated with CAVI independently of VFA.
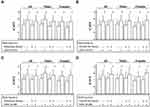 |
Figure 4 Relationships of CAVI and MetS based on the combination of visceral obesity or visceral fat obesity and different two cutoffs ABSI obesity in all participants, male subjects, and female subjects. 10,182 middle-aged untreated Japanese workers (mean age 46.2 years) who underwent periodic health check-ups. Among subjects with MetS based on visceral obesity (A and C) or visceral fat obesity (B and D), those with MetS who had ABSI ≥0.078 (A and B) or ABSI ≥ 0.080 (C and D) had significantly higher CAVI than those who did not, in all participants, male subjects, and female subjects. *p < 0.0001, †p < 0.001, #p < 0.01 vs subjects without MetS based on ABSI obesity in each subgroup. Visceral obesity was defined as waist circumference (WC) ≥85 cm for men and ≥ 90 cm for women, and visceral fat obesity as visceral fat area (VFA) ≥100 cm2. MetS was defined as the presence of obesity according to either obesity index and two or more of the following three criteria: (1) triglycerides ≥150 mg/dL and/or high-density lipoprotein cholesterol < 40 mg/dL; (2) systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg; and (3) fasting blood glucose ≥ 110 mg/dL. Reprinted from Clinical Nutrition ESPEN, Volume 46, Sugiura T, Dohi Y, Takagi Y, et al. A body shape index could serve to identify individuals with metabolic syndrome and increased arterial stiffness in the middle-aged population, Pages 251–258, Copyright (2021), with permission from Elsevier.40 Abbreviations: CAVI, cardio-ankle vascular index; ABSI, a body shape index. |
Similarly, Kim et al29 examined the contributions of ABSI-MetS for increased CAVI, defined as CAVI of the age-sex-strata specific highest quartile, in 7179 asymptomatic real-world Korean population (Table 6). ABSI ≥ 0.080 was adopted as visceral obesity to define ABSI-MetS. ABSI-MetS showed stronger independent association with increased CAVI compared to WC-MetS [OR (95% CI): 1.69 (1.51–1.90) vs 1.32 (1.18–1.48)], regardless of gender. In addition, although increased WC was not associated with increased risk of increased CAVI, increased ABSI showed a significant association with increased CAVI in both sexes.
 |
Table 6 Association of Metabolic Syndrome and Metabolic Syndrome Components with Increased Arterial Stiffness |
Longitudinal Study on the Change of CAVI in Japanese Urban Residents
Prospective role of MetS in the change of CAVI over 1 year period was examined in Japanese urban residents without WC-MetS (N=16,722) or ABSI-MetS (N=16,844) at baseline [Original data derived from database using Reference 5]. After one year, WC-MetS and ABSI-MetS occurred in 4.1% and 4.3% of participants, respectively. No significant differences in CAVI change were observed between subjects with and without WC-MetS incidence (ΔCAVI; 0.085±0.021 vs 0.058±0.004, p = 0.229 in Figure 5A). On the other hand, subjects with ABSI-MetS incidence showed significant higher CAVI change over 1 year compared to those without (ΔCAVI; 0.097±0.020 vs 0.060±0.004, p = 0.032 in Figure 5B).
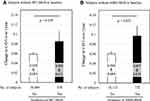 |
Figure 5 Comparison of changes in CAVI by incidence of WC-MetS or ABSI-MetS over 1 year. Change in CAVI over 1 year by incidence of (A) WC-MetS or (B) ABSI-MetS. Japanese urban residents (median age 48 years at baseline) who participated in a public health screening program. Data are presented as mean ± standard error; p values were calculated using Mann–Whitney U-test. In Japanese criteria, WC-MetS is defined as the presence of at least two of the following three abnormalities: [1] TG ≥ 150 mg/dl (1.69 mmol/l) and/or HDL-C < 40 mg/dl (1.03 mmol/l); [2] SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg; [3] FPG ≥ 110 mg/dl (6.11 mmol/l), in the presence of high WC (≥85 cm in men, and ≥ 90 cm in women). Treatments for dyslipidemia, hypertension and diabetes were counted as positive for the respective abnormalities. ABSI-MetS is defined by replacing high WC with high ABSI (≥ 0.080, both sexes) as the criterion of visceral obesity. Data from Nagayama D, Fujishiro K, Tsuda S, et al.5 Abbreviations: CAVI, cardio-ankle vascular index; WC, waist circumference; MetS, metabolic syndrome; ABSI, a body shape index; TG, triglyceride; HDL-C, high-density lipoprotein-cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose. |
Longitudinal Study on Renal Function Decline in Japanese Urban Residents
Finally, we examined whether the diagnosis of ABSI-MetS is useful for predicting new-onset renal function decline in Japanese urban residents.5 Figure 6 shows Kaplan–Meier survival curves for the new-onset renal function decline (eGFR < 60 mL/min/1.73m2) when MetS was diagnosed by various criteria shown in Table 5.
 |
Figure 6 Kaplan-Meier curves for the rate of renal function decline in MetS (+) and MetS (–) diagnosed by various MetS criteria using WC or ABSI. 5438 Japanese urban residents (median age 48 years at baseline) who participated in a public health screening program. MetS was diagnosed by (A) Japanese, (B) IDF and (C) NCEP-ATPIII criteria using WC, and also diagnosed by (D) Japanese, (E) IDF and (F). NCEP-ATPIII criteria using ABSI instead of WC. Reproduced from Nagayama D, Fujishiro K, Tsuda S, et al. Enhanced prediction of renal function decline by replacing waist circumference with “A Body Shape Index (ABSI)” in diagnosing metabolic syndrome: a retrospective cohort study in Japan. Int J Obes (Lond). 2022;46(3):564–573.5 Abbreviations: WC-MetS, conventional metabolic syndrome (MetS) diagnosed using waist circumference (WC); ABSI-MetS, MetS diagnosed using a body shape index (ABSI) instead of WC; Japanese, criteria developed by the Japanese Committee for the Diagnostic Criteria of MetS; IDF, International Diabetes Federation; NCEP-ATPIII, National Cholesterol Education Program Adult Treatment Panel III. |
When using high ABSI in MetS diagnosis, the cumulative rate of renal function decline occurring over 4 years was significantly higher in ABSI-MetS subjects than in non ABSI-MetS subjects diagnosed by Japanese, IDF and NCEP-ATPIII criteria (Figure 6D–F). On the other hand, when using high WC in MetS diagnosis, the cumulative rates of renal function decline were comparable in WC-MetS and non-WC-MetS subjects diagnosed by Japanese and NCEP-ATPIII criteria (Figure 6A and C), although the rate was significantly higher in IDF WC-MetS subjects than in non IDF WC-MetS subjects (Figure 6B), similar to the result of IDF ABSI-MetS (Figure 6E). In subsequent gender-specific Cox-proportional hazards analyses including age, proteinuria, and treatments of metabolic disorders as confounders, ABSI-MetS (Japanese criteria for both sexes, IDF criteria for men) contributed independently to the renal function decline. Namely, replacing high WC with high ABSI in MetS criteria may more efficiently predict individuals at risk of renal function decline and systemic arterial stiffening.
The Pathophysiology to Explain Relationship of ABSI with CAVI and Renal Function Decline
Why does the use of ABSI instead of WC contribute to the identification of individuals at risk of high CAVI and renal function decline? The answer is that ABSI reflects atherogenic pathophysiology induced by visceral obesity more strongly than WC. Following the ABSI concept, it may be possible to genuinely assess the vascular toxicity induced by visceral fat accumulation by minimizing the influence of vasoprotective body composition as reflected by BMI. In other words, high BMI may be associated with increased subcutaneous fat-derived vasorelaxant factors, and further validation is warranted. In addition, ABSI may also reflect not only visceral fat accumulation but also renal ectopic fat accumulation that induces obesity-related glomerulopathy. Considering that ABSI reflects metabolic disorders regardless of obesity,41 it is possible that ABSI predicted obesity related glomerulopathy even in the current study population, which mainly included non-overweight individuals.
Conclusions
MetS is currently defined using WC as a potent pathology causing CVD. However, several epidemiologic studies revealed that arterial stiffness assessed by CAVI is not necessarily high in MetS patients. Considering cross-sectional and longitudinal studies in Japanese and Korean populations, this article shows that the conventional MetS definition using WC is inadequate for the extraction of atherosclerotic diseases, and that this problem may be solved by using ABSI instead of WC. It was then proposed to use ABSI as visceral obesity index in MetS diagnosis. However, there is concern that WC and the standards for WC and CAVI may differ from ethnic group to ethnic group. Further research is also needed on Caucasian, whose body shape differs slightly from that of Asians.
Future Challenges
1. Whether diagnosis of MetS using ABSI may predict CVD morbidity and mortality.
2. Whether ABSI-lowering therapeutic approaches may contribute to improved outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cameron AJ, Magliano DJ, Shaw JE, et al. The influence of Hip circumference on the relationship between abdominal obesity and mortality. Int J Epidemiol. 2012;41:484–494. doi:10.1093/ije/dyr198
2. Nagayama D, Watanabe Y, Yamaguchi T, et al. New index of abdominal obesity, a body shape index, is BMI-independently associated with systemic arterial stiffness in real-world Japanese population. Int J Clin Pharmacol Ther. 2020;58(12):709–717. doi:10.5414/CP203778
3. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi:10.1016/s0140-6736(05)66378-7
4. Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011;18(8):629–639. doi:10.5551/jat.7922
5. Nagayama D, Fujishiro K, Tsuda S, et al. Enhanced prediction of renal function decline by replacing waist circumference with “A Body Shape Index (ABSI)” in diagnosing metabolic syndrome: a retrospective cohort study in Japan. Int J Obes. 2022;46(3):564–573. doi:10.1038/s41366-021-01026-7
6. Nagayama D, Watanabe Y, Yamaguchi T, et al. Issue of waist circumference for the diagnosis of metabolic syndrome regarding arterial stiffness: possible utility of a body shape index in middle-aged nonobese Japanese urban residents receiving health screening. Obes Facts. 2022;15(2):160–169. doi:10.1159/000520418
7. Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi:10.2337/diacare.24.4.683
8. Ninomiy T, Kubo M, Doi Y, et al. Impact of metabolic syndrome on the development of cardiovascular disease in a general Japanese population: the Hisayama study. Stroke. 2007;38(7):2063–2069. doi:10.1161/STROKEAHA.106.479642
9. Reaven GM. The metabolic syndrome: is this diagnosis necessary? Am J Clin Nutr. 2006;83(6):1237–1247. doi:10.1093/ajcn/83.6.1237
10. Sundström J, Vallhagen E, Risérus U, et al. Risk associated with the metabolic syndrome versus the sum of its individual components. Diabetes Care. 2006;29(7):1673–1674. doi:10.2337/dc06-0664
11. Guembe MJ, Toledo E, Barba J, et al. Association between metabolic syndrome or its components and asymptomatic cardiovascular disease in the RIVANA-study. Atherosclerosis. 2010;211(2):612–617. doi:10.1016/j.atherosclerosis.2010.03.004
12. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121(4):505–511. doi:10.1161/CIRCULATIONAHA.109.886655
13. Shirai K, Song M, Suzuki J, et al. Contradictory effects of β1- and α1- aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)–CAVI independent of blood pressure. J Atheroscler Thromb. 2011;18:49–55. doi:10.5551/jat.3582
14. Shirai K, Utino J, Otsuka K, et al. A novel blood pressure independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13:101–107. doi:10.5551/jat.13.101
15. Nagayama D, Watanabe Y, Saiki A, et al. Lipid parameters are independently associated with cardio-ankle vascular index (CAVI) in healthy Japanese subjects. J Atheroscler Thromb. 2018;25:621–633. doi:10.5551/jat.42291
16. Nagayama D, Watanabe Y, Saiki A, et al. Difference in positive relation between cardio-ankle vascular index (CAVI) and each of four blood pressure indices in real-world Japanese population. J Hum Hypertens. 2019;33(3):210–217. doi:10.1038/s41371-019-0167-1
17. Nagayama D, Yamaguchi T, Saiki A, et al. High serum uric acid is associated with increased cardio-ankle vascular index (CAVI) in healthy Japanese subjects: a cross-sectional study. Atherosclerosis. 2015;239:163–168. doi:10.1016/j.atherosclerosis.2015.01.011
18. Nakamura K, Tomaru T, Yamamura S, et al. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J. 2008;72:598–604. doi:10.1253/circj.72.598
19. Sato Y, Nagayama D, Saiki A, et al. Cardio-ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb. 2016;23:596–605. doi:10.5551/jat.31385
20. Saiki A, Ohira M, Yamaguchi T, et al. New horizons of arterial stiffness developed using cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2020;27(8):732–748. doi:10.5551/jat.RV17043
21. Spronck B, Avolio AP, Tan I, et al. Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens. 2017;35:98–104. doi:10.1097/HJH.0000000000001132
22. Shirai K, Suzuki K, Tsuda S, et al. Comparison of cardio–ankle vascular index (CAVI) and CAVI0 in large healthy and hypertensive populations. J Atheroscler Thromb. 2019;26:603–615. doi:10.5551/jat.48314
23. Nagayama D, Fujishiro K, Miyoshi T, et al. Predictive ability of arterial stiffness parameters for renal function decline: a retrospective cohort study comparing cardio-ankle vascular index, pulse wave velocity and cardio-ankle vascular index 0. J Hypertens. 2022;40(7):1294–1302. doi:10.1097/HJH.0000000000003137
24. Satoh N, Shimatsu A, Kato Y, et al. Evaluation of the cardio-ankle vascular index, a new indicator of arterial stiffness independent of blood pressure, in obesity and metabolic syndrome. Hypertens Res. 2008;31(10):1921–1930. doi:10.1291/hypres.31.1921
25. Topouchian J, Labat C, Gautier S, et al. Effects of metabolic syndrome on arterial function in different age groups: the Advanced Approach to Arterial Stiffness study. J Hypertens. 2018;36(4):824–833. doi:10.1097/HJH.0000000000001631
26. Gomez SL, Garcia OL, Patino AMC, et al.; MARK Group. Association of metabolic syndrome and its components with arterial stiffness in Caucasian subjects of the MARK study: a cross-sectional trial. Cardiovasc Diabetol. 2016;15(1):148. doi:10.1186/s12933-016-0465-7
27. Nagayama D, Imamura H, Sato Y, et al. Inverse relationship of cardioankle vascular index with BMI in healthy Japanese subjects: a cross-sectional study. Vasc Health Risk Manag. 2016;13:1–9. doi:10.2147/VHRM.S119646
28. Sugiura T, Dohi Y, Takagi Y, et al. Relationships of obesity-related indices and metabolic syndrome with subclinical atherosclerosis in middle-aged untreated Japanese workers. J Atheroscler Thromb. 2020;27:342–352. doi:10.5551/jat.50633
29. Kim S, Choi SY, Lee H, et al. Sex and age differences in the impact of metabolic syndrome and its components including a body shape index on arterial stiffness in the general population. J Atheroscler Thromb. 2022;29. doi:10.5551/jat.63371
30. Tang B, Luo F, Zhao J, et al. Relationship between body mass index and arterial stiffness in a health assessment Chinese population. Medicine. 2020;99(3):e18793. doi:10.1097/MD.0000000000018793
31. Juonala M, Viikari JSA, Laitinen T, et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation. 2004;110:2918–2923. doi:10.1161/01.CIR.0000147540.88559.00
32. Krakauer NY, Krakauer JC. Anthropometrics, metabolic syndrome, and mortality hazard. J Obes. 2018;2018:9241904. doi:10.1155/2018/9241904
33. Lee CM, Huxley RR, Wildman RP, et al. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61(7):646–653. doi:10.1016/j.jclinepi.2007.08.012
34. Corrada MM, Kawas CH, Mozaffar F, et al. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163(10):938–949. doi:10.1093/aje/kwj114
35. Valdez R. A simple model-based index of abdominal adiposity. J Clin Epidemiol. 1991;44(9):955–956. doi:10.1016/0895-4356(91)90059-i
36. Fujioka S, Matsuzawa Y, Tokunaga K, et al. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism. Metabolism. 1987;36(1):54–59. doi:10.1016/0026-0495(87)90063-1
37. Park HE, Choi SY, Kim HS, et al. Epicardial fat reflects arterial stiffness: assessment using 256-slice multidetector coronary computed tomography and cardio-ankle vascular index. J Atheroscler Thromb. 2012;19:570–576. doi:10.5551/jat.12484
38. Kidney Disease Improving Global Outcomes. Chapter 1: definition and classification of CKD. Kidney Int Suppl. 2013;3:19–62. doi:10.1038/kisup.2012.64
39. Christakoudi S, Tsilidis KK, Muller DC, et al. A Body Shape Index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: results from a large European cohort. Sci Rep. 2020;10(1):14541. doi:10.1038/s41598-020-71302-5
40. Sugiura T, Dohi Y, Takagi Y, et al. A body shape index could serve to identify individuals with metabolic syndrome and increased arterial stiffness in the middle-aged population. Clin Nutr ESPEN. 2021;46:251–258. doi:10.1016/j.clnesp.2021.10.001
41. Bertoli S, Leone A, Krakauer NY, et al. Association of Body Shape Index (ABSI) with cardiometabolic risk factors: a cross-sectional study of 6081 Caucasian adults. PLoS One. 2017;12:e0185013. doi:10.1371/journal.pone.0185013
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
