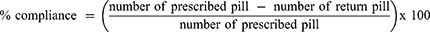Back to Journals » Patient Preference and Adherence » Volume 16
Validity and Reliability of the Thai Version of the 19-Item Compliance-Questionnaire-Rheumatology
Authors Panichaporn S, Chanapai W, Srisomnuek A, Thaweeratthakul P, Katchamart W
Received 12 May 2022
Accepted for publication 11 August 2022
Published 17 August 2022 Volume 2022:16 Pages 2149—2158
DOI https://doi.org/10.2147/PPA.S374445
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Saranya Panichaporn,1 Wanwisa Chanapai,2 Ananya Srisomnuek,2 Phakhamon Thaweeratthakul,1 Wanruchada Katchamart1
1Division of Rheumatology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 2Division of Clinical Trials, Research Department, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Wanruchada Katchamart, Division of Rheumatology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, 2 Wanglang Road, Bangkok Noi, Bangkok, 10700, Thailand, Tel +66 2 419 7775, Fax +66 2 418 3222, Email [email protected]
Purpose: To evaluate the psychometric properties and feasibility of the Thai version of Compliance-Questionnaire-Rheumatology (CQR)-19.
Patients and Methods: A cross-sectional study was conducted in the Rheumatology clinic of Siriraj hospital from October 2020 to March 2022. Literate patients diagnosed with rheumatoid arthritis and aged ≥ 18 years old were included. Participants completed the Thai CQR-19 at baseline and during a follow-up visit. A pill count at the follow-up visit indicating that ≤ 80% of prescribed pills were taken was defined as poor compliance. Feasibility was assessed by the time to completion and the number of missing questions.
Results: 156 participants were enrolled and 88% were in good compliance. The sensitivity of the CQR-19 to detect good compliance was 78.2% and the specificity was 27.8%. The Thai CQR-19 showed moderate agreement (Gwet’s AC1= 0.61, p < 0.01) with 72.2% agreement against pill counts. The Thai CQR-19 had good internal consistency (Cronbach’s alpha = 0.83) with moderate test-retest reliability (intraclass coefficients = 0.64, 95% CI 0.54– 0.73; p < 0.05). There was a weak correlation between the Thai CQR-19 and disease activity (rs = 0.20, p < 0.05), and no correlation with functional disability (rs = 0.09, p = 0.25). The median (IQR) completion time was 4.42 (3.25– 6.16) minutes and 114 (73%) completed all 19 questions.
Conclusion: The Thai version of CQR-19 is a valid and reliable tool to assess medication compliance in Thai patients with RA. To ensure completeness and accuracy, this questionnaire may be administered by interview.
Keywords: medication compliance, rheumatoid arthritis, validity, reliability, feasibility, psychometric property
Introduction
Rheumatoid arthritis (RA) is the most common chronic inflammatory arthritis with prevalence of 0.25% worldwide1 and 0.12% in Thailand.2 RA primarily affects the lining of the synovial joints and can cause progressive disability, socioeconomic burden and premature death.3 Early treatment can prevent irreversible disability and improves quality of life.4 Current recommendations suggest the use of disease-modifying antirheumatic drugs (DMARDs) and treat-to-target strategy aiming at remission or at least low disease activity to attain favorable outcomes.5–7 To achieve this goal, adherence with the medication regimen is essential. Medication nonadherence is associated with higher disease activity, disease flares, and functional impairment.8–11
Adherence to medication in patients with RA varies from 22 to 100%.9,11–14 There are many methods to assess medication adherence including self-reports, physician’s estimation, drug biomarkers, pharmacy refills, electronic monitoring, and questionnaires.11 Assessment of medication adherence in the clinical setting is unlikely to apply because it is time-consuming. However, physicians tend to substantially overestimate medication adherence.15–18 It has been shown that sensitivity of physician’s estimation of poor adherence was quite low as 24%.15 There are many self-reported questionnaires developed for measuring medication adherence.19 The 19-item Compliance-Questionnaire-Rheumatology was developed by de Klerk et al in 1999.20 The CQR-19 was validated against an electronic medication event monitoring system that records the time and date of opening and closing of the drug medication package through microelectronic circuitry integrated into the cap of a pill-bottle and is known as the Medication Event Monitoring System (MEMs). The sensitivity and specificity of the CQR-19 to detect good compliance were 95% and 62%, respectively. The predictive value to detect poor compliance was 86% and to detect good compliance was 83%.21 The CQR-19 has been translated into many languages and used in many countries,22–25 including Thailand.26 Therefore, we aimed to evaluate the psychometric properties and satisfaction of the Thai version of the CQR-19 (Thai CQR-19).
Materials and Methods
Study Design and Population
This cross-sectional study was conducted in the rheumatology clinic of Siriraj hospital in Bangkok from October 2020 to March 2022. Patients with RA that fulfilled the 1987 American College of Rheumatology (ACR) Diagnostic Criteria27 or the 2010 ACR/European League Against Rheumatism (EULAR) Classification Criteria,28 who were ≥ 18 years of age and literate were included. Patients with cognitive dysfunction, schizophrenia, psychosis, vision problems, or were unable to complete the questionnaire by themselves were excluded. This study was approved by the Scientific Ethics Committee of the Siriraj Institutional Review Board (COA Si no. 739/2020). Informed consent was obtained from all participants prior to enrollment. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Thai Version of Compliance-Questionnaire-Rheumatology-19 (Thai CQR-19)
The English version of the CQR-19 (Supplement 1) was translated into Thai language (Supplement 2) using the framework suggested by Beaton et al29 in a previous study.26 The Thai version of the CQR-19 consists of 19 items that ask patients to specify to what degree they agree or disagree with each statement on a 4-point Likert scale (1, strongly disagree; 2, disagree; 3, agree; 4, strongly agree). There are six items stated negatively (items 4, 8, 9, 11, 12, and 19) and were therefore reversed. The total score is calculated by summing the items, subtracting 19, and then dividing by 0.57. This ensures that the CQR total score can vary from 0 (complete noncompliance) to 100 (perfect compliance).21 To interpret the CQR-19, the score for each item was weighted and calculated using a calculator provided in a Microsoft Excel spreadsheet.21 Individual use for medical purpose is free of charge. For the use of more than one person for research or commercial activities, permission from the corresponding author is required.
Data Collection and Clinical Evaluation
The study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at the Siriraj Center of Excellence in Bioinformatics and Data Management, Faculty of Medicine Siriraj Hospital, Mahidol University.30 REDCap is a secure, web-based application designed to support data capture for research studies. It provides 1) an intuitive interface for validated data entry; 2) audit trails to monitor data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for the importing of data from external sources.
Demographic and disease-related variables including age, sex, educational levels, comorbid diseases, smoking and alcohol status, disease duration, and serological status were recorded. RA disease activity was assessed by patient global assessment (PGA) (0–10 cm visual analogue scale or VAS, physician global assessment (PhGA) (0–10 cm VAS), the number of swollen and tender joint assessed by their rheumatologists, and erythrocyte sedimentation rate (ESR). The disease activity score 28 (DAS28) was used to represent the RA disease activity. DAS28 is composite measurement comprised of the number of tender joint count 28 joints (TJC28), the number of swollen joint count 28 joints (SJC28), the ESR, and the PGA. DAS28 was calculated as followed, DAS28 = 0.56*sqrt(TJC28) + 0.28*sqrt(SJC28) + 0.70*Ln(ESR) + 0.014*PGA. A DAS28 of greater than 5.1 implies active disease, less than 3.2 low disease activity, and less than 2.6 remission.31 Functional status was assessed using the Thai version of the Health Assessment Questionnaire (Thai HAQ).32 Participants were asked to complete the Thai CQR-1926 by themselves at baseline. All unanswered questions were explained to the participants until they understood and could answer these questions. The Thai CQR-19 satisfaction was assessed by numeric scale from 0 to 10. The score of 0 means unsatisfactory and a score of 10 means satisfactory. The completion time and number of unanswered questions were recorded. Participants were advised to take medications only dispensed during this visit. Participants were received Thai CQR-19 in prepared envelop and were asked to complete and returned to investigator within two days via mail. A few days prior to the follow-up visit date, the investigators telephoned the participants to remind them to bring the remaining medications to the clinic. At the 3–4 months of follow-up visit, participants were asked to complete the Thai CQR-19 by themselves. Investigators assessed the medication compliance by comparing the pill count of the prescribed DMARDs and prednisolone from the previous visit. For the participants who could not attend the clinic at follow-up visit, investigators telephoned and asked the participants to perform pill counts by themselves via video call. The pill count was calculated using the following equation and poor compliance was described as compliance ≤ 80%.11,19,21,24
Statistical Analysis
Sample Size Calculation
To measure validity, the Cohen’s kappa (κ) of medication compliance between the Thai CQR-19 and pill count was set at 0.8.20 The calculated sample size for criterion validation was 139 participants when the proportion of chance agreement was 0.5, the desired precision width was 0.1, and type I error was 0.05.33 Based on an estimated Cronbach’s alpha of 0.8 from previous studies,21,24 the calculated sample size for reliability testing was 103 participants, when type I error was 0.05 and power was 0.80.34 For intraclass correlation coefficient (ICC) of test-retest reliability of 0.63,23 140 participants were required with desired precision width of 0.2 and type I error of 0.05.35 Finally, a total sample size of 154 participants was required with a 10% drop-out rate.
Data Analysis
Categorical variables were reported as number and frequencies. Continuous variables were reported using the mean and standard deviations for normally distributed data and the median and interquartile ranges (IQR) were used for non-normal distribution. The criterion validity of the Thai CQR-19 was evaluated using pill counts. Agreement between the Thai CQR-19 at follow-up visit and the pill count was described using Cohen’s kappa and percentage of agreement. However, when a “kappa paradox” was present (a discrepancy between Cohen’s kappa and percentage of agreement), agreement was assessed using Gwet’s AC1.36–39 To assess construct validity, Spearman correlation coefficients (rs) were calculated for the relationships between the Thai CQR-19 scores, DAS28 scores and Thai HAQ scores. Internal consistency was tested using Cronbach’s alpha, and test-retest reliability was tested using ICC with two-way random model, single measures, and consistency. The completion time and number of unanswered questions were recorded as a proxy for feasibility and described as median with IQR and percentage, respectively. A P value of less than 0.05 was considered to be statistically significant. All analyses were performed using IBM SPSS statistics Version 18.0 for Windows.
Results
Participant Characteristics
A total of 156 participants were enrolled (Table 1). The mean age ± SD was 55.9 ± 11.7 years. Most (89.7%) were female. The median education duration (IQR) was 12 (6.0–16.0) years. The median disease duration (IQR) was 9.9 years (3.9–15.6). The majority of participants had mild disease activity [median DAS28 (IQR) = 2.67 (2.16–3.40)] and mild functional impairment [median HAQ (IQR) = 0.13 (0.00–0.61)]. Eighty-one (52%) were prescribed two different types of DMARDs [median (IQR), 2 (2.0–2.8)]. A minority (15.4%) took prednisolone. Methotrexate (85.9%), chloroquine (38.5%), sulfasalazine (30.1%), and leflunomide (27.6%) were the most commonly prescribed medications. Only 3.2% and 5.8% used target synthetic DMARDs and biologic DMARDs, respectively.
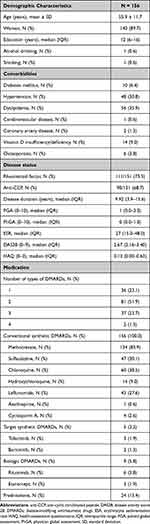 |
Table 1 Baseline Characteristics and Prescribed Medications of 156 Patients with RA |
Feasibility
One hundred and fourteen (73.1%) participants completed all 19 items. The most frequent unanswered questions were items 8, 9, 11, 12 and 19 (Table 2). The median number of unanswered questions (IQR) was 0 (0–1). Most participants skipped 1–2 items (9.0% each). The median completion time (IQR) was 4.4 (3.2–6.2) minutes. Most participants satisfied the Thai CQR-19 questionnaire [median (IQR), 9 (8–10)].
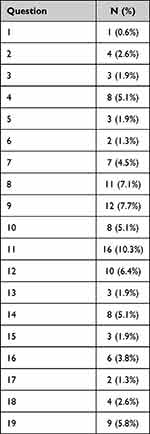 |
Table 2 Unanswered Question of Thai CQR-19 Questionnaire |
Criterion Validity
Five participants were lost to follow-up. Thus, 151 participants were included for criterion validity analyses and 156 were included for construct validity analyses. Based on the pill count, the median compliance (IQR) was 95.6% (88.9–100.0%) and most participants had good compliance (% compliance > 80% = 88%). There was no agreement between the Thai CQR-19 and the pill count based on the kappa coefficient (kappa = 0.043, p = 0.56), despite the high percentage of agreement (72.2%).40 Gwet’s AC1 was calculated and showed moderate agreement between the Thai CQR-19 and the pill count (AC1 = 0.61, p < 0.001). Sensitivity and specificity to detect good compliance by the Thai-CQR-19 were 78.2% (95% CI: 71.2–85.2) and 27.8% (95% CI: 27.8–70.9), respectively with 88.9% (95% CI: 83.2–94.6) positive predictive value, and 14.7% (95% CI: 2.8–26.6) negative predictive value. The overall accuracy was 72.2% (95% CI: 65–79) (Table 3).
 |
Table 3 Comparison of Medication Compliance Based on the Thai CQR-19 and Pill Count |
Construct Validity
There was a weak correlation between the total Thai CQR-19 score and disease activity measured by DAS28 (rs = 0.200, p = 0.01). The total Thai CQR-19 score was not correlated with functional disability measured by Thai HAQ (rs = 0.092, p 0.25) (Figure 1).
 |
Figure 1 Scatter plot showing the correlation of total Thai CQR-19 and DAS28 scores (A) and HAQ scores (B). |
Reliability
One hundred and forty-eight (94.9%) participants returned the retest questionnaires. The median CQR-19 score (IQR) was 82.46 (70.18–89.47) on the first measurement and 80.70 (70.18–89.47) on the retest. Internal consistency, measured by Cronbach’s alpha, was 0.826. The test-retest reliability, measured by ICC, was 0.643 (95% CI 0.54–0.73, p < 0.001).
Discussion
This study demonstrates that the Thai CQR-19 questionnaire is moderately correlated with pill count and reliable in both internal consistency and test-retest. Similar to previous studies, the median compliance was high (95.6%). Salgado et al reported a median compliance (IQR) of 98% (83 −100%) when the medication compliance was assessed using MEMs.24 Lee et al assessed compliance using pharmacy refills and reported a mean compliance ± SD of 89.4 ± 14.5%.22 However, the prevalence of good compliance (> 80% compliance) in this study was 88.1%, which was higher than de Klerk et al who reported of 52% using MEMs.21 This discrepancy may be caused by the different gold standard for medication compliance used in these studies. It has been shown that self-reported and pill count overestimate compliance, while MEMs underestimate the compliance.11,41–43 Additionally, the “Hawthorne effect” may explain the high compliance we observed if participants modified their behavior in response to their awareness of being monitored. Moreover, some patients may deliberately not return medications.11,44
Previous studies have described a good correlation between CQR-19 and compliance using MEMs, pharmacy refill, and self-reported questionnaire.22–25 Initially, we found no agreement between the Thai CQR-19 and the pill count when analysed using Cohen’ s kappa. We found a discordance between kappa and the percentage of agreement due to asymmetric data.38,45,46 In our study, the high prevalence of good compliance contributed to this discordance. Therefore, Gwet’s AC1 was applied, revealing a moderate agreement between the Thai CQR-19 and the pill count.
We found that the Thai CQR-19 score had a weak correlation with disease activity as measured by the DAS28. Consistent with previous studies, disease activity was not statistically significantly related to medication noncompliance.26,47,48 Marras et al also found that DAS28 scores were not different between patients in good or poor compliance CQR-19 categories.49 Similar to the Spanish adaptation with respect to divergent construct validity, the total Thai CQR-19 score and HAQ were not correlated (rs = 0.092, p 0.25).24 High disease activity and poor functional status may be either the cause or outcome of poor medication compliance. Patients who have active disease or impaired functional status may be more likely to try to take medications according to their physicians’ prescription, while patients who have poor compliance may have more active disease or functional disability. Due to the cross-sectional study design, we could not determine any temporal relationship between compliance and disease activity or functional status.
The test-retest reliability yielded an ICC of 0.64 for the total Thai CQR-19 score, which was comparable to the Korean (0.71), Spanish (0.76), Arabic (0.76), and Turkish (0.63) adaptations, respectively).22–25 Internal consistency as assessed by Cronbach’s alpha (0.83) was also comparable to the Spanish (0.86), Arabic (0.79), and Turkish (0.83) adaptation, respectively.23–25 These reliability results were adequate and acceptable.50,51
The median completion time of approximately five minutes was shorter than reported in previous studies (English, 12 minutes; Turkish, 10–15 minutes).21,23 The most common unanswered questions were items 8, 9, 11, 12 and 19. All were negative questions. Items 9 and 19 were also the most often unanswered questions in the original English language version of the CQR-19.21 Questions 9 and 19 asked about drug compliance on holidays and special weekends, and some participants did not recognize the special days so they could not respond. Likewise, some participants did not understand the word “miracle” in question 11. Question 8 asked if they disliked taking medication, some participants felt neutral about taking medication, so they did not answer this question. Item 12 was framed as a hypothetical situation, “If you can’t stand the medicines you might say: ‘throw it away, no matter what’”, a behavior that rarely occurs with Thai patients. This may explain why some participants left the answer box empty. Because of the complexity of some questions in the Thai-CQR19, we suggest that the questionnaire be completed by a trained interviewer rather than self-administered.
The strength of this study is that we included the largest sample size, when compared to validating versions in other languages.21–25 We used RA as study population in this validation study because RA is the most common rheumatic disease requiring long-term treatment. Therefore, the results of this study should be applicable to patients with other chronic illnesses.There are some limitations in this study. First, this study was conducted during the COVID-19 pandemic and some participants could not attend the follow-up visit at the clinic. Therefore, we telephoned and asked the participants to perform pill counts by themselves. This may have led to an overestimate of compliance. However, when possible, we requested the participants to use video call to verify the pill count. Second, a few participants (less than 5%) took the remaining drugs from the previous visit, as a result, the pill count may be inaccurate in some cases. Third, this study was conducted in a tertiary care hospital where patients tend to have more severe disease and become more aware of how to manage their illness, resulting in higher compliance than might be observed elsewhere. Forth, the retest was done and returned using the post office service. Some participants did not return the retest questionnaire by mail within two days, but returned it in person at the follow-up visit. Therefore, we could not be sure whether the duration between test and retest was actually within two days. Finally, we did not assess the content validity of the Thai version questionnaire.
Conclusion
In conclusion, the Thai CQR-19 is a valid and reliable instrument to evaluate medication compliance in daily practice and epidemiological research. Although this questionnaire is intended to be self-administered, to ensure completeness and accuracy of the compliance assessment this questionnaire may be administered by interview.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Khemajira Karaketklang, BSc (nursing), M.P.H. for the statistical analysis, Bhoom Suktitipat, MD, PhD, Graduate Program in Biochemistry, Medical Molecular Biology, and Medical Bioinformatics, Department of Biochemistry, Faculty of Medicine Siriraj Hospital, Mahidol University, and Nutchavadee Vorasan, MSc (Tropical medicine), Center of Siriraj Genomics, Faculty of Medicine Siriraj Hospital, Mahidol University for database management.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78(11):1463–1471. doi:10.1136/annrheumdis-2019-215920
2. Chaiamnuay P, Darmawan J, Muirden KD, Assawatanabodee P. Epidemiology of rheumatic disease in rural Thailand: a WHO-ILAR COPCORD study. Community oriented programme for the control of rheumatic disease. J Rheumatol. 1998;25(7):1382–1387.
3. Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6(1):15. doi:10.1038/s41413-018-0016-9
4. Aletaha D, Smolen JS. Diagnosis and Management of Rheumatoid Arthritis: a Review. JAMA. 2018;320(13):1360–1372. doi:10.1001/jama.2018.13103
5. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2021;73(7):924–939. doi:10.1002/acr.24596
6. Lau CS, Chia F, Dans L, et al. 2018 update of the APLAR recommendations for treatment of rheumatoid arthritis. Int J Rheum Dis. 2019;22(3):357–375. doi:10.1111/1756-185X.13513
7. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi:10.1136/annrheumdis-2019-216655
8. Contreras-Yáñez I, Ponce De León S, Cabiedes J, Rull-Gabayet M, Pascual-Ramos V. Inadequate therapy behavior is associated to disease flares in patients with rheumatoid arthritis who have achieved remission with disease-modifying antirheumatic drugs. Am J Med Sci. 2010;340(4):282–290. doi:10.1097/MAJ.0b013e3181e8bcb0
9. Li L, Cui Y, Yin R, et al. Medication adherence has an impact on disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Patient Prefer Adherence. 2017;11:1343–1356. doi:10.2147/PPA.S140457
10. Pasma A, Schenk CV, Timman R, et al. Non-adherence to disease-modifying antirheumatic drugs is associated with higher disease activity in early arthritis patients in the first year of the disease. Arthritis Res Ther. 2015;17:281. doi:10.1186/s13075-015-0801-4
11. van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8(4):337–351. doi:10.1586/eci.12.23
12. Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and persistence with methotrexate in rheumatoid arthritis: a systematic review. J Rheumatol. 2016;43(11):1997–2009. doi:10.3899/jrheum.151212
13. Peter ME, Zuckerman AD, DeClercq J, et al. Adherence and persistence in patients with rheumatoid arthritis at an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(7):882–890. doi:10.18553/jmcp.2021.27.7.882
14. Salaffi F, Di Carlo M, Carotti M, et al. Predictive validity of the 5-item Compliance Questionnaire for Rheumatology (CQR5) in detecting poor adherence of patients with rheumatoid arthritis to biological medication. Arthritis Res Ther. 2020;22(1):227. doi:10.1186/s13075-020-02319-4
15. Miller LG, Liu H, Hays RD, et al. How well do clinicians estimate patients’ adherence to combination antiretroviral therapy? J Gen Intern Med. 2002;17(1):1–11. doi:10.1046/j.1525-1497.2002.09004.x
16. Copher R, Buzinec P, Zarotsky V, Kazis L, Iqbal SU, Macarios D. Physician perception of patient adherence compared to patient adherence of osteoporosis medications from pharmacy claims. Curr Med Res Opin. 2010;26(4):777–785. doi:10.1185/03007990903579171
17. Curtis JR, Cai Q, Wade SW, et al. Osteoporosis medication adherence: physician perceptions vs. patients’ utilization. Bone. 2013;55(1):1–6. doi:10.1016/j.bone.2013.03.003
18. Kekäle M, Talvensaari K, Koskenvesa P, Porkka K, Airaksinen M. Chronic myeloid leukemia patients’ adherence to peroral tyrosine kinase inhibitors compared with adherence as estimated by their physicians. Patient Prefer Adherence. 2014;8:1619–1627. doi:10.2147/PPA.S70712
19. Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047. doi:10.1155/2015/217047
20. de Klerk E, van der Heijde D, van der Tempel H, van der Linden S. Development of a questionnaire to investigate patient compliance with antirheumatic drug therapy. J Rheumatol. 1999;26(12):2635–2641.
21. de Klerk E, van der Heijde D, Landewé R, van der Tempel H, van der Linden S. The compliance-questionnaire-rheumatology compared with electronic medication event monitoring: a validation study. J Rheumatol. 2003;30(11):2469–2475.
22. Lee JY, Lee SY, Hahn HJ, Son IJ, Hahn SG, Lee EB. Cultural adaptation of a compliance questionnaire for patients with rheumatoid arthritis to a Korean version. Korean J Intern Med. 2011;26(1):28–33. doi:10.3904/kjim.2011.26.1.28
23. Cinar FI, Cinar M, Yilmaz S, et al. Cross-cultural adaptation, reliability, and validity of the turkish version of the compliance questionnaire on rheumatology in patients with Behçet’s disease. J Transcult Nurs. 2016;27(5):480–486. doi:10.1177/1043659615577699
24. Salgado E, Maneiro Fernández JR, Souto Vilas A, Gómez-Reino JJ. Spanish transcultural adaptation and validation of the English version of the compliance questionnaire in rheumatology. Rheumatol Int. 2018;38(3):467–472. doi:10.1007/s00296-018-3930-7
25. Aljohani R, Aljohani Z, Aljohani R, Alsaidalani R. Saudi cultural adaptation of the “compliance questionnaire of Rheumatology” for Rheumatoid arthritis patients on disease modifying anti-rheumatic drugs (DMARDs). Saudi Pharm J. 2021;29(5):377–383. doi:10.1016/j.jsps.2021.03.007
26. Katchamart W, Narongroeknawin P, Sukprasert N, Chanapai W, Srisomnuek A. Rate and causes of noncompliance with disease-modifying antirheumatic drug regimens in patients with rheumatoid arthritis. Clin Rheumatol. 2021;40(4):1291–1298. doi:10.1007/s10067-020-05409-5
27. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi:10.1002/art.1780310302
28. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
29. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000;25(24):3186–3191. doi:10.1097/00007632-200012150-00014
30. Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010
31. Prevoo ML, van ‘t Hof MA, Kuper HH, Van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi:10.1002/art.1780380107
32. Osiri M, Deesomchok U, Tugwell P. Evaluation of functional ability of Thai patients with rheumatoid arthritis by the use of a Thai version of the health assessment questionnaire. Rheumatology. 2001;40(5):555–558. doi:10.1093/rheumatology/40.5.555
33. Shoukri MM, Asyali MH, Donner A. Sample size requirements for the design of reliability study: review and new results. Stat Methods Med Res. 2004;13(4):251–271. doi:10.1191/0962280204sm365ra
34. Bonett DG. Sample size requirements for testing and estimating coefficient alpha. J Educ Behav Stat. 2002;27(4):335–340. doi:10.3102/10769986027004335
35. Bonett DG. Sample size requirements for estimating intraclass correlations with desired precision. Stat Med. 2002;21(9):1331–1335. doi:10.1002/sim.1108
36. Dettori JR, Norvell DC. Kappa and beyond: is there agreement? Global Spine J. 2020;10(4):499–501. doi:10.1177/2192568220911648
37. Konstantinidis M, Le LW, Gao X. An empirical comparative assessment of inter-rater agreement of binary outcomes and multiple raters. Symmetry. 2022;14(2):262. doi:10.3390/sym14020262
38. Shankar V, Bangdiwala SI. Observer agreement paradoxes in 2x2 tables: comparison of agreement measures. BMC Med Res Methodol. 2014;14:100. doi:10.1186/1471-2288-14-100
39. Wongpakaran N, Wongpakaran T, Wedding D, Gwet KL. A comparison of Cohen’s Kappa and Gwet’s AC1 when calculating inter-rater reliability coefficients: a study conducted with personality disorder samples. BMC Med Res Methodol. 2013;13(1):61. doi:10.1186/1471-2288-13-61
40. Fleiss JL, Levin B, Paik MC. The measurement of interrater agreement. Stat Methods Rates Proportions. 1981;2(212–236):22–23.
41. Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11(6):1189–1197. doi:10.1200/JCO.1993.11.6.1189
42. Mudhune V, Gvetadze R, Girde S, et al. Correlation of adherence by pill count, self-report, MEMS and plasma drug levels to treatment response among women receiving ARV Therapy for PMTCT in Kenya. AIDS Behav. 2018;22(3):918–928. doi:10.1007/s10461-017-1724-7
43. Hartman L, Cutolo M, Bos R, et al. Medication adherence in older people with rheumatoid arthritis is lower according to electronic monitoring than according to pill count. Rheumatology. 2021;60(11):5239–5246. doi:10.1093/rheumatology/keab207
44. Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):
45. Feinstein AR, Cicchetti DV. High agreement but low kappa: i. The problems of two paradoxes. J Clin Epidemiol. 1990;43(6):543–549. doi:10.1016/0895-4356(90)90158-L
46. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363.
47. Tuncay R, Eksioglu E, Cakir B, Gurcay E, Cakci A. Factors affecting drug treatment compliance in patients with rheumatoid arthritis. Rheumatol Int. 2007;27(8):743–746. doi:10.1007/s00296-006-0299-9
48. Xia Y, Yin R, Fu T, et al. Treatment adherence to disease-modifying antirheumatic drugs in Chinese patients with rheumatoid arthritis. Patient Prefer Adherence. 2016;10:735–742. doi:10.2147/PPA.S98034
49. Marras C, Monteagudo I, Salvador G, et al. Identification of patients at risk of non-adherence to oral antirheumatic drugs in rheumatoid arthritis using the compliance questionnaire in rheumatology: an ARCO sub-study. Rheumatol Int. 2017;37(7):1195–1202. doi:10.1007/s00296-017-3737-y
50. Bland JM, Altman DG. Cronbach’s alpha. BMJ. 1997;314(7080):572. doi:10.1136/bmj.314.7080.572
51. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi:10.1016/j.jcm.2016.02.012
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.