Back to Journals » Cancer Management and Research » Volume 14
The Predictive Value of CA-125 and Hb for Ocular Metastasis in Hepatocellular Carcinoma Patients
Authors Xiong X, Rong R, Tang LY, Sun T, Pan YC , Shu HY, Zhang LJ , Ge QM , Liang RB, Shao Y
Received 24 February 2022
Accepted for publication 1 August 2022
Published 5 December 2022 Volume 2022:14 Pages 3405—3415
DOI https://doi.org/10.2147/CMAR.S363115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Xin Xiong,1,* Rong Rong,2,* Li-Ying Tang,3,* Tie Sun,1 Yi-Cong Pan,1 Hui-Ye Shu,1 Li-Juan Zhang,1 Qian-Min Ge,1 Rong-Bin Liang,1 Yi Shao1
1Department of Pathology and Ophthalmology, The First Affiliated Hospital of Nanchang University, Jiangxi Centre of Natural Ocular Disease Clinical Research Center, Nanchang, Jiangxi, 330006, People’s Republic of China; 2Eye Center of Xiangya Hospital, Central South University, Changsha, Hunan, 410008, People’s Republic of China; 3Department of Ophthalmology, Zhongshan Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, Fujian Province, 361004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi Shao, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, No. 17, Yong Wai Zheng Street, Dong Hu District, Nanchang, Jiangxi, 330006, People’s Republic of China, Tel/Fax +86 791-88692520, Email [email protected]
Objective: To explore the risk factors of ocular metastasis (OM) in patients with hepatocellular carcinoma (HCC) by analyzing the demographic characteristics and serum markers.
Methods: From July 2002 to December 2012, 1064 HCC patients were included in our study. The chi-squared test and Student’s t-test were used to assess the difference between OM and any other metastasis (NOM). Receiver operating curve (ROC) was used to analyze the diagnostic value of serum biomarkers in HCC patients with OM.
Results: The incidence of OM in HCC patients was 1.88% in our research. There are no significant differences in age, gender, or histopathology in the OM group and the group without any metastasis. Binary logistic regression analysis presented that compared with the patients without cancer metastasis, carbohydrate antigen 125 (CA-125) and hemoglobin (Hb) were risk factors in hepatocellular carcinoma patients with OM (P < 0.05). The ROC curve analysis showed that the areas under the CA-125, Hb, and CA125+Hb curves were 0.877, 0.554, and 0.431, and the cutoff values of CA-125 and Hb each were 115.78 u/mL and 120.50 g/L.
Conclusion: Our data suggest that CA-125 and Hb are risk indicators in hepatocellular carcinoma patients with OM, and that CA-125+Hb has potentially greater utility in diagnosing hepatocellular carcinoma.
Keywords: hepatocellular carcinoma, ocular metastases, CA-125, Hb, risk factors
Article Summary
- We found that CA-125 and Hb are risk indicators in HCC patients with OM.
- The study indicated that CA-125+Hb has potentially greater utility in diagnosing HCC.
Introduction
Cancer liver is the fourth common malignant tumor seen in clinical practice, with the characteristics of high malignancy, rapid progression, strong invasiveness, poor prognosis, and high mortality, bring patients with decreased survival and quality of life.1 Hepatocellular carcinoma (HCC) is currently the most common type of primary HCC.2 Studies have shown that HCC metastasizes mainly rely on blood spread, with lung and bone metastases accounting for 76% and 11% of metastases. And following HCC metastasis to the brain, patients often experience significant nausea and headache. The mortality rate of primary HCC is about 54% worldwide nowadays,3 and accurate diagnosis at the early clinical stage is thought to greatly improve treatment efficacy and survival of patients. However, the high metastasis and recurrence rates of primary HCC (mainly HCC) after surgery are the primary factors affecting patients’ prognosis.4 Therefore, an in-depth study of HCC metastasizing mechanisms is essential for clinical prevention and treatment. Metastasis of liver tumors to the eye has not been reported in detail in the current literature; however, according to some studies, HCC with ocular metastasis (OM) can cause a series of ocular symptoms, which may seriously affect the physical and mental quality of cancer patients. Therefore, early detection, diagnosis, and treatment is crucial for HCC patients with OM. Currently, clinical studies have found that some reliable predictors of immune checkpoint inhibitors response, such as PD-L1 and TMB, may help predict that HCC patients will benefit more from immunotherapy.40–42
In this study, we used tumor serological markers to assess factors associated with the early diagnosis of HCC with OM. Tumor serological markers are less invasive than biopsy, relatively inexpensive, reusable, and easy to screen and follow-up. Tumor markers are molecules that indicate the presence of tumor cells. These markers are produced by tumors, and a tumor may have a variety of markers. Targeted detection of tumor-associated markers in serum can facilitate the early detection and timely treatment of malignant tumors. Tumor markers are produced by tumors and accumulate in other tissues and bodily fluids.5 The detection of tumor markers in human blood, bodily fluids, or tissue cells can aid in diagnosing the presence, pathogenesis, and prognosis of tumors. Currently, numerous serum tumor markers have been found to be associated with HCC. For example, Ma et al6 found that CEA, CA-125, and CYFRA21-1 levels are high in lung cancer and can be used for the diagnosis of this disease. Previous studies have also shown that these markers are associated with poor prognosis in non-small cell lung cancer.7 Cancer embryonic antigen (CEA) is significantly elevated in 70% of patients with definitive diagnosis of colorectal cancer (CRC)8 and in 75% of patients with metastatic eye tumors,9 while concentrations of lipid biomarkers, such as triglycerides and HDL-C, have been shown to correlate with the incidence of breast and colorectal cancers.10 In addition, tumor markers may be potentially useful for predicting tumor metastasis. Studies have reported that elevated levels of apolipoprotein are strongly correlated with the development of tumors. Studies have demonstrated that specific apolipoproteins affect tumor growth by modulating immune cell function.11 The levels of apolipoproteins are used to assess the prognosis of patients with HCC and colorectal cancer.12,13
However, it is unclear whether there is a difference in the tumor markers themselves between HCC patients with and without OM. In this study, we collected the medical records of HCC patients admitted to our hospital, and retrospectively analyzed the diagnostic value of common HCC markers. We mainly tested the serum markers in HCC patients, and compared the levels of these markers between patients with and without metastasis. The factors increasing the risk of OM of HCC were identified. Our results provide a powerful medical basis of testing targets for assessing the presence of OM in HCC patients.
Materials and Methods
Study Design
All participants joined voluntarily and were informed of the study design, and all participating patients provided written informed consent and the study was performed in accordance with the tenets of the Declaration of Helsinki and its amendments. The study was approved by the Medical Research Ethics Committee of the First Affiliated Hospital of Nanchang University. The patients in this study were diagnosed with HCC from July 2002 to December 2012 based on pathological sections obtained by surgical resection or biopsy. Examine of computed tomography (CT) and magnetic resonance imaging (MRI) were used to determine whether OM occurred in patients with HCC. Exclusion criteria included patients with secondary HCC, primary ocular malignancy and benign ocular tumors.
Patient and Public Involvement Statement
The patients were not involved.
Data Collection
We collected clinical data, including age, gender, treatment, pathological type, and the presence time of OM, from clinical medical records of patients with HCC. Additionally, the levels of several tumor markers, including blood calcium concentration, hemoglobin (Hb), alkaline phosphatase (ALP), ferritin (FER), carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), and cytokeratin fragment 19 (CYFRA21-1), were assessed by analyzing serum from patients. The levels of CA-125, CA-153, CA-199, CA724, serum lipids (eg, total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol), Lp (a), ApoA1, and ApoB were also assessed.
ROC Curve
Receiver operating characteristic (ROC) curves was used to assess the diagnostic value of the abnormalities (abnormal ReHo values) detected by ReHo method in specific brain regions. The brain region or ReHo method has diagnostic significance when the related area under the ROC curve (AUC) is greater than 0.5.
Hematoxylin-Eosin (HE) and Immunohistochemistry (IHC) Staining
Hematoxylin-eosin (H&E) staining of eyelids and Hepatocyte IHC of eyeballs were performed in the 4-um-thick sections of HCC samples.
Statistical Analyses
We performed the t-test and chi-squared test to compare age and gender characteristics to avoid affecting the experimental results. Differences in the levels of tumor markers between the OM group and group with other metastases were also analyzed by the independent t-test. The binary logistic regression model was then applied to identify the independent risk factors for OM. A receiver operating characteristic (ROC) curve was constructed, and the area under the curve (AUC) was calculated. P < 0.05 indicated statistical significance of differences. All statistical analyses were performed using SPSS 21.0 software (SPSS, IBM, USA) and Excel 2010 software. Continuous data are displayed as the means ± standard deviations (SD). The statistical method is similar to the related article we reported earlier.14
Result
Demographics and Clinical Characteristics
1064 primary HCC patients were enrolled in this study, including 20 OM cases and 1044 NOM cases. The mean ages of OM patients and NOM patients were 51.78±13.95 and 51.92±13.65 years, respectively. The most common histopathological types were HCC and cholangiocarcinoma. According to the chi-squared test and the non-parametric summary test, there was no significant difference in gender, age, and location of other metastases between the OM group and the NOM group (P > 0.05) (Table 1, Figure 1).
 |
Table 1 The Clinical Characteristics of HCC Patients |
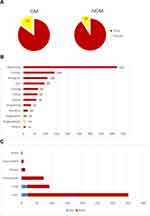 |
Figure 1 The demographic characteristics of the included cases (A and B) and different types of cancer with ocular metastases (C). |
Differences in Clinical Features and Risk Factors Between Patients with and without Ocular Metastasis
By comparing the clinical data of OM and NOM patients, we found that CA-125 was significantly elevated and Hb concentration was decreased in the OM patients compared with that in the NOM group (P < 0.05). There were no significant differences in serum levels of CEA, CA-724, CA-199, CA-153, FER, ALP, TC, TG, HDL, LDL, ApoA1, ApoB, Lp (a), or serum calcium (P > 0.05; Table 2) between the two groups. The results of the binary logistic regression modeling showed that the high levels of CA125 and Hb could be used as independent risk factors for the prediction of HCC metastasis (Tables 2 and 3).
 |
Table 2 Differences in Tumor Markers Between HCC Patients with and without OM |
 |
Table 3 Risk Factors for OM in HCC Patients |
CA-125 and Hb as Diagnostic Markers
Figure 2 shows CA-125 and Hb as single factors in the ROC curve and as a combination for predicting OM in HCC patients. Table 4 shows that their cutoff values were 115.78 u/mL and 120.50 g/L, respectively. The AUC of CA-125 had higher accuracy, and its sensitivity in our study was as high as 90%, making it the best diagnostic marker of OM. The specificity of Hb detection for OM was low, at 53.5%. After the combined analysis of CA-125 with Hb data, CA-125 and Hb were found to have high sensitivity and relatively low specificity, and the AUC was 0.431. All results were statistically significant (P < 0.05; Figure 2, Table 4). And some eye examination results of a HCC patients with OM (Figure 3). In addition, Hematoxylin-eosin (HE) staining and Immunohistochemical staining (IHC) are shown in Figure 4.
 |
Table 4 The Cutoff Value, Sensitivity, Specificity, and AUC for Individual Risk Factors in Predicting OM in HCC Patients |
 |
Figure 2 ROC curve shows CA-125 and Hb as single factors and as a combination for predicting OM in HCC patients. |
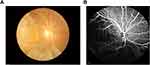 |
Figure 3 Fundus camera image of HCC patients. (A) Retinal fundus photography. (B) Fundus fluorescence angiography. |
 |
Figure 4 Pathological images of HCC patients. (A) Hematoxylin-eosin (HE) staining. (B) Hepatocyte IHC staining. |
Discussion
At present, many studies have reported many cases of OM of clinical malignant tumors (Table 5). For various types of malignant tumors, OM often indicates a poor prognosis, and patients will suffer vision decline, ocular pain and a series of other problems that seriously affect the quality of life of patients.
 |
Table 5 Studies on the OM from Different Cancers |
Metastatic HCC, also known as secondary HCC, occurs as a result of advanced malignant tumors. HCC is more prone to metastasis than other types of HCC. Studies have shown that the incidence of metastatic HCC in China is 1.2-fold higher than that of primary HCC, while the ratio in Europe and the United States is 20-fold higher than that of primary HCC. Treatment efficacy in metastatic HCC directly improves the quality of life and survival of patients; however, the treatment of this cancer is very challenging. With the development of tumor diagnosis and treatment technology though, increasingly more clinicians are facing the troublesome treatment of metastatic HCC. Although there are many treatment methods for metastatic HCC, the prognosis of this disease is currently not satisfactory. Generally, patients will die within one year after diagnosis of liver metastasis, and patients with multiple liver metastases are more likely to die within two to three years. The removal of as much of the primary lesion as possible and adoption of comprehensive surgery-based treatment may enable the disease to be alleviated to the maximum extent, the quality of life of patients be improved, and the survival time to be prolonged. Studies have shown that tumor size, degree of cell differentiation, whether the tumor breaks through the capsule, whether it is accompanied by peripheral micro-metastasis, and whether there is portal vein tumor thrombus are significantly correlated with the metastasis and recurrence of HCC. Wayne et al24 found that regardless of the presence of liver cirrhosis, large tumor size and portal vein tumor thrombus were the main factors associated with recurrence after HCC surgery. The analysis of hematogenous metastasis in the context of HCC metastasis and identification of the most frequent sites of metastasis are crucial for early clinical diagnosis of this disease as well as to guide the choice of clinical treatment strategies and aid prognosis. Studies have shown that men are more likely to develop HCC than women. Some studies reported that the rate of intravascular metastasis in male HCC patients was significantly higher than that in female patients, indicating that the former is prone to intravascular metastasis. Furthermore, the rate of intravascular metastasis among elderly patients was only 25.00%, which is significantly lower than that among middle-aged and young patients, indicating that the latter are more likely to have intravascular metastasis and miss the opportunity for surgery.
Molecular biology shows that HCC has two ways of hematogenous metastasis due to the anatomical characteristics of double blood supply, together with the portal vein collection of abdominal most organs of the blood reflux and the effect of liver itself filtration, resulting in liver metastatic tumors. As cancer metastasis involves multiple genes and mechanisms and comprises different phenotypes (such as hematopoietic metastasis and lymphatic metastasis), it is difficult to identify a single related gene that is linked to this process. Recent data suggest that many molecular changes associated with HCC metastasis, such as various serological factors, are common to solid tumors. It is possible to identify a set of related genes that may be used to make a clinically valuable prediction of a certain phenotype of HCC that may additionally serve as a preventive target. If predictors of HCC metastasis can be identified, it may be possible to provide effective intervention, in a timely manner, to patients prone to postoperative metastasis to reduce the risk of metastasis. At present, many studies have analyzed the risk factors of OM such as breast cancer, lung cancer and colorectal cancer (Table 6), and there are many molecular markers related to HCC metastasis have been found, which are expected to be useful indicators for predicting postoperative recurrence and metastasis of HCC, while such related study about HCC with OM has not been reported. Our study found that CA-125+Hb has great predictive value for the diagnosis of HCC complicated with OM, which will contribute to the further evaluation of future clinical trials, and provide a basis for further research to improve the accuracy of CA-125+Hb in OM diagnosis of HCC, and contribute to the clinical application of this biomarker.
 |
Table 6 Studies on the Risk Factors of OM from Different Cancers |
In Table 7, we list various studies on HCC metastasis demonstrating that the detection of tumor markers in the serum of patients is a reliable method for diagnosing and preventing HCC metastasis. In the traditional serological test, alpha fetoprotein (AFP) is used. As the oldest and most widely used serological marker, the limitations of AFP have been increasingly identified. Approximately 1/3 of patients are negative for AFP,38 which limits its application in the early diagnosis of HCC and indicates the need for the development of other markers.
 |
Table 7 The Risk Factors for Metastases of Primary HCC |
In this study, we analyzed the relevance between OM and HCC. We collected serum samples from patients with HCC and evaluated the change of each index. We measured the concentration of CA-125 and Hb in serum from a large number of patients as independent risk factors for patient with OM from HCC (P < 0.001, P < 0.05). We also use ROC curve to analyze oriented biomarkers. Consequently, we concluded that CA-125 and Hb are significant risk factors for patient with OM from HCC. The result of ROC curves accurately reflected the relationship between the specificity and sensitivity of the analysis method, and is the comprehensive representative of the test accuracy, using the ROC curves of these biomarkers, reliable clinical trials can be developed. Blood CA-125 levels of higher than the critical value of 115.78 U/mL and blood Hb levels higher than the critical value of 120.50 g/L indicate a higher risk of OM in patients with HCC. Accordingly, the AUC for CA-125 is the highest based on detailed diagnostic techniques (eg, CT and MRI of the eye); therefore, this marker shows high accuracy in distinguishing OM patients from elderly lung cancer patients. To improve the accuracy of diagnosing HCC with OM, we combined the detection of CA-125 and Hb and found that the combination of CA-125+Hb showed relatively high sensitivity and specificity, and may therefore be more accurate for the prediction of OM in HCC.
There are some limitations in our study, as for the study was conducted using only data collected at our hospital, which may not represent the entire OM patient population. Further research that includes a larger sample size and multi-center study are needed to improve the rigor and credibility of these risk factors in predicting OM in elderly lung cancer patients. The metastatic stage of HCC is usually fatal.39 Most of the patients in this study were not classified by tumor type; therefore, we could not determine the risk factors for OM based on tumor stage. However, there are few studies in this area at present, and our study fills this gap. In the future, we can collect a large number of samples from multiple clinical centers for further understanding.
Strengths and Limitations of This Study
Our results provide a powerful medical basis of testing targets for assessing the presence of OM in HCC patients.
The study was conducted using only data collected at our hospital, which may not represent the entire OM patient population. Most of the patients in this study were not classified by tumor type; therefore, we could not determine the risk factors for OM based on tumor stage.
Conclusion
In summary, the incidence of OM in the HCC patients in our study was 1.88%. The most common histopathological subtype in the present OM patients was HCC, followed by cholangiocarcinoma. Serum concentrations of CA-125 and Hb were determined to be independent risk factors for patient with OM from HCC. We also found that the combination of CA-125+Hb has great predictive value and should be further assessed in future clinical trials for the diagnosis of HCC with OM. Further in-depth studies are needed to improve the accuracy of CA-125+Hb in the diagnosis of HCC with OM to enable clinical application of this biomarker.
Data Sharing Statement
No additional data available.
Ethical Approval
All research methods were approved by the committee of the medical ethics of the First Affiliated Hospital of Nanchang University and were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All subjects were explained the purpose, method, potential risks and signed an informed consent form.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
National Natural Science Foundation (No. 82160195); Jiangxi Province Double Thousand Plan Science and Technology Innovation High-end Talent Project (2022); Major (Key) R&D Program of Jiangxi Province (No. 2022103; No.20181 bbg70004; No.20203BBG73059); Excellent Talents Development Project of Jiangxi Province (No. 20192BCBL23020); Natural Science Foundation of Fujian (No. 2022J05297).
Disclosure
Xin Xiong, Rong Rong, and Li-Ying Tang are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Kim JU, Shariff MIF, Crossey MME, et al. Hepatocellular carcinoma: review of disease and tumor biomarkers. World J Hepatol. 2016;8(10):471. doi:10.4254/wjh.v8.i10.471
3. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
4. Ding XX, Zhu QG, Zhang SM, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715–55730. doi:10.18632/oncotarget
5. Mabert K, Cojoc M, Peitzsch C, et al. Cancer biomarker discovery: current status and future perspectives. Int J Radiat Biol. 2014;90(8):659–677. doi:10.3109/09553002.2014.892229
6. Ma L, Xie XW, Wang HY, et al. Clinical evaluation of tumor markers for diagnosis in patients with non-small cell lung cancer in China. Asian Pac J Cancer Prev. 2015;16(12):4891. doi:10.7314/apjcp.2015.16.12.4891
7. Susana C, Nunez I, Longo M, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced Non–Small-Cell Lung Cancer (NSCLC). Clin Lung Cancer. 2011;12(3):172–179. doi:10.1016/j.cllc.2011.03.019
8. McKeown E, Nelson DW, Johnson EK, et al. Current approaches and challenges for monitoring treatment response in colon and rectal cancer. J Cancer. 2014;5(1):31–43. doi:10.7150/jca.7987
9. Michelson JB, Felberg NT, Shields JA. Carcinoembryonic antigen. Its role in the evaluation of intraocular malignant tumors. Arch Ophthalmol. 1976;94(3):414–416. doi:10.1001/archopht.1976.03910030200005
10. Chandler PD, Song Y, Lin J, et al. Lipid biomarkers and long-term risk of cancer in the women’s health study. Am J Clin Nutr. 2016;103(6):1397–1407. doi:10.3945/ajcn.115.124321
11. Zamanian-Daryoush M, Lindner D, Tallant TC, et al. The cardioprotective protein apolipoprotein A1 promotes potent anti-tumorigenic effects. J Biol Chem. 2013;288(29):21237–21252. doi:10.1074/jbc
12. Ma XL, Gao XH, Gong ZJ, et al. Apolipoprotein A1: a novel serum biomarker for predicting the prognosis of hepatocellular carcinoma after curative resection. Oncotarget. 2016;7(43):70654–70668. doi:10.18632/oncotarget.12203
13. Sirniö P, Väyrynen JP, Klintrup K, et al. Decreased serum apolipoprotein A1 levels are associated with poor survival and systemic inflammatory response in colorectal cancer. Sci Rep. 2017;7(1):1–8. doi:10.1038/s41598-016-0028-x
14. Lin Q, Chen XY, Liu WF, et al. Diagnostic value of CA‐153 and CYFRA 21‐1 in predicting intraocular metastasis in patients with metastatic lung cancer. Cancer Med. 2019;9:1279–1286. doi:10.1002/cam4.2354
15. Tei M, Wakasugi M, Akamatsu H. Choroidal metastasis from early rectal cancer: case report and literature review. Int J Surg Case Rep. 2014;5(12):1278–1281. doi:10.1016/j.ijscr.2014.10.059
16. Shah SU, Mashayekhi A, Shields CL, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352–357. doi:10.1016/j.ophtha.2013.07.014
17. Hazan A, Katz MS, Leder H, et al. Choroidal metastases of choriocarcinoma. Retin Cases Brief Rep. 2014;8(2):95–96. doi:10.1097/ICB.0000000000000012
18. Cristina NG, Francisco ED, Vanesa RG, et al. Choroidal metastasis from primary bone leiomyosarcoma. Int Ophthalmol. 2015;35(5):721–725. doi:10.1007/s10792-015-0096-0
19. Mercado CL, Toy BC, Kistler HB, et al. Choroidal metastases from cutaneous melanoma. Ophthalmic Surg Lasers Imaging Retina. 2016;47(5):497. doi:10.3928/23258160-20160419-17
20. Fountas A, Tigas S, Giotaki Z, et al. Choroidal metastasis from papillary thyroid cancer: an unusual feature of a common disease. Ann Endocrinol. 2017;78(1):64–66. doi:10.1016/j.ando.2016.09.001
21. Essadi I, Lalya I, Kriet M, et al. Successful management of retinal metastasis from renal cancer with everolimus in a monophthalmic patient: a case report. J Med Case Rep. 2017;11(1):340. doi:10.1186/s13256-017-1501-2
22. Levison AL, Erenler F, Zhao Y, et al. Late-onset choroidal metastasis from breast cancer. Retin Cases Brief Rep. 2018;12(4):342–345. doi:10.1097/ICB.0000000000000516
23. Chang SY, Tsai SH, Chen LJ, et al. Choroidal metastasis from esophageal squamous cell carcinoma. Taiwan J Ophthalmol. 2018;8(2):104–107. doi:10.4103/tjo.tjo_80_17
24. Wayne JD, Lauwers GY, Ikai L, et al. Preoperative predictors of survival after resection of small hepatocellular carcinomas[J]. Ann Surg. 2002;235(5):722–731. doi:10.1097/00000658-200205000-00015
25. Liu J, Yuan Q, Min Y, et al. Apolipoprotein A1 and B as risk factors for development of intraocular metastasis in patients with breast cancer. Cancer Manag Res. 2019;11:2881–2888. doi:10.2147/CMAR.S191352
26. Zhu P, Gong Y, Min Y, et al. The predictive value of high-density lipoprotein for ocular metastases in colorectal cancer patients. Cancer Manag Res. 2019;11:3511–3519. doi:10.2147/CMAR.S194637
27. Min Y, Gong Y, Zhu P, et al. CEA as a risk factor in predicting ocular metastasis from colorectal cancer. J Cancer. 2020;11(1):51–56. doi:10.7150/jca.31196
28. Lin SC, Shih SC, Kao CR, et al. Transcatheter arterial embolization treatment in patients with hepatocellular carcinoma and risk of pulmonary metastasis. World J Gastroenterol. 2003;9(6):1208–1211. doi:10.3748/wjg.v9.i6.1208
29. Ogawa M, Yamamoto T, Kubo S, et al. Clinicopathologic analysis of risk factors for distant metastasis of hepatocellular carcinoma. Hepatol Res. 2004;29(4):228–234. doi:10.1016/j.hepres.2004.04.002
30. Xiang ZL, Zeng ZC, Tang ZY, et al. Chemokine receptor CXCR4 expression in hepatocellular carcinoma patients increases the risk of bone metastases and poor survival. BMC Cancer. 2009;9:176. doi:10.1186/1471-2407-9-176
31. Lin ZZ, Jeng YM, Hu FC, et al. Significance of Aurora B overexpression in hepatocellular carcinoma. Aurora B Overexpression in HCC. BMC Cancer. 2010;10:461. doi:10.1186/1471-2407-10-461
32. Hsiao SY, Chen SF, Chang CC, et al. Central nervous system involvement in hepatocellular carcinoma: clinical characteristics and comparison of intracranial and spinal metastatic groups. J Clin Neurosci. 2011;18(3):364–368. doi:10.1016/j.jocn.2010.04.037
33. Chua TC, Morris DL. Exploring the role of resection of extrahepatic metastases from hepatocellular carcinoma. Surg Oncol. 2012;21(2):95–101. doi:10.1016/j.suronc.2011.01.005
34. Morimoto Y, Nouso K, Wada N, et al. Involvement of platelets in extrahepatic metastasis of hepatocellular carcinoma. Hepatol Res. 2014;44(14):353–359. doi:10.1111/hepr.12315
35. Wan P, Zhang J, Long X, et al. Serum levels of preoperativeα-fetoprotein and CA19-9 predict survival of hepatic carcinoma patients after liver transplantation. Eur J Gastroenterol Hepatol. 2014;26(5):553–561. doi:10.1097/MEG.0000000000000070
36. Chen Y, Gao SG, Chen JM, et al. Risk factors for the long-term efficacy, recurrence, and metastasis in small hepatocellular carcinomas. Cell Biochem Biophys. 2015;72(2):627–631. doi:10.1007/s12013-015-0514-y
37. Lee CH, Chang CJ, Lin YJ, et al. Nomogram predicting extrahepatic metastasis of hepatocellular carcinoma based on commonly available clinical data. JGH Open. 2018;3(1):38–45. doi:10.1002/jgh3.12102
38. Egeblad M, de Visser KE. Sticking together helps cancer to spread. Nature. 2019;566(7745):459–460. doi:10.1038/d41586-019-00341-4
39. Matsuda M, Asakawa M, Amemiya H, et al. Lens culinaris agglutinin-reactive fraction of AFP is a useful prognostic biomarker for survival after repeat hepatic resection for HCC. J Gastroenterol Hepatol. 2011;26(4):731–738. doi:10.1111/j.1440-1746.2010.06532.x
40. Rizzo A, Brandi G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat Res Commun. 2021;27:100328. doi:10.1016/j.ctarc.2021.100328
41. De Lorenzo S, Tovoli F, Barbera MA, et al. Metronomic capecitabine vs. best supportive care in Child-Pugh B hepatocellular carcinoma: a proof of concept. Sci Rep. 2018;8(1):9997. doi:10.1038/s41598-018-28337-6
42. Rizzo A, Ricci AD. PD-L1, TMB, and other potential predictors of response to immunotherapy for hepatocellular carcinoma: how can they assist drug clinical trials? Expert Opin Investig Drugs. 2022;31(4):415–423. doi:10.1080/13543784.2021.1972969
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
