Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Use of Topical Corticosteroids in the Treatment of Noninfectious Inflammatory Dermatoses of the Scalp: A Survey of Practicing Dermatologists and Dermatology Residents Using Delphi Methodology
Authors Mir-Bonafé JF, Piquero-Casals J , Prudkin L , Delgado J, Santamaria Martínez J , Garcia-Patos Briones V
Received 3 November 2023
Accepted for publication 21 February 2024
Published 18 March 2024 Volume 2024:17 Pages 671—681
DOI https://doi.org/10.2147/CCID.S448016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Juan Francisco Mir-Bonafé,1 Jaime Piquero-Casals,2 Ludmila Prudkin,3 Jesus Delgado,3 Julia Santamaria Martínez,3 Vicente Garcia-Patos Briones4
1Department of Dermatology, Hospital Son Llàtzer, Palma de Mallorca, Spain; 2Department of Aesthetic Dermatology and Laser, Dermik, Clínica Dermatológica Multidisciplinar, Barcelona, Spain; 3Innovation and Development, ISDIN, Barcelona, Spain; 4Department of Dermatology, Hospital Vall d’Hebron, Barcelona, Spain
Correspondence: Jaime Piquero-Casals, Department of Aesthetic Dermatology and Laser, Dermik, Clínica Dermatológica Multidisciplinar, Barcelona, Spain, Tel +34 935464710, Email [email protected]
Background: Noninfectious inflammatory dermatoses of the scalp are common, and their symptomsin particular, those affecting appearance, can have a psychological effect that may be disproportionate to their clinical severity. Effective, cosmetically acceptable treatments are important to manage these conditions. Topical corticosteroids form the cornerstone of acute treatment for many of these conditions. We surveyed practicing dermatologists and dermatology residents to determine the current clinical practice in prescribing topical corticosteroids for these disorders in their various preparation formats.
Methods: A Delphi method was used, consisting of 2 questionnaire rounds. The first round contained 34 questions and was completed by 612 dermatologists and dermatology residents via email. The first round of responses was analyzed, and points that had > 70% agreement were used to form a second questionnaire of 21 statements. This second round was completed by 346 participants, and their responses were used to generate a final report. Participants were practicing in both public and private practices.
Results: Clobetasol propionate 0.05% topical solution was considered to be the most appropriate treatment for noninfectious inflammatory scalp dermatoses in general, with 75.1% agreement in the second round of questionnaire. The main advantages of clobetasol propionate over other topical corticosteroids were reported as potency, effectiveness, and broad action spectrum (94.8% agreement). The preferred pharmaceutical format was the solution of clobetasol propionate.
Conclusion: Clobetasol propionate was the preferred topical treatment for the management of scalp dermatoses, usually as first-line treatment; solution was the preferred preparation.
Keywords: seborrheic dermatitis, psoriasis, shampoo, topical, solution, clobetasol, scalp, therapy
Introduction
Noninfectious inflammatory dermatoses of the scalp include seborrheic dermatitis, eczema, psoriasis, lichen planopilaris (and the variant frontal fibrosing alopecia), alopecia areata, neurodermatitis (aka lichen simplex chronicus), and discoid lupus erythematosus. Many of these share common or overlapping symptoms,1 and can have a considerable impact on quality of life. In addition to itch, which can be highly distressing, there are the emotional and social consequences of symptoms such as scaling, flaking, and possibly hair-loss, in a very visible area.
Topical corticosteroid-based preparations, which inhibit epidermal proliferation and inflammation and modulate immune response,2 form the cornerstone of treatment for many scalp dermatoses.3,4
The formulation of topical treatments for the scalp is of utmost importance:5 the visibility of the area and presence of hair make cosmesis key, and formats including foam, gel, solution, shampoo, and spray have been developed over the years, which are often preferable to ointments or creams.3 Delivery of therapy can also represent a challenge3 as medicated shampoos are only in contact with the affected areas for a very short time.3 Clobetasol propionate is a class I very potent steroid that is widely used, owing to its strong anti-inflammatory, antipruritic, vasoconstrictive, and antiproliferative activities.6 Its formulation as a 0.05% scalp application is safe and effective treatment.7
Although topical corticosteroids are widely used and also well established as safe and effective treatments, professional dermatological associations have noted “great variability in aspects such as dosing, potency and application quantity”.8 While individualized treatment is important, we sought to establish a picture of current practice among dermatologists in the treatment of scalp dermatoses, particularly regarding use of topical corticosteroids in their various preparations/formulations.
A Delphi method was used; this method is based on a series of “rounds”, with analysis of the responses provided as feedback to the participants, allowing them to carefully consider their answers in light of others’ responses.9
Methods
Figure 1 shows a flowchart of the process. Two rounds of questionnaires were issued to practicing dermatologists in Spain, which included specialists and residents in dermatology. Questionnaires were sent to 1500 dermatologist and dermatology residents via Email following the guidelines established in the CHERRIES (Checklist for Reporting Results of Internet E-surveys) statement.10 These participants were identified via an online educational campaign that awards points for participation (which can be used in exchange for cosmetic and dermatology products). A total of 612 completed the first round, which consisted of 34 questions: 5 on demographics and 29 on clinical practice. These included questions on the most common signs and symptoms reported (multiple choice of 4: alopecia, itch, redness, desquamation/scale, other); the extent to which scalp dermatoses were considered to affect patient quality of life (visual analog scale [VAS] of 1–10 for 11 different factors: social life, work/hobbies, sleep problems, sex life, physical appearance, worrying about the disease, depression, itch, hypersensitivity, burning, pain); frequency of prescribing of various topical corticosteroids as first-line and second-line treatment (never/sometimes/always); perceived advantages, if any, of prescribing clobetasol propionate over other topical corticosteroids (VAS scale of 1–10 for each possible advantage); reasons, if any, for not prescribing clobetasol propionate; estimated proportion of patients for whom different formulations (solution, foam, shampoo) of clobetasol propionate is prescribed; and the perceived advantages of each formulation type (topical [not systemic] absorption, ie the feeling that it soaks in to the skin without residue, cosmesis, effectiveness, improved quality of life, treatment adherence, other [specify]). There were also questions on prescribing regimens for clobetasol propionate solution: number of doses per day and duration of treatment, and the indications for which clobetasol propionate was prescribed, as well as use of adjuvant treatments.
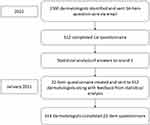 |
Figure 1 Study methodology. |
The results of round 1 were analyzed (see below for details of statistical analysis), and in January 2021, the findings were provided as feedback to the interviewees, along with a more concise, 22-item questionnaire. For inclusion in the second round, a cutoff of 70% agreement for first round questions was used. A total of 346 dermatologists completed round 2. In this round, the participants indicated the degree of agreement with each of the 22 statements, using a Likert scale from 1–9, 1–3 being disagreement (1 being complete disagreement), 4–6 being neither agree nor disagree, and 7–9 being agreement (9 being complete agreement). Statements related to the main symptoms reported by patients; the effect on quality of life caused by pain from the disease; the appropriateness of clobetasol propionate as first-line and second-line treatment options; the advantages of clobetasol propionate over other corticosteroids; preference for different formulations/preparations (solution vs shampoo or foam); treatment duration for once-daily, twice-daily, and three-times-daily regimens of clobetasol propionate; routine prescribing of clobetasol propionate for different disease indications (lichen planopilaris, discoid lupus erythematosus, scalp psoriasis, alopecia areata, frontal fibrosing alopecia, and scalp eczema).
Consent/Ethics
Participants gave consent to participate and for the results to be published. No patients were included in this study.
Statistical Analysis
A descriptive analysis was performed of the individuals who completed each round, using the statistical software package SPSS 22.0 for Windows. For the descriptive analysis, nominal variables were reported as frequency and percentage. Continuous variables were summarized as mean, median, mode, standard deviation, and minimum and maximum. Analysis of variance (ANOVA) was used to determine significance. For inferential analysis, the summary tables of the inferences of the alphanumeric variables were represented as frequency and percentage. Chi-squared test used. For other more specific inferences, ANOVA or Mann–Whitney U-test were used according to whether the assumptions were met.
Results
Round 1 was completed by 612 dermatologists and dermatology residents; round 2, by 346. Their demographics are presented in Table 1. The majority worked in the public health system or both public and private systems. Most (77%) were specialists (ie consultants/attendings), the remainder were residents. All regions of Spain (its 17 autonomous communities) were represented.
 |
Table 1 Demographics of Practicing Dermatologists and Dermatology Residents in Spain Participating in the Survey |
Phase 1 Results
Noninfectious inflammatory dermatoses of the scalp made up a mean 15% (SD 12%) of the dermatologists’ caseload (median 11%; min-max, 0–100%). The main symptoms seen in practice were itch (89.1% agreement), desquamation/scale (87.4%) and alopecia (76.6%).
Patient quality of life was affected mainly by the following factors, with scores on a VAS of 0–10 (0 = not affected, 10 = severely affected) as follows: physical appearance (7.9 ± 1.6), itch (7.9 ± 1.6), and worrying about the disease (7.3 ± 1.8). Somewhat lower scores were obtained for social life (6.8), hypersensitivity (6.5), burning (6.2), work and hobbies (5.5), depression (5.2), sleep problems (4.4) and sex life (4.2).
Figure 2 shows the frequency of prescribing the different corticosteroids as first-line treatment. The most commonly prescribed corticoid as first-line treatment was clobetasol propionate 0.05% solution (28.4% always, 68.3% sometimes), followed by betamethasone dipropionate 0.05% solution (8.5% always, 72.4% sometimes) and clobetasol propionate 0.05% shampoo (7.8% always, 65.8% sometimes).
 |
Figure 2 First-line corticosteroid treatment of scalp dermatoses (overall noninfectious inflammatory disease): frequency of prescribing. Abbreviations: CP, clobetasol propionate; soln, solution. |
The most commonly prescribed corticosteroid as second-line treatment of scalp dermatoses was also clobetasol propionate 0.05% solution, (9% always, 84.1% sometimes), followed by clobetasol propionate 0.05% shampoo (6.2% always, 69.6% sometimes) and betamethasone dipropionate 0.05% solution (4.8% always, 72.8% sometimes).
Figure 3 shows the frequency of prescribing clobetasol propionate 0.05% solution by individual disease. Lichen planopilaris and psoriasis were the indications most commonly treated with this solution.
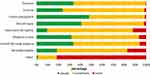 |
Figure 3 Use of clobetasol propionate solution by disease. |
The perceived advantages of clobetasol propionate (VAS score 0–10) were its potency (9.1 ± 1.1), effectiveness (8.9 ± 1.1), action spectrum against a range of diseases (8.1 ± 1.6), predictable/consistent response (7.6 ± 1.7) and tolerability (7.4 ± 1.8).
Regarding the usage of the various preparations of clobetasol propionate (solution, shampoo, and foam), solution was the most used format. The dermatologists’ reported that they prescribed solution in 67% of patients (SD±27%), shampoo in 20% (±21%), and foam in 13% (±17%). In the perceived advantages of the various preparations, solution scored marginally higher than shampoo and foam for the aspects of effectiveness, improved QOL, cosmesis, treatment adherence, and absorption (topical) (Figure 4).
 |
Figure 4 Mean scores for perceived advantages of each formulation of clobetasol propionate. Abbreviation: QOL, quality of life. |
The most common prescribing regimen for clobetasol propionate solution was once daily (95.8% of dermatologists reported prescribing this). Twice-daily regimens were less common (50.0%), and three-times-daily regimens (29.7%) and alternate day regimens (26.0%) were uncommon.
Treatment duration for a once-daily treatment ranged between 1 and 4 weeks, with small percentages of treatment durations >4 weeks.
Adjuvant treatments were largely categorized as being prescribed “sometimes”, shampoos (keratolytic, antiseborrheic and antipruritic) being the most frequently used.
Differences Between Specialists and Residents
As would be expected, more of the specialists worked in a private healthcare setting than the residents (75.5% of specialists were in the private or mixed public and private setting vs 5.8% for residents). The differences found between specialists vs residents for corticosteroid prescription are shown in Supplementary Table 1.
Residents were more likely to prescribe clobetasol propionate solution (35.3% always prescribe vs 26.4% in specialists, P = 0.037), and less likely to prescribe shampoo: 38.8% never prescribe vs 22.6% in specialists) (Supplementary Table 1). Regarding duration of treatment, there were no significant differences based on resident vs specialist for once-daily regimens but significant differences for twice-daily regimens, with 58.0% of specialists vs 34.7% of residents opting for 1 week, and a slightly higher percentage of residents going for longer durations of 2, 3, and 4 weeks. Supplementary Table 1 shows the comparison of responses from residents and specialists.
Phase 2 Results
Figure 5 shows the percentage agreement and disagreement for all the questionnaire statements from round 2. Eighty-eight percent of the dermatologists agreed that the aspects of patient quality of life most affected by scalp dermatoses were itch and physical appearance. Clobetasol propionate 0.05% solution was viewed as the most appropriate first-line corticosteroid treatment of noninfectious inflammatory scalp dermatoses in general by 75.1% of respondents.
Comparing the different formulations, most of the dermatologists surveyed preferred to prescribe solution over foam or shampoo (90.5% agreement); there was not a strong consensus on reasons: 69.1% considered it more effective, 62.4% considered it to improve patient QOL more, 61.8% thought it had better cosmesis, 64.7% thought it had better treatment adherence, and 60.7% considered it to have better topical absorption.
The most preferred regimen was once daily for 2 weeks (64.7% agreement), followed by once daily for 4 weeks (49.4%), and twice daily for 1 week (47.4%).
There was consensus on the prescribing of clobetasol propionate 0.05% solution as standard for the treatment of lichen planopilaris (93.4%), scalp psoriasis (88.2%), discoid lupus (87.0%) alopecia areata (79.2%), frontal fibrosing alopecia (77.5%) and eczema of the scalp (73.7%).
Discussion
This study set out to determine current practice in dermatologists in Spain regarding the use of topical corticosteroids in the treatment of noninfectious inflammatory scalp dermatoses, with a particular focus on highly potent steroids and their different formulations/preparations. The most salient findings were that clobetasol propionate was considered to be, in general, the most appropriate topical steroid in the treatment of noninfectious inflammatory scalp dermatoses, the main perceived advantages over other topical corticoids being potency, effectiveness and broad action spectrum; solution was used preferentially over shampoo or foam.
Many of the findings were for the noninfectious inflammatory scalp dermatoses as a whole, which does represent a limitation of the study. If we look at the individual conditions, we see that lichen planopilaris, discoid lupus erythematosus, and scalp psoriasis were the conditions for which dermatologists most commonly prescribed clobetasol propionate as standard. So, is this in line with the existing evidence in the literature? For psoriasis, it is fairly well established that first-line therapy for patients with scalp psoriasis is topical corticosteroids.3,11 The National (USA) Psoriasis Foundation concluded that, based on the limited number of comparative studies, they are more effective than other topical therapies, such as calcipotriene, coal tar, and probably tazarotene.11 A Cochrane review of chronic plaque psoriasis also stated that corticosteroids were more effective than vitamin D for scalp psoriasis and less likely than vitamin D to cause irritation, but that further research was needed on maintenance treatment and safety.12 Another Cochrane review on scalp psoriasis topical therapies found that although a combination of steroid plus vitamin D was statistically better than steroid monotherapy, the benefit was not clinically relevant, and that, given the slim benefit but similar safety profile, monotherapy with topical steroids “may be fully acceptable” for short-term treatment.13 However, Chan et al considered that combination therapy may have some advantages by reducing toxicity and side effects.11 Descaling (eg with salicylic acid) may be necessary to allow effective immunosuppressive treatment to penetrate;14 for such patients, therefore, corticosteroids would probably not be considered the first-line.
Recent (2019) recommendations on discoid lupus erythematosus state that lifestyle measures and topical treatment (corticosteroids and topical calcineurin inhibitors) have the highest evidence level,15 with a strength A recommendation (level of evidence 1+) but are “commonly insufficient in isolation”.15
In the case of lichen planopilaris, recent (2020) European guidelines16 briefly discuss the role of topical steroids, reporting that they are the most frequently used medication for this condition. However, the authors of the document describe them (in their superpotent, potent or mild forms) as only sometimes effective, and raise doubts about their value, suggesting that intralesional corticosteroids may be a more effective option.16
The other conditions falling under the category of scalp dermatoses included in this study—alopecia areata, seborrheic dermatitis, eczema, and neurodermatitis—did not have such strong agreement regarding topical corticosteroid use as first-line treatment. This may be due to the additional importance of other therapies in these conditions. A study on alopecia areata comparing clobetasol propionate 0.05% and topical pimecrolimus 1% found that pimecrolimus was as effective as corticosteroids, and superior to corticosteroids in terms of side effects.17 A recent (2017) review article includes contact immunotherapy, topical corticosteroids, intralesional steroids, and minoxidil all as possible first line therapies.4
In the treatment of seborrheic dermatitis, antifungal treatment is a key component,1 and potent topical steroids may be reserved for these with more severe or inflammatory disease.1 Danish guidelines from 2015 state that first line treatment is based on topical antifungal agents, either alone or in combination with corticosteroids or topical calcineurin inhibitors.18
For the treatment of atopic dermatitis, the American Academy of Dermatology guidelines8 make a strong recommendation for the use of topical corticosteroids, but also for topical calcineurin inhibitors, PDE-4 inhibitors, JAK inhibitors, as well as nonprescription therapies such as moisturizers (no particular products or ingredients are recommended). The guideline document describes topical corticosteroids as the most commonly used therapy, used as first-line treatment for mild to severe dermatitis with “overwhelming literature and high certainty evidence” to support topical corticosteroid treatment.8
Neurodermatitis, or lichen simplex chronicus, has been described as difficult to manage owing to its multifactorial nature and lack of evidence-based guidelines.19 A systematic review of the evidence for the treatment of lichen simplex chronicus came out in 2021, and concluded that the most robust evidence was for topical corticosteroids, but also that there was some (limited) data suggesting benefit with other treatments, namely topical immunomodulators, topical antipruritic agents, oral antihistamines, antiepileptics and antidepressants.19 This lack of clear guidance may explain why topical corticosteroid use was less consistent in this condition in the present study.
Regarding treatment duration, current guidelines on the treatment of psoriasis say that maximal efficacy is reached within 3 to 4 weeks.2 In the present study, 1–2 weeks were the most common durations, although some did report use of several weeks and even up to more than 8 weeks. Chan et al reported that there were no studies to support the safety of topical corticosteroid use on the scalp beyond 4 weeks,11 but noted that a previous study had found use for >8 weeks to also be common.11,20 Regarding longer-term therapy, they notes that many experienced clinicians prescribe long-term intermittent topical steroids (in scalp psoriasis), but ultimately called for a better evidence base.11
Regarding type of corticosteroid and potency, Chan et al suggest (again for scalp psoriasis) that the lowest effective strength should be used, and that the prescribing regimen should be adapted to the individual’s needs.11 The Danish guidelines on seborrheic dermatitis also suggest that less potent corticosteroids should be used preferentially to avoid cutaneous side effects (corticosteroids are categorized as an adjuvant treatment in that document);18 however, we should bear in mind that the guideline is not scalp-specific, and that the scalp is less predisposed to side effects of skin thinning than the face. The American Academy of Dermatology guidelines on AD do not state a preferred type of topical corticosteroid but note that very high potency steroids (of which clobetasol propionate is one) can be an effective treatment of severe AD flare-ups.8 Again, these guidelines are not specific to the scalp. Besides strength, the efficacy of one steroid over another seems difficult to ascertain based on existing studies. An early (1976), double-blind study of scalp psoriasis treatments found clobetasol propionate solution 0.05% to be superior to betamethasone dipropionate solution 0.05%,21 but Bergstom et al found, in their study of clobetasol foam 0.05% vs combination clobetasol cream 0.05% to the skin and clobetasol solution 0.05% to the scalp, that there was a greater absolute improvement in psoriasis severity in the foam group.22 They also found that the foam application was less time consuming (than the patients’ previous treatments)22 – this is a very important aspect since, in a European patient survey, being time consuming was reported as “the most troublesome” aspect of treatment by 50%.23,24 However, the study by Bergstom et al was of psoriasis, not restricted to the scalp, hence the use of a cream, so their finding relating to time spent may be less generalizable to those with diseases of the scalp only. The 2009 European consensus on scalp psoriasis expressed preference for a short-contact formulation, such as a shampoo, but that other vehicles (solution, lotion, foam, gel) were also appropriate.14 Clobetasol propionate shampoo 0.05% has been proven to be highly effective and safe for scalp psoriasis, for the initial treatment phase and as maintenance therapy to prevent relapse.5,23 Several other published studies, in addition to the vast clinical experience, have highlighted the importance of delivery format in providing a suitable and effective treatment for scalp dermatoses.11 Regarding specific type of corticosteroid, Katz et al’s study compared clobetasol propionate and betamethasone augmented ointment for scalp psoriasis and found that both were equally effective but the betamethasone worked faster;25 however, a scalp ointment is unlikely to be acceptable to many patients cosmetically.
Two aspects of this questionnaire were the main symptoms seen in practice and the effect on patient quality of life. There was consensus that itch and scale were the predominant symptoms of scalp dermatoses seen in practice. Of course, the ideal way to determine patients’ symptoms and quality of life is to ask them directly, and use a validated assessment tool. However, this finding does align with a previous survey of >3000 psoriasis patients (not scalp-specific) in North America and Europe, in which itch was reported to be the most bothersome symptom in 43%, followed by scales (23%) and flaking (20%).26 Another survey of psoriasis patients also found itch, scaliness and visibility to be perceived as the major disease issues.20 This is reassuring as our dermatologist-reported findings appear to accurately reflect patient opinion. A significant correlation has previously been reported between desquamation on the scalp and mental health, and that it does not necessarily have to be severe to have a profound effect.27
This study used a DELPHI methodology, which is intended to obtain a reliable consensus from a group of experts, using iterative questionnaires along with feedback of responses.28 Such a method means that, firstly, responses are anonymous, so more prominent participants are not allowed to dominate or influence other participants based on personality, but also risks a “bandwagon effect”, that is, participants may adjust their response to comply with the majority.9 The process allows participants to reflect and reconsider their views in light of others’ contributions.9 We think that providing feedback based on the analysis of the first-round responses allowed participants to consider and reflect upon their answers more than they may have done with a single interview. Regarding the threshold for consensus, there is not an established absolute cutoff,9 with previous suggestions ranging from 51% to 100%;9 we opted for what we considered a reasonable value, of 70%. This survey was emailed to the participants; as such, the limitations include those inherent to an electronic survey, in that those who respond may not be representative of the whole population of dermatologists.10
Another limitation is that many of the questions were about noninfectious inflammatory scalp dermatoses as a whole group. Some questions did specify individual diseases, but generally they were grouped together, meaning that results may not be equally as applicable to individual dermatoses, this may be particularly true with the use of adjuvants such as coal tar for example. Future studies may focus on a single disease and its various treatments rather than the treatments for a group of diseases. In clinical practice, it is essential to tailor treatment to suit the individual, taking into account not only their disease and its objective severity but also their opinions and preferences regarding formulation/preparation and dose regimen.
Many of these diseases are chronic relapsing-remitting conditions that are not definitively cured. Not only is the scalp a highly visible area, but the presence of hair complicates the cosmetic acceptability of treatment.5,26 Given the potentially huge effect on quality of life,5 these conditions must be managed well, that is, with effective treatments that patients will be happy to use.
Conclusions
This clinical practice survey completed by 346 dermatologists practicing in Spain found that clobetasol propionate 0.05% solution was the preferred first-line topical corticosteroid formulation for the management of most noninfectious inflammatory scalp dermatoses.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethics Guidelines
This survey used aggregate data for analysis and did not involve any intervention on human subjects and was therefore not considered biomedical research that would require mandatory IRB review, in accordance with local (Spain) regulations. The survey was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki. Survey conduct was consistent with good clinical practices and applicable laws and regulations. All participants provided consent to participate.
Acknowledgments
To Jane Marshall (medical writer freelance, Glasgow, UK).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Publication costs funded by ISDIN S.A.
Disclosure
JPC is a consultant for ISDIN. LP, JD and JS are ISDIN employers. VGP has received honoraria from ISDIN. JFMB reports personal fees from ISDIN. The authors report no other conflicts of interest in this work.
References
1. Grimalt R. A practical guide to scalp disorders. J Investig Dermatol Symp Proc. 2007;12(2):10–14. doi:10.1038/sj.jidsymp.5650048
2. van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. Diagnosis and management. Am J Clin Dermatol. 2001;2(3):159–165. doi:10.2165/00128071-200102030-00005
3. Mosca M, Hong J, Hadeler E, Brownstone N, Bhutani T, Liao W. Scalp psoriasis: a literature review of effective therapies and updated recommendations for practical management. Dermatol Ther. 2021;11(3):769–797. doi:10.1007/s13555-021-00521-z
4. Lee S, Lee WS. Management of alopecia areata: updates and algorithmic approach. J Dermatol. 2017;44(11):1199–1211. doi:10.1111/1346-8138.13933
5. Gooderham M, Blakely K. Management of scalp psoriasis: current perspectives. Psoriasis. 2016;33. doi:10.2147/PTT.S85330
6. Nair AB, Kumar S, Dalal P, et al. Novel dermal delivery cargos of clobetasol propionate: an update. Pharmaceutics. 2022;14(2). doi:10.3390/pharmaceutics14020383
7. Olsen EA, Cram DL, Ellis CN, et al. A double-blind, vehicle-controlled study of clobetasol propionate 0.05% (Temovate) scalp application in the treatment of moderate to severe scalp psoriasis. J Am Acad Dermatol. 1991;24(3):443–447.
8. Sidbury R, Alikhan A, Bercovitch L, et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89(1):128–129. doi:10.1016/j.jaad.2022.08.068
9. Barrett D, Heale R. What are delphi studies? Evidence Based Nurs. 2020;23(3):68–69. doi:10.1136/ebnurs-2020-103303
10. Eysenbach G. Improving the quality of web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res. 2004;6(3):e34. doi:10.2196/jmir.6.3.e34
11. Chan CS, Van Voorhees AS, Lebwohl MG, et al. Treatment of severe scalp psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60(6):962–971. doi:10.1016/j.jaad.2008.11.890
12. Mason A, Mason J, Cork M, Hancock H, Dooley G. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69(5):799–807. doi:10.1016/j.jaad.2013.06.027
13. Schlager JG, Rosumeck S, Werner RN, et al. Topical treatments for scalp psoriasis. Cochrane Database Syst Rev. 2016;2(2):Cd009687. doi:10.1002/14651858.CD009687.pub2
14. Ortonne J, Chimenti S, Luger T, Puig L, Reid F, Trüeb R. Scalp psoriasis: European consensus on grading and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23(12):1435–1444. doi:10.1111/j.1468-3083.2009.03372.x
15. Company-Quiroga J, Alique-García S, Romero-Maté A. Current insights into the management of discoid lupus erythematosus. Clin Cosmet Invest Dermatol. 2019;12:721–732. doi:10.2147/CCID.S184824
16. Ioannides D, Vakirlis E, Kemeny L, et al. European S1 guidelines on the management of lichen planus: a cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol. 2020;34(7):1403–1414. doi:10.1111/jdv.16464
17. Ucak H, Kandi B, Cicek D, Halisdemir N, Dertlıoğlu SB. The comparison of treatment with clobetasol propionate 0.05% and topical pimecrolimus 1% treatment in the treatment of alopecia areata. J Dermatol Treat. 2012;23(6):410–420. doi:10.3109/09546634.2011.590788
18. Hald M, Arendrup MC, Svejgaard EL, Lindskov R, Foged EK, Saunte DM. Evidence-based Danish guidelines for the treatment of Malassezia-related skin diseases. Acta Derm Venereol. 2015;95(1):12–19. doi:10.2340/00015555-1825
19. Juarez MC, Kwatra SG. A systematic review of evidence based treatments for lichen simplex chronicus. J Dermatological Treat. 2021;32(7):684–692. doi:10.1080/09546634.2019.1708856
20. van de Kerkhof PC, de Hoop D, de Korte J, Cobelens SA, Kuipers MV. Patient compliance and disease management in the treatment of psoriasis in the Netherlands. Dermatology. 2000;200(4):292–298. doi:10.1159/000018390
21. Lassus A. Local treatment of psoriasis of the scalp with clobetasol propionate in alcoholic solution: a comparison of once and twice a day application. Curr Med Res Opin. 1976;4(3):214–217. doi:10.1185/03007997609109306
22. Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72(5):407–411.
23. Poulin Y, Papp K, Bissonnette R, Barber K, Kerrouche N, Villemagne H. Clobetasol propionate shampoo 0.05% is efficacious and safe for long-term control of moderate scalp psoriasis. J Dermatol Treat. 2010;21(3):185–192. doi:10.3109/09546630903493311
24. Dubertret L, Mrowietz U, Ranki A, et al. European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol. 2006;155(4):729–736. doi:10.1111/j.1365-2133.2006.07405.x
25. Katz HI, Lindholm JS, Weiss JS, et al. Efficacy and safety of twice-daily augmented betamethasone dipropionate lotion versus clobetasol propionate solution in patients with moderate-to-severe scalp psoriasis. Clin Ther. 1995;17(3):390–401. doi:10.1016/0149-2918(95)80104-9
26. Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Am Acad Dermatol. 2014;70(5):
27. Heydendael VM, de Borgie CA, Spuls PI, Bossuyt PM, Bos JD, de Rie MA. The burden of psoriasis is not determined by disease severity only. J Investig Dermatol Symp Proc. 2004;9(2):131–135. doi:10.1111/j.1087-0024.2004.09115.x
28. Norman Dalkey OH, Helmer O. An experimental application of the DELPHI method to the use of experts. Manage Sci. 1963;9(3):458–467. doi:10.1287/mnsc.9.3.458
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Reducing the Risk of Developing Psoriatic Arthritis in Patients with Psoriasis
Gisondi P, Bellinato F, Maurelli M, Geat D, Zabotti A, McGonagle D, Girolomoni G
Psoriasis: Targets and Therapy 2022, 12:213-220
Published Date: 10 August 2022
The Anti-Psoriatic Efficacy and Safety Profile of Topical and Intralesional Methotrexate: A Literature Review
Chaiyabutr C, Punnakitikashem P, Silpa-archa N, Wongpraprarut C, Chularojanamontri L
Clinical, Cosmetic and Investigational Dermatology 2022, 15:2253-2274
Published Date: 26 October 2022
Therapeutic Management of a Case of Severe Psoriasis Coexistent with Bullous Pemphigoid in the Elderly
Di Lernia V, Peccerillo F, Ficarelli E
Psoriasis: Targets and Therapy 2023, 13:27-31
Published Date: 22 August 2023

