Back to Journals » Vascular Health and Risk Management » Volume 18
Is Nigella sativa an Effective Bodyweight Lowering Agent and a Mitigator of Obesity Risk? A Literature Review
Authors Al Asoom LI
Received 16 May 2022
Accepted for publication 2 July 2022
Published 12 July 2022 Volume 2022:18 Pages 495—505
DOI https://doi.org/10.2147/VHRM.S373702
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Lubna Al Asoom
Physiology Department, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
Correspondence: Lubna Al Asoom, Physiology Department, College of Medicine, Imam Abdulrahman Bin Faisal University, P.O. 2114, Dammam, 31541, Saudi Arabia, Tel +966 133335131, Email [email protected]
Abstract: Obesity is one of the major health-threatening conditions nowadays. Nigella sativa (NS) is a medicinal plant that demonstrates multiple therapeutic effects. In the current review, we aim to evaluate the weight lowering effect of NS in both clinical trials and experimental studies and to explore the possible reported mechanisms of this effect. We searched PubMed and Web of science and retrieved 14 clinical trials and 5 experimental studies that justify our inclusion criteria. After the analysis of these articles, we can conclude that long-term administration of NS for 6– 12 weeks can significantly lower bodyweight and other anthropometric indices. NS-oil is more potent than NS-powder in lowering bodyweight probably due to the higher concentration of fatty acids and thymoquinone. The weight lowering effect of NS is not a toxic effect, it conversely and preferably lowers the elevated liver enzymes in condition of fatty liver. It is also frequently accompanied by positive metabolic modifications, such as enhancement of lipid profile, lowering blood glucose and improving insulin resistance. Possible mechanisms for NS-bodyweight lowering effect might include an appetite-suppression effect, lowering caloric-intake and inhibition of intestinal glucose absorption. However, further experimental evidence is required to support these mechanisms or unveil new ones.
Keywords: Nigella sativa, obesity, bodyweight, BMI, waist-circumference
Introduction
Obesity is a major health-threatening condition. It affects about 1.9 billion people in the year 2016 worldwide, and its incidence continues to increase exponentially. The prevalence of overweight and obesity has increased by four folds from the year 1975 to the year 2016 in both adults and children, according to the World Health Organization report.1 The main concern about the increasing prevalence of obesity is that obesity is a risk factor for all-cause mortality and a predisposing factor for diabetes mellitus, hypertension, ischemic heart disease, stroke, and cancer.2 One of the proposed theories for the explanation of the high incidence of obesity is the contemporary modifications of the modern lifestyle; people tend to rely in their nourishment on the highly processed food, which is rich in sugar and fat, and ignore the healthy ingredients of nature, which provide fibers, vitamins, and antioxidants.3 Many natural products, which were frequently consumed by human in old ages, are found to be rich in antioxidants and provide numerous protective effects against multiple diseases, among which are the black seeds.4 The black seed is a medicinal plant known generically as Nigella sativa Linn and it belongs to the family of Ranunculaceae. It is composed of fixed oil, proteins, alkaloids, saponin and essential oil. The main constituents of the essential oil are thymoquinone in addition to other fatty acids, such as palmitoleic acid, palmitic acid, arachic acid, and linoleic acid.5 It is commonly used as a food herb and flavoring in the countries of the Middle East, India, and Iran. Furthermore, it is considered a natural remedy for multiple purposes, such as cough, asthma, stomach ache, vomiting, allergy, and eczema.6 Currently, the therapeutic effects of Nigella sativa (NS) are supported by accumulating evidence in the literature from extensive research work.7 Both experimental and clinical studies showed reproducible preferable effects of long-term supplementation of NS such as antioxidation and anti-inflammation.8 Not only this but also the intake of NS culminates in specific and favorable modulations of cardiac structure and function,9,10 vascular growth and regeneration,11,12 and immunoregulatory effects.13 NS in multiple studies demonstrated favorable modifications of the glucose and lipid metabolism in diabetic patients.14 However, less attention was given to the direct effect of NS on bodyweight and BMI. Few systematic reviews and meta-analyses are available in the literature, which discussed the effect of NS as an anti-obesity agent in human clinical trials. However, these systematic reviews included either numerous metabolic parameters in the discussion, missed some negative studies, excluded animal studies, or included multiple herbs besides NS.15–17 On the other hand, these reviews did not discuss the mechanisms of the anti-obesity effect of NS, particularly in regard to the bodyweight lowering effect. Therefore, in the present review, we aim to analyse and evaluate the effect of NS supplementation on the bodyweight and anthropometric values in both human and animal studies. Moreover, we aim to explore the reported mechanisms of NS weight-lowering effect if present.
Methods
We searched the following databases: PubMed and Web of Science using the following terms: Nigella sativa or black seed and bodyweight, or anthropometric measures, waist-circumference, or appetite with no time restriction. PubMed search first yielded 170 articles and Web of Science yielded 281 articles. We excluded reviews and included only research articles, clinical trials, systematic reviews and meta-analyses. After meticulous review, we included studies that used NS supplementation as a crude substance, either powdered, oil or extract. Studies that used thymoquinone only – which is the active ingredient of NS– were excluded. We also excluded the studies that used NS in combination with other drugs or other natural products and did not show the sole effect of NS in any of the tested groups. The selected clinical trials and experimental studies were then evaluated using CONSORT checklist for herbal medicine and ARRIVE 2, respectively. The final number of included studies was 14 clinical trials, and 5 animal experimental studies (Figure 1).
 |
Figure 1 Flow diagram for the process of article selection. Abbreviations: NS, Nigella sativa; WoS, Web of science. |
Results
We have reviewed 14 clinical trials and 5 animal studies for understanding and evaluating the effect of long-term administration of NS on bodyweight. Most of the clinical studies include both male and female participants (8 studies), while 5 includes women only and one study includes men only. The targeted populations were diverse but all shared metabolic derangements such as diabetic patients, prediabetics, patients with autoimmune hypothyroidism, patients with non-alcoholic fatty liver or obese and overweight individuals. All the included studies employed an oral administration of NS capsules that either contain NS-powder or oil. Ten out of the 14 clinical studies revealed significant effect of NS on bodyweight, BMI, and waist circumference, and the effect was more consistent with the use of NS-oil (Table 1). The experimental studies were mostly on rats (4 studies) and one on mice. Two of the animal studies were on male rats, one used both male and female rats, one used ovariectomised female rats, and one used female mice. The preparations of NS used in the experimental studies were either NS-oil, petroleum ether extract, or aqueous extract of NS-powder. The duration of NS feeding ranges from 6 to 12 weeks. Four out of 5 animal studies showed a significant effect of NS intake on the weight gain of the growing animals (Table 2).
 |  |  |
Table 1 Clinical Trials That Tested the Effect of Nigella sativa (NS) on the Anthropometric Data. The Studies are Presented in a Descending Chronological Order |
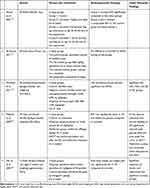 |
Table 2 Experimental Studies That Tested the Effect of Nigella sativa (NS) on Bodyweight |
The mechanisms of the weight reduction effect of NS were investigated by some authors, and the proposed mechanisms include induction of anorexia, reduction of intestinal glucose absorption, reduction of insulin secretion and increase in adiponectin level (Table 3).
 |
Table 3 Clinical Trials and Experimental Studies That Explore the Mechanisms of Nigella sativa (NS) Effect on Bodyweight and Anthropometric Data |
Discussion
In this review, we aimed to evaluate the bodyweight reduction effect of NS and explore the possible underlying mechanisms of this effect. Most of the analysed studies, whether clinical trials or experimental studies, revealed that NS administration in the form of NS-oil or powdered capsules for 6–12 weeks can culminate in bodyweight reduction as well as enhancement of BMI, waist, and hip circumference. We observed that studies that utilized NS-oil in doses of 1–3g/day for 6–12 weeks showed more pronounced effect on the bodyweight and the other anthropometric parameters than those which employed NS-powder of equivalent doses. This might be related to the difference in the composition of these two preparations for NS. NS-oil has higher concentrations of fatty acids, such as linoleic acid, linolenic acid, and palmitic acid in addition to a higher concentration of thymoquinone.38,39 Dalle et al demonstrated different composition and concentration of multiple specific active compounds, such as thymoquinone, multiple fatty acids, catechin, rutin and gallic acid when they used different methods and solvents for the extraction of NS.5 Therefore, higher NS-powder or extract doses might be needed to induce the bodyweight-lowering effect of NS, and this can explain why Al Asoom33 who utilized 800 mg/Kg dose of powdered NS failed to demonstrate weight lowering effect in rats, while Meddah et al35 who adopted 2g/Kg for rats showed a significant reduction of rat weight gain. In addition, the responsiveness of the targeted subjects in each of the included clinical trials might also be different, because those subjects suffer from different aspects of metabolic derangements; Some are healthy obese and overweight, but others are diabetics or patients with fatty liver or Hashimoto’s hypothyroidism.
Furthermore, we believe that the weight-lowering effect of NS is a positive therapeutic effect and not a sign of toxicity based on the findings of multiple studies that showed an improvement of liver enzymes when diabetic patients or patients of fatty liver were treated by NS.23,24 In addition, Le et al compared the acute effect of NS to Dimethyl sulfoxide (DMSO) on isolated hepatocytes and found no toxic effect of NS.36 Furthermore, the acute administration of high doses of NS-oil whether orally or intraperitoneally up to 28.8 mL/kg of bodyweight showed no signs of toxicity. Similarly, the oral administration of 6g/kg methanol extract of NS for 14 days showed no change in the activity of hepatic enzymes in histological preparation. NS-oil or thymoquinone exerted protective effect against the toxicity induced by cyclophosphamide.40 Thymoquinone also demonstrated in multiple studies a protective effect against the toxicity of chemotherapy in multiple types of cancer.41
Most of the analysed studies in this review reported the statistical significance and the safety of the weight-lowering effect of NS. However, one might argue the clinical significance of NS weight-lowering effect, but we can further illustrate that NS-weight lowering effect is also clinically significant based on the relatively large effect size of 0.6 for NS bodyweight-lowering and 0.4 for waist circumference reduction as reported by Safi et al18 as well as the frequently and repeatedly demonstrated metabolic effect of NS on lowering blood lipids including triglycerides, total cholesterol, LDL, blood glucose and HbA1C and the positive influence on HDL and insulin resistance.23,29 In the study performed by Mostafa et al,19 NS administration for 12 weeks was superior to metformin or diet restriction in modifying total cholesterol, LDL, and triglycerides. Moreover, the clinical significance of NS-weight lowering effect can also be augmented by lifestyle modifications such as caloric restriction and exercise training because evidence has been shown that the combined NS administration and lifestyle changes yielded better effect than each of these interventions separately.27,42
Mechanisms of NS-Induced Bodyweight Reduction
Most of the retrieved literature reflecting the weight reduction effect of NS lacks the explanation and the evidence of the exact mechanism of this effect. Some studies proposed an anorexic effect of NS and aimed to estimate the food intake or appetite modifications. Mahdavi et al,26 Farhangi et al,25 and Safi et al18 failed to demonstrate any difference in the total caloric intake of their NS-treated subjects compared to the control group, albeit Safi et al showed a reduction in appetite sensation using a visual scale of appetite before and after meals. On the other hand, Le et al36 showed a significant reduction in food intake by rats fed with NS compared to control rats. Despite these efforts to estimate the caloric intake or the changes in the appetite, there is a lack of exploration of the appetite regulation pathways and the influence of multiple peptides on the satiety and hunger centers of the hypothalamus.43 Few studies only, such as Moustafa et al23 and Le et al,36 showed a reduction in insulin secretion, and Mahdavi et al37 demonstrated an increase in adiponectin. However, more mediators and peptides in the pathway of appetite and energy balance need to be explored. Meddah et al35 showed an in vitro dose-dependent inhibition of intestinal glucose transport by NS. Similarly, Dalli et al,5 using different methods for NS extraction, also demonstrated an inhibition of intestinal glucose absorption by three different preparations of NS with a range of inhibition equals to 24.82–60.12%.
Limitations
The current review might be limited by the number of databases that were explored for related literature. In addition, the populations studied in the included articles were diverse in multiple metabolic diseases that might skew the interpretation of the effect of NS on bodyweight in healthy obese and overweight subjects.
Conclusion
The current review showed that the administration of about 1–3 g/day of NS for 6–12 weeks can culminate in a reduction of the bodyweight and other anthropometric indices. It further showed the advancement in the NS-related research particularly in the field of metabolic derangement. About two decades ago, NS-reported studies focused mainly on the effect of NS on diabetic metabolic profiles such as hyperglycemia, HbA1c and insulin resistance. Later, the studies started to explore the possible prophylactic influence of NS through recruiting prediabetic and healthy obese and overweight individuals and reflect explicitly on the direct effect of NS on the bodyweight and anthropometric data as primary outcomes.
In this review, we found that NS-oil might be superior to other NS-preparations in regard to the weight-lowering effect, and the doses used in all the reported clinical and experimental studies are safe and provide positive metabolic modifications that can minimize the hazards of obesity such as lowering the lipid profile and liver enzymes.
Therefore, NS can be recommended for bodyweight reduction along with lifestyle modifications in the form of diet restriction and high physical activity. Nevertheless, identification of the best preparation of NS for the induction of the weight-lowering effect is needed as well as the identification of the main active ingredients responsible for this effect. Moreover, large prospective clinical trials are also required to explore the efficacy and safety of NS on bodyweight in different age groups, such as children and adolescents. Finally, experimental work that focused on unveiling the exact mechanisms of NS bodyweight-lowering effect is still needed, which might open new paths for extra-therapeutic applications of NS.
Disclosure
The author reports no conflicts of interest in this work.
References
1. World Health Organization. Health topics; Obesity. Available from: https://www.who.int/health-topics/obesity. Accessed July 8, 2021.
2. Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5(7):161. doi:10.21037/atm.2017.03.107
3. Safaei M, Sundararajan EA, Driss M, Boulila W, Shapi’i A. A systematic literature review on obesity: understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput Biol Med. 2021;136:104754. doi:10.1016/j.compbiomed.2021.104754
4. Zheng J, Zhou Y, Li Y, Xu DP, Li S, Li HB. Spices for prevention and treatment of cancers. Nutrients. 2016;8:8. doi:10.3390/nu8080495
5. Dalli M, Daoudi NE, Azizi SE, Benouda H, Bnouham M, Gseyra N. Chemical composition analysis using HPLC-UV/GC-MS and inhibitory activity of different. Biomed Res Int. 2021;2021:9979419. doi:10.1155/2021/9979419
6. Dalli M, Bekkouch O, Azizi SE, Azghar A, Gseyra N, Kim BL. Phytochemistry and pharmacological activities: a review (2019–2021). Biomolecules. 2021;12(1):20. doi:10.3390/biom12010020
7. Bamosa A. Nigella sativa is a safe herbal product. J Integr Med. 2014;12(1):66. doi:10.1016/S2095-4964(14)60007-8
8. Hawsawi ZA, Ali BA, Bamosa AO. Effect of Nigella sativa (Black Seed) and thymoquinone on blood glucose in albino rats. Ann Saudi Med. 2001;21(3–4):242–244. doi:10.5144/0256-4947.2001.242
9. Al-Asoom LI, Al-Shaikh BA, Bamosa AO, El-Bahai MN. Comparison of Nigella sativa- and exercise-induced models of cardiac hypertrophy: structural and electrophysiological features. Cardiovasc Toxicol. 2014;14(3):208–213. doi:10.1007/s12012-014-9244-4
10. Al-Asoom LI, Al-Shaikh BA, Bamosa AO, El-Bahai MN. Effect of Nigella sativa supplementation to exercise training in a novel model of physiological cardiac hypertrophy. Cardiovasc Toxicol. 2014;14(3):243–250. doi:10.1007/s12012-014-9248-0
11. Al Asoom L. Inventor; Imam Abdulrahman Bin Faisal University, assignee. Method for effecting angiogenesis by administering Nigella sativa. Saudi Arabia; 2020.
12. Al Asoom LI. Molecular mechanisms of Nigella sativa- and Nigella sativa exercise-induced cardiac hypertrophy in rats. Evid Based Complement Altern Med. 2021;2021:5553022. doi:10.1155/2021/5553022
13. Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L seed. Int Immunopharmacol. 2005;5(13–14):1749–1770. doi:10.1016/j.intimp.2005.06.008
14. Kaatabi H, Bamosa AO, Badar A, et al. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS One. 2015;10(2):e0113486. doi:10.1371/journal.pone.0113486
15. Mohtashami A, Entezari MH. Effects of Nigella sativa supplementation on blood parameters and anthropometric indices in adults: a systematic review on clinical trials. J Res Med Sci. 2016;21:21. doi:10.4103/1735-1995.179889
16. Mousavi SM, Sheikhi A, Varkaneh HK, Zarezadeh M, Rahmani J, Milajerdi A. Effect of Nigella sativa supplementation on obesity indices: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2018;38:48–57. doi:10.1016/j.ctim.2018.04.003
17. Payab M, Hasani-Ranjbar S, Shahbal N, et al. Effect of the herbal medicines in obesity and metabolic syndrome: a systematic review and meta-analysis of clinical trials. Phytother Res. 2020;34(3):526–545. doi:10.1002/ptr.6547
18. Safi S, Razmpoosh E, Fallahzadeh H, et al. The effect of Nigella sativa on appetite, anthropometric and body composition indices among overweight and obese women: a crossover, double-blind, placebo-controlled, randomized clinical trial. Complement Ther Med. 2021;57:102653. doi:10.1016/j.ctim.2020.102653
19. Mostafa TM, Hegazy SK, Elnaidany SS, Shehabeldin WA, Sawan ES. Nigella sativa as a promising intervention for metabolic and inflammatory disorders in obese prediabetic subjects: a comparative study of Nigella sativa versus both lifestyle modification and metformin. J Diabetes Complications. 2021;35(7):107947. doi:10.1016/j.jdiacomp.2021.107947
20. Hadi S, Daryabeygi-Khotbehsara R, Mirmiran P, et al. Effect of Nigella sativa oil extract on cardiometabolic risk factors in type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2021;35(7):3747–3755. doi:10.1002/ptr.6990
21. Tavakoli-Rouzbehani OM, Abbasnezhad M, Kheirouri S, Alizadeh M. Effects of Nigella sativa oil supplementation on selected metabolic parameters and anthropometric indices in patients with coronary artery disease: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2021;35(7):3988–3999. doi:10.1002/ptr.7115
22. Shirazi M, Khodakarami F, Feizabad E, Ghaemi M. The effects of nigella sativa on anthropometric and biochemical indices in postmenopausal women with metabolic syndrome. Endocrine. 2020;69(1):49–52. doi:10.1007/s12020-020-02265-w
23. Moustafa HAM, El Wakeel LM, Halawa MR, Sabri NA, El-Bahy AZ, Singab AN. Effect of Nigella sativa oil versus metformin on glycemic control and biochemical parameters of newly diagnosed type 2 diabetes mellitus patients. Endocrine. 2019;65(2):286–294. doi:10.1007/s12020-019-01963-4
24. Hussain M, Tunio AG, Akhtar L, Shaikh GS. Effects of nigella sativa on various parameters in Patients of non-alcoholic fatty liver disease. J Ayub Med Coll Abbottabad. 2017;29(3):403–407.
25. Farhangi MA, Dehghan P, Tajmiri S, Abbasi MM. The effects of Nigella sativa on thyroid function, serum Vascular Endothelial Growth Factor (VEGF) - 1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: a randomized controlled trial. BMC Complement Altern Med. 2016;16(1):471. doi:10.1186/s12906-016-1432-2
26. Mahdavi R, Namazi N, Alizadeh M, Farajnia S. Effects of Nigella sativa oil with a low-calorie diet on cardiometabolic risk factors in obese women: a randomized controlled clinical trial. Food Funct. 2015;6(6):2041–2048. doi:10.1039/C5FO00316D
27. Namazi N, Mahdavi R, Alizadeh M, Farajnia S. Oxidative stress responses to nigella sativa oil concurrent with a low-calorie diet in obese women: a randomized, double-blind controlled clinical trial. Phytother Res. 2015;29(11):1722–1728. doi:10.1002/ptr.5417
28. Latiff LA, Parhizkar S, Dollah MA, Hassan ST. Alternative supplement for enhancement of reproductive health and metabolic profile among perimenopausal women: a novel role of Nigella sativa. Iran J Basic Med Sci. 2014;17(12):980–985.
29. Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54(4):344–354.
30. Datau EA, Surachmanto EE, Pandelaki K, Langi JA. Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med Indones. 2010;42(3):130–134.
31. Qidwai W, Hamza HB, Qureshi R, Gilani A. Effectiveness, safety, and tolerability of powdered Nigella sativa (kalonji) seed in capsules on serum lipid levels, blood sugar, blood pressure, and body weight in adults: results of a randomized, double-blind controlled trial. J Altern Complement Med. 2009;15(6):639–644. doi:10.1089/acm.2008.0367
32. Anwar N, Nadeem A, Naheed K, et al. Impact of Nigella sativa on body weight, volume and weight of ovary in letrozole induced polycystic ovarian syndrome in mice. Pak J Med Health Sci. 2021;15(9):2355–2357. doi:10.53350/pjmhs211592355
33. Al Asoom LI. Coronary angiogenic effect of long-term administration of Nigella sativa. BMC Complement Altern Med. 2017;17(1):308. doi:10.1186/s12906-017-1795-z
34. Parhizkar S, Latiff LA, Rahman SA, Dollah MA. Preventive effect of Nigella sativa on metabolic syndrome in menopause induced rats. J Med Plant Res. 2011;5(8):1478–1484.
35. Meddah B, Ducroc R, El Abbes Faouzi M, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009;121(3):419–424. doi:10.1016/j.jep.2008.10.040
36. Le PM, Benhaddou-Andaloussi A, Elimadi A, Settaf A, Cherrah Y, Haddad PS. The petroleum ether extract of Nigella sativa exerts lipid-lowering and insulin-sensitizing actions in the rat. J Ethnopharmacol. 2004;94(2–3):251–259. doi:10.1016/j.jep.2004.04.030
37. Mahdavi R, Alizadeh M, Namazi N, Farajnia S. Changes of body composition and circulating adipokines in response to Nigella sativa oil with a calorie restricted diet in obese women. J Herb Med. 2016;6(2):67–72. doi:10.1016/j.hermed.2016.03.003
38. Razmpoosh E, Safi S, Mazaheri M, et al. Effects of oral Nigella sativa oil on the expression levels and serum concentrations of adiponectin, PPAR-gamma, and TNF-alpha in overweight and obese women: a study protocol for a crossover-designed, double-blind, placebo-controlled randomized clinical trial. Trials. 2019;20(1). doi:10.1186/s13063-019-3568-0
39. Tiruppur VSK, Pattekhan H, Divakar S, US K. Chemical composition of Nigella sativa L. seed extracts obtained by supercritical carbon dioxide. J Food Sci Technol. 2010;47(6):598–605. doi:10.1007/s13197-010-0109-y
40. Hossain MS, Sharfaraz A, Dutta A, et al. A review of ethnobotany, phytochemistry, antimicrobial pharmacology and toxicology of Nigella sativa L. Biomed Pharmacother. 2021;143:112182. doi:10.1016/j.biopha.2021.112182
41. Jehan S, Huang J, Farooq U, Basheer I, Zhou W. Combinatorial effect of thymoquinone with chemo agents for tumor therapy. Phytomedicine. 2022;98:153936. doi:10.1016/j.phymed.2022.153936
42. Mahdavi R, Namazi N, Alizadeh M, Farajnia S. Nigella sativa oil with a calorie-restricted diet can improve biomarkers of systemic inflammation in obese women: a randomized double-blind, placebo-controlled clinical trial. J Clin Lipidol. 2016;10(5):1203–1211. doi:10.1016/j.jacl.2015.11.019
43. Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. 2017;10(6):679–689. doi:10.1242/dmm.026609
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
