Back to Journals » Patient Preference and Adherence » Volume 16
Exploring Factors Influencing Medication Compliance in Saudi Rheumatoid Arthritis Patients: A Nationwide Cross-Sectional Survey – Results from the COPARA Study
Authors Omair MA , Alshehri MM, Altokhais NA, Aljanobi GA , El Dessougi MI, AlHarthi A , Omair MA, Attar SM, Bahlas SM, Alfurayj AS, Alazmi MS, Asiri AM, AlOmair MM, Al Juffali L , Almalag HM
Received 21 February 2022
Accepted for publication 12 April 2022
Published 26 April 2022 Volume 2022:16 Pages 1105—1114
DOI https://doi.org/10.2147/PPA.S363477
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Mohammed A Omair,1 Maha M Alshehri,2 Nouf A Altokhais,2 Ghada A Aljanobi,3 Maha I El Dessougi,4 Amal AlHarthi,4 Maha A Omair,5 Suzan M Attar,6 Sami M Bahlas,6 Abdullah S Alfurayj,7 Mansour S Alazmi,8 Alhussain M Asiri,9 Mohammed M AlOmair,9 Lobna Al Juffali,10 Haya M Almalag10
1Rheumatology Unit, Department of Medicine, King Saud University, Riyadh, Saudi Arabia; 2College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 3Rheumatology Unit, Department of Medicine, Qatif Central Hospital, Qatif, Saudi Arabia; 4Rheumatology Unit, Department of Medicine, Security Forces Hospital, Riyadh, Saudi Arabia; 5Department of Statistics and Operations Research, College of Science, King Saud University, Riyadh, Saudi Arabia; 6Rheumatology Unit, Department of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 7Rheumatology Unit, Department of Medicine, Buraidah Central Hospital, Buraidah, Saudi Arabia; 8Rheumatology Unit, Department of Medicine, Prince Mohammed Medical City, Sakaka-Aljouf, Saudi Arabia; 9Rheumatology Unit, Department of Medicine, Aseer Central Hospital, Abha, Saudi Arabia; 10Department of Clinical Pharmacy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
Correspondence: Mohammed A Omair, Rheumatology Unit, Department of Medicine, King Saud University, Riyadh, Saudi Arabia, Tel +966505270513, Email [email protected]
Purpose: Compliance is essential to achieve treatment goals in rheumatoid arthritis (RA) patients. The current study evaluated compliance and related factors in a large and diverse population.
Patients and Methods: Patients with RA who received active treatment were invited to participate in an online survey. The Arabic versions of the 5-Item Compliance Questionnaire for Rheumatology (ACQR-5) and the RA Impact of Disease (RAID) were used to measure compliance and disability, respectively. The patients were sub-grouped based on background disease-modifying anti-rheumatic drugs (DMARDs). Variables associated with high compliance were selected for the logistic regression analysis.
Results: A total of 1241 patients completed the survey and were included in the final analysis. Of those, 1055 (85%) were females with a mean (±SD) age and disease duration of 47.14 ± 13.71 and 8.77 ± 7.43 years, respectively. The mean RAID was 4.4± 2.58, with 980 (79%) having an unacceptable level state. Patients with an unacceptable RAID level had a lower compliance rate (78.8% vs 85.8%, p = 0.001). Demographics associated with high compliance were female sex and increased age, with reported odds ratios of 1.018 (95% CI: 1.007– 1.028) and 1.464 (95% CI: 1.016– 2.108), respectively. Compliance was similar between patients on Janus kinase inhibitors or biological DMARDs (88.14% vs 80.83%, p = 0.17), between monotherapy, double therapy, or triple therapy recipients (80% vs 82.23% vs 81.32%, p = 0.665), and between patients receiving injectable and oral therapy (77.32% vs 81.14%, p = 0.246).
Conclusion: A high compliance level was observed in this population, with patient demographics influencing compliance rather than the medication type or route of administration. Interventional studies should focus on the of high-risk patients identified in this study.
Keywords: rheumatoid arthritis, compliance, Saudi Arabia
Introduction
Rheumatoid Arthritis (RA) is a chronic autoimmune disease that burdens patients’ lives.1 Outcomes of RA management have improved in the last two decades owing to early diagnosis, the introduction of highly effective therapies, and the adoption of a Treat To Target strategy.2 Treatment options include conventional synthetic Disease-Modifying Anti-rheumatic Drugs (csDMARDs), target synthetic DMARDs (tsDMARDs), and biological DMARDs (bDMARDs).3 Similar to other chronic diseases, treatment compliance is essential to achieve the desired therapeutic goal.4,5 Methotrexate (MTX), the anchor drug in RA management, is usually started as a monotherapy orally or subcutaneously in moderate to severe RA patients. Its failure requires the addition of csDMARDs, bDMARDs, or tsDMARDs with a large variety of routes and frequencies of administration. However, this can negatively impact compliance with drugs administered daily compared to weekly or monthly injections. tsDMARDs such as the Janus Kinase Inhibitors (JAKi) tofacitinib, baricitinib, and upadacitinib are required to be consumed once daily compared to bDMARDs, with frequencies ranging from once weekly to once every six months. The ARCO (Study on Adherence of Rheumatoid Arthritis patients to SubCutaneous and Oral Drugs) study was multicenter, looking at compliance related to drug route of administration and frequency using a Medication Possession Ratio of ≥80% as the cutoff level to define non-compliance. Interestingly, they found that patients on a monthly interval of administration had a better compliance level than patients on a weekly interval of administration (6.4% vs 17.4%, p=0.034).6 However, most studies published in the literature had sample sizes ranging from 100 to 400 patients, which could not have adequate statistical power to assess numerous factors.6–8 A large study included a mixed population of 3390 patients with RA, psoriatic arthritis, and ankylosing spondylitis. A higher odds ratio of compliance was observed in RA patients using Tumor Necrosis Factor inhibitors (TNFi) combined with csDMARDs (5.45; 3.35–8.87) and monotherapy (3.63,1.72–7.69) compared to csDMARDs alone.9
Systematic assessment of compliance among Saudi patients with RA is challenging owing to the lack of validated tools in Arabic. The 19 and 5 item Compliance Questionnaire for Rheumatology (CQR) versions were only recently validated in the Arabic language.10,11 The current study aimed to establish compliance on large sample size and study the impact of patient-related factors, disease characteristics, medication frequency, and route administration.
Materials and Methods
Study Design
The COmpliance and Patient Activation in Rheumatoid Arthritis (COPARA) study is a multicenter, nationwide, cross-sectional study that assessed compliance and patient activation. The first part of this study reports the compliance component. The study report was prepared in compliance the Strengthening the Reporting of Observational Studies in Epidemiology Checklist (STROBE Statement).12
Ethical Consideration
Ethics approval for this study was obtained from the Institutional Review Board (IRB) of King Saud University (KSU) in Riyadh, Saudi Arabia (IRB approval: E-19-4363). Electronic consent was obtained from all participants. All participants were informed about the purpose of the study, in accordance with the Declaration of Helsinki.
Setting
Participants were recruited from rheumatology clinics at multiple national centers in Saudi Arabia. The centers included Security Forces Hospital, Riyadh; King Khalid University Hospital, King Abdulaziz University Hospital, Jeddah; Prince Mohammed Medical City, Al-Jouf; Qatif Central Hospital, Qatif; Buraidah Central Hospital, Buraidah; Aseer Central Hospital, Abha; and the Charitable Association for Rheumatic Diseases database.
Participants
Consecutive patients aged ≥18 years diagnosed with RA were eligible for inclusion based on the American College of Rheumatology/European League Against Rheumatism classification criteria for ≥6 months.13 Patients in drug-free remission or rituximab monotherapy were excluded. Patient recruitment was performed between May and June 2021.
Confounding variables included patient-related factors such as age, sex, educational level, employment status, income, living status, smoking, comorbidities, and medications. In addition, patients were stratified into an oral group if they were receiving only oral DMARDs, and an injectable group if they were only on injectable DMARDs. The study’s primary outcome was the level of compliance measured using the ACQR-5. The secondary outcome was to explore the relationship between the ACQR-5 level and patient demographics, disease characteristics, medication use, and treatment strategy.
Instrument
The online survey was created using Google Forms, consisting of a series of questionnaires in Arabic, focused on sociodemographic information, health condition characteristics, and currently prescribed medications. Additionally, they included the A-CQR5 tool, which aims to measure the extent to which a RA patient adheres to treatments by choosing the degree of agreement to five statements, where the score ranges from (scored 1) “Definitely do not agree” to (scored 4) “Definitely agree”.14 The CQR5 was translated in a previous study.10 The last part was the Arabic version of the Rheumatoid Arthritis Impact of Disease (RAID), developed initially in English.15 The tool aimed to measure the impact of RA by answering seven questions on a scale of (0 to 10) where (0) “Did not cause me pain” and10 “Caused me severe pain.” Patients who accessed the questionnaire but did not complete all the items were excluded from the analysis.
Statistical Analysis
Data were cross-tabulated, coded, entered, and statistically analyzed using the IBM SPSS Statistics for Windows version 26 (IBM Corp., Armonk, NY. USA). A unique study number was assigned to each patient to ensure patient confidentiality. Descriptive statistics of confounding variables were expressed as means and standard deviations for continuous variables and numbers and percentages for categorical variables. Univariate and multivariate analyses of the factors affecting CQR as dependent variables were performed. The odds ratio (OR) of being highly compliant with regard to demographic characteristics and medication classes is displayed. In addition, the adjusted OR of being highly compliant in medication classes was noted, considering significant confounders in the univariate analysis.
Results
Patients’ Demographics
A total of 1356 patients were invited, with 1241 completing the survey and being included in the final analysis, generating a response rate of 91%. Of those, 1055 (85%) were women with a mean (±SD) age of 47.14±13.71 years. Most of the patients were from the central region (46.8%), followed by the western and southern regions (21.8% and 11%, respectively) of Saudi Arabia. The level of education was variable, with 162 (13.1%) being illiterate and 798 (64.3%) completing high school or higher education. The majority were unemployed (71.4%), with a monthly income of <3000 SAR in 53% of participants. Two or more comorbidities were reported in 395 (31.9%) patients. Hypertension and diabetes mellitus (DM) were the most common comorbidities, affecting 20.2% and 17.2% of patients, respectively.
Disease Characteristics and Background Medications
The mean disease duration was 8.77±7.43 years. The mean RAID was 4.4±2.58, with 980 (79%) having an unacceptable level state. Most patients were treated with ≥2 agents. csDMARDs and prednisolone were being used in 938 (75.6%) and 318 (25.6%) patients, respectively. TNFi was the most frequently identified bDMARD (30.5%), followed by non-TNFi (24.4%) and JAKi (4.8%).
Compliance Level and Associated Factors
High compliance was observed in 996 patients (80.25%). Patients with higher postgraduate education and working status were more likely to have low compliance (p=0.05 and 0.008, respectively). The region of residence affected adherence (p<0.001), with the northern and southern regions scoring the highest (93.2% and 83.2%, respectively) in terms of compliance, followed by the western and central regions (79.7% and 78.1%, respectively). The eastern region scored the lowest (74.2%) (Figure 1). Having DM and osteoporosis were associated with higher compliance (p=0.008 and 0.045, respectively). Using csDMARDs, oral medication, and abatacept treatment were associated with higher compliance. The mean RAID was similar between patients classified as high and low compliant (4.4±2.64 vs 4.41±2.32, p=0939); however, patients with an unacceptable level of disease impact had a lower compliance level (78.8% vs 85.8%, p=0.001) (Table 1). Demographics associated with high compliance were female sex and increased age, with reported OR of 1.018 (95% CI: 1.007–1.028) and 1.464 (95% CI: 1.016–2.108), respectively. In addition, the northern region, having an acceptable RAID, positive diabetes, osteoporosis, and presence of two or more comorbidities were associated with a high compliance status. Higher education with a university degree [OR 0.595 (95% CI: 0.368–0.962)] or postgraduate degree [OR 0.669 (95% CI: 0.479–0.900)] was inversely correlated with compliance. Participants receiving abatacept (OR 3.903), with the use of background csDMARDs (odds ratio 1.413), and any oral medication (OR: 1.437) were more likely to be compliant, even after adjusting for confounders (Table 2). Subgroup analysis did not show a statistically significant difference between patients on JAKi or bDMARDs (88.14% vs 80.83%, p=0.17) (Figure 2), between monotherapy, double, or triple therapy (80% vs 82.23% vs 81.32%, p=0.665) (Figure 3), or between patients receiving injectable and oral therapy (77.32% vs 81.14%, p=0.246) (Figure 4).
 |
Table 1 Baseline Demographics and Comorbidities Stratified by Compliance Score High or Low with p-value of Difference |
 |
Table 2 Binary Logistic Regression of Demographics and Medication with the Resulting Odds Ratio of Being Highly Compliant |
 |
Figure 1 Compliance level stratified by region. Total population = 1241. |
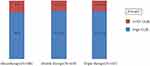 |
Figure 3 Comparison between compliance levels of patients receiving monotherapy, double therapy, and triple therapy. Abbreviation: CQR, Compliance Questionnaire for Rheumatology. |
 |
Figure 4 Comparison between the level of compliance in patients using injectable and oral therapies. |
Discussion
Advancements in RA treatment have led to better control of disease activity, improved quality of life, and reduced disability. In addition, studies on RA have reported adherence or compliance to treatment plans with variable results based on the population studied, medication assessed, and tool used.4,5,16 Viller et al reported one of the first prospective studies on compliance over three years. This large multicenter study recruited 556 patients from Norway, France, and the Netherlands. They noted consistent behavior in 429 patients (77.2%). Of these patients, 35.7% were consistently compliant. Older age, female sex, decreased disability, more personal knowledge of RA, and better relationships with healthcare professionals were associated with compliant behavior.17 Other studies have assessed compliance with specific medications. For example, MTX is administered orally and subcutaneously at weekly doses. Curtis et al performed a systematic review assessing adherence to MTX with results ranging between 50–94% at year one and dropping to 25–79% at year 5.5 Another systematic review assessing adherence to adalimumab, etanercept, and infliximab found high methodological variability that generated a wide-ranging adherence between 32.0% and 90.9%.4
In our study, compliance was primarily affected by patient-related factors. Being older, living in rural areas, having a low socioeconomic status, and being unemployed were associated with better compliance. Similarly, Park et al found that older age and the ability to cope with the disease were associated with higher adherence. In contrast, living a busy life negatively impacted adherence.18 In the post hoc analysis of the Spanish ARCO study by Marras et al, they found that educational level did not impact compliance measured by CQR, but working status comparing currently working vs others was associated with lower compliance, almost reaching a statistical difference (27.8% vs, 16.7% p=0.062).19 Also, a French study has not identified education level or working status as significant factors affecting compliance.7 In contrast to all the previously reported findings, Mena-Vazquez et al have shown better compliance in RA patients with a university degree (p=0.039).8
We hypothesized that existing oral medications and combination therapy would negatively impact compliance. The findings of this study suggest that background therapy does not significantly affect compliance levels. These findings confirm that using JAKi does not negatively impact compliance in general. However, we suggest that these findings should be cautiously interpreted. Choosing the most suitable therapy requires incorporating variables such as patient engagement, comorbidities, pregnancy planning, health coverage, and utilization. The study did not include any disease activity measure because of its nature as a multicenter survey. RAID is a validated tool15 that functions well as patient-reported outcomes and correlates with disease activity score (DAS28).20,21 The finding that patients with an unacceptable RAID state had a lower compliance level could be explained by the fact that lower compliance leads to higher disease activity, causing a lower RAID. Finally, the observed high compliance level could be due to the ACQR-5 properties, which could be overestimated compared to previous studies using other tools.4,5,16 In a previous study conducted during the COVID-19 pandemic, we observed a similar level of compliance using the Medication Adherence Rating Scale in 637 patients (85.8%) with different rheumatic diseases, with 42.7% of them having RA.22
The strengths of this study include its large sample size and adequate representation of Saudi Arabia’s regions. However, the study was limited by its cross-sectional design, the use of a subjective measure of compliance, and the fact that the assessment did not include factors such as seropositivity and disease activity.
Conclusion
In conclusion, to the best of our knowledge, the current study is so far the largest to assess compliance in RA in the MIddle East. Improvements in clinical projects and interventional studies can be used to describe patients’ characteristics in the current study to identify high-risk patients.
Data Sharing Statement
Data are available through a request directed to the corresponding author.
Acknowledgments
The authors would like to thank the Charitable Association for Rheumatic Diseases, patients and coordinators who helped complete this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Omair MA, Erdogan A, Tietz N, Alten R. Physical and Emotional Burden of Rheumatoid Arthritis in Saudi Arabia: an Exploratory Cross-Sectional Study. Open Access Rheumatol. 2020;12:337–345. doi:10.2147/OARRR.S284734
2. Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338–2348.
3. Mysler E, Caubet M, Lizarraga A. Current and Emerging DMARDs for the Treatment of Rheumatoid Arthritis. Open Access Rheumatol. 2021;13:139–152.
4. Blum MA, Koo D, Doshi JA. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther. 2011;33(7):901–913.
5. Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and Persistence with Methotrexate in Rheumatoid Arthritis: a Systematic Review. J Rheumatol. 2016;43(11):1997–2009.
6. Calvo-Alén J, Monteagudo I, Salvador G, et al. Non-adherence to subcutaneous biological medication in patients with rheumatoid arthritis: a multicentre, non-interventional study. Clin Exp Rheumatol. 2017;35(3):423–430.
7. Monchablon C, Gondé H, Pouplin S, Varin R, Vittecoq O, Lequerré T. Assessment of adherence to disease-modifying anti-rheumatic drugs in rheumatoid arthritis. Clin Rheumatol. 2020;39(1):207–216.
8. Mena-Vazquez N, Manrique-Arija S, Yunquera-Romero L, et al. Adherence of rheumatoid arthritis patients to biologic disease-modifying anti-rheumatic drugs: a cross-sectional study. Rheumatol Int. 2017;37(10):1709–1718.
9. Smolen JS, Gladman D, McNeil HP, et al. Predicting adherence to therapy in rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis: a large cross-sectional study. RMD Open. 2019;5(1):e000585.
10. Omair MA, Al Suwayeh F, Almashaan A, et al. Cross-Cultural Validation of the 5-Item Compliance Questionnaire for Rheumatology to the Arabic Language in Patients with Rheumatoid Arthritis. Patient Prefer Adherence. 2021;15:1461–1467. doi:10.2147/PPA.S316263
11. Aljohani R, Aljohani Z, Aljohani R, Alsaidalani R. Saudi cultural adaptation of the “compliance questionnaire of Rheumatology” for Rheumatoid arthritis patients on disease modifying anti-rheumatic drugs (DMARDs). Saudi Pharm J. 2021;29(5):377–383. doi:10.1016/j.jsps.2021.03.007
12. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi:10.1016/j.jclinepi.2007.11.008
13. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581.
14. Hughes LD, Done J, Young A. A 5 item version of the Compliance Questionnaire for Rheumatology (CQR5) successfully identifies low adherence to DMARDs. BMC Musculoskelet Disord. 2013;14:286.
15. Gossec L, Paternotte S, Aanerud GJ, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis. 2011;70(6):935–942.
16. Fidder HH, Singendonk MM, van der Have M, Oldenburg B, van Oijen MG. Low rates of adherence for tumor necrosis factor-alpha inhibitors in Crohn’s disease and rheumatoid arthritis: results of a systematic review. World J Gastroenterol. 2013;19(27):4344–4350.
17. Viller F, Guillemin F, Briançon S, Moum T, Suurmeijer T, van den Heuvel W. Compliance to drug treatment of patients with rheumatoid arthritis: a 3 year longitudinal study. J Rheumatol. 1999;26(10):2114–2122.
18. Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47(2):172–183.
19. Marras C, Monteagudo I, Salvador G, et al. Identification of patients at risk of non-adherence to oral anti-rheumatic drugs in rheumatoid arthritis using the Compliance Questionnaire in Rheumatology: an ARCO sub-study. Rheumatol Int. 2017;37(7):1195–1202.
20. Dougados M, Brault Y, Logeart I, van der Heijde D, Gossec L, Kvien T. Defining cutoff values for disease activity states and improvement scores for patient-reported outcomes: the example of the Rheumatoid Arthritis Impact of Disease (RAID). Arthritis Res Ther. 2012;14(3):R129.
21. Nikiphorou E, Norton S, Young A, et al. Association between rheumatoid arthritis disease activity, progression of functional limitation and long-term risk of orthopaedic surgery: combined analysis of two prospective cohorts supports EULAR treat to target DAS thresholds. Ann Rheum Dis. 2016;75(12):2080–2086.
22. Hassen LM, Almaghlouth IA, Hassen IM, et al. Impact of COVID-19 outbreak on rheumatic patients’ perceptions and behaviors: a cross-sectional study. Int J Rheum Dis. 2020;23(11):1541–1549.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

