Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Development and Validation of a Nomogram to Predict the Recurrence of HCC Patients Undergoing CECT After Ablation
Authors Qiao W , Fan Z, Wang Q, Jin R, Hu C
Received 22 September 2023
Accepted for publication 9 January 2024
Published 13 January 2024 Volume 2024:11 Pages 65—79
DOI https://doi.org/10.2147/JHC.S441540
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Manal Hassan
Wenying Qiao,1– 3,* Zibo Fan,2,* Qi Wang,2,* Ronghua Jin,2,3 Caixia Hu1
1Interventional Therapy Center for Oncology, Beijing Youan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Changping Laboratory, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Caixia Hu
Interventional Therapy Center for Oncology; Beijing You’an Hospital, Capital Medical University, 8 Xitoutiao, Youanmenwai Street, Fengtai District, Beijing, People’s Republic of China
, Tel/Fax +86-13811395702
, Email [email protected]
Ronghua Jin
Beijing Ditan Hospital, Capital Medical University, 8 Jingshun East Street, Chaoyang District, Beijing, People’s Republic of China
, Tel/Fax +86-13811611118
, Email [email protected]
Purpose: We first aimed to compare the prognostic difference between the application of Contrast-enhanced computed tomography (CECT) and Non-enhanced computed tomography (NECT) in hepatocellular carcinoma(HCC) patients with early-stage immediately after ablation. We secondly propose to explore the risk factors for recurrence in patients undergoing CECT, and then develop a nomogram.
Patients and Methods: Clinical data were collected from 711 patients who received TACE combined with ablation from January 1, 2015, to December 31, 2022, at Beijing Youan Hospital. According to the imaging methods applied after ablation, patients were categorized into the CECT group and the NECT group and then were compared by Kaplan-Meier (KM) curves. Lasso regression is used to screen risk factors for recurrence and the nomogram was plotted. Finally, discrimination, calibration plot, and decision curve analysis (DCA) were used to measure the performance of the nomogram.
Results: The KM curve indicates that recurrence-free survival (RFS) was longer in the CECT group than in the NECT group (HR =0.759, 95% CI 0.606– 0.951, P=0.016). Six variables were selected to construct the nomogram. 1-, 3-, and 5-year area under the curves (AUCs) (0.867, 0.731, 0.773 and 0.896, 0.784, 0.773) of the training and validation cohorts proved the good predictive performance of the nomogram. Calibration curves and DCA curves suggested accuracy and net clinical benefit rates. The nomogram enabled to classify of patients into three groups according to the risk of recurrence: low risk, intermediate risk, and high risk. There was a statistically significant difference in RFS between the two groups in the training and validation cohorts (P< 0.001).
Conclusion: We demonstrated that HCC patients who underwent CECT evaluation after ablation had a better prognosis, making this evaluation method highly recommended for guiding clinical management.
Keywords: hepatocellular carcinoma, transcatheter arterial chemoembolization, contrast-enhanced computed tomography, recurrence-free survival, risk factors
Introduction
Primary liver cancer (PLC) has emerged as a major public health threat, with increasing prevalence globally.1 It is estimated that more than one million patients will be diagnosed with PLC annually by 2025.2 Hepatocellular carcinoma (HCC) accounts for more than 90% of PLC, causing about 800, 000 deaths every year and ranking as the third leading cause of cancer death worldwide.3 HCC patients are treated based on tumor burden, liver function, and performance status.4 Liver transplantation, surgical resection, and ablation are first-line therapies for HCC patients at an early stage, whose survival has improved because of advances in these therapeutic modalities.5,6 For its advantages of high safety profile, low cost, and minimal invasiveness, local ablation, including radiofrequency ablation (RFA), microwave ablation (MRA), cryoablation, irreversible electroporation (IRE) plays a large role in the treatment of HCC.7,8 However, over the past decade, ablation therapy has been facing a great challenge of recurrence, which is the main factor influencing the prognosis of HCC.9–11 Therefore, it is crucial to establish a prediction model to improve the prognosis of patients more effectively.
Through diverse forms of energy and physical mechanisms, ablation could directly kill tumor tissue.12,13 Imaging is performed about one month after the procedure to determine the extent and status of the ablation.14 Nevertheless, tumors may regrow in the month or so prior to post-ablation. In addition, complications, such as hepatic subcellular hemorrhage and perforation caused by liver tumors invading the cavities may occur, which greatly increase the clinical risk and lethality of the patients.15 Consequently, relevant imaging examinations should be applied immediately after ablation to detect the ablation site and to further improve the outcomes. Nonetheless, there is still no consensus in clinical practice on which imaging methods we should apply. A few studies have explored the use of certain imaging methods to reduce recurrence rates after ablation. Recently, a meta-analysis suggested that Ultrasound elastography of the liver stimulates the tissue with ultrasound and extracts parameters related to the elasticity of the tissue in order to display diagnostic information in the image, which could greatly boost prediction performance in the recurrence of HCC after ablation.16 Few studies have researched the clinical value of postoperative application of Contrast-enhanced computed tomography (CECT) or Non-enhanced CT (NECT).
Thus, the aim of this study was to compare the prognostic difference of HCC applying CT or CECT immediately after ablation and build a prognostic model by integrating multiple factors to better manage recurrence after ablation as well as improve the prognosis.
Materials and Methods
Patients
All patients were enrolled from Beijing Youan Hospital, Capital Medical University. A total of 1298 HCC patients diagnosed in 2015–2022 were screened and 711 eligible patients were ultimately enrolled in this study (Figure S1). HCC was diagnosed according to the American Association for the Study of Liver Diseases (AASLD) HCC practice guidelines.17 All patients underwent transcatheter arterial chemoembolization (TACE) combined with ablation and achieved complete ablative.
The inclusion criteria were the following. (1) Patients with Barcelona Clinic Liver Cancer stage 0, A, or B; (2) Patients with no history of other malignancies besides an HCC diagnosis; (3) Patients without extrahepatic metastases; (4) Patients with complete clinical or follow-up data; (5) Patients who have received TACE combined with locoregional ablation.
The subjects fulfilling any of the exclusion criteria listed below were excluded. (1) Aged<18 years or >75 years; (2) Secondary liver cancer; (3) Child-Pugh class C group; (4) Patients with a previous history of malignancy; (6) Patients with severe heart, lung, kidney, and brain dysfunction, as well as coagulopathy and intolerance.
Due to the retrospective nature of this study, the requirement for informed consent was waived. This study was approved by the Ethical Review Committee of Beijing Youan Hospital, Capital Medical University, and conducted under the guidance of the Declaration of Helsinki.
Clinicopathological Data Collection
Demographic and clinicopathological data were collected from all the patients. Demographic data included age, gender, smoking, drinking, history of hypertension, and history of diabetes. Clinicopathological data included following: (1) Tumor information: tumor number, tumor size, and alpha-fetoprotein(AFP); (2) Liver function indexes: alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), Gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), Child-Pugh classification, and liver cirrhosis defined based on clinical manifestations, encompassing a history indicative of Chronic Liver Disease (CLD) along with documented complications of CLD (such as ascites, varices, hepatic encephalopathy), along with imaging studies indicating cirrhotic changes and/or histological evidence confirming cirrhosis in liver tissue;18 (3) Laboratory parameters: neutrophils (Neu), platelets (PLT), lymphocytes (Lym) and others; (4) Etiology of liver disease: hepatitis B virus(HBV), hepatitis C virus(HCV) and alcohol; (5) therapy-related factors: ablation modality.
Treatment Procedures
All included patients received TACE combined with locoregional ablation. In particular, for HBV-associated HCC or HCV-associated HCC, antiviral therapy is administered for at least two weeks prior to the intervention. TACE was performed only after the viral load had turned negative by two experienced interventional radiologists. After local disinfection and anesthesia, percutaneous right femoral artery puncture intubation with a modified Seldinger technique was performed, and the left femoral artery or the left brachial artery was punctured, if necessary. A 5-F catheter was introduced, and angiography was conducted to assess the tumor number, size, localization, and tumor-feeding arteries. Afterward, a microcatheter was inserted into the blood-supply artery of the carcinoma to inject a mixture of doxorubicin (Pfizer Inc., New York, NY, USA) and lipiodol (Guerbet, Villepinte, France), followed by embolization using embolic materials, such as gel foam or polyvinyl alcohol particles. The dose of the drug is based on the patient’s white blood cell count, platelet count, and liver function. Finally, Angiography shows occlusion of intratumoral vessels, filling of embolic agents, and loss of tumor staining, which is considered to be the endpoint of embolization.
Local ablation by a qualified hepatologist was carried out until two weeks after TACE, guided by Computed Tomography (CT) or magnetic resonance imaging (MRI). The specific procedures are summarized as follows: (1) The appropriate location was selected by CT or MRI to determine the ablation procedure; (2) Routine disinfection and anesthesia were applied around the puncture points; (3) The ablation needle was inserted into the skin and multiple overlapping ablations should be considered depending on the tumor size and a number of tumors, followed by timely image scanning to track the ablation process; (4) Blood pressure, pulse, respiratory rate, and oxygen saturation were monitored during the procedure; (5) After ablation, the needle track was ablated to prevent postoperative bleeding and tumor implantation along the needle track; (6) CECT or CT was performed immediately following the ablation procedure in order to assess both therapeutic responses and possible complications. For the purpose of ensuring the effect of ablation therapy, the safe ablation range of 0.5–1.0cm should be reserved to ensure complete coverage of the tumor. All patients will undergo CT or CECT immediately after ablation to assess treatment efficacy.
Follow Up
Follow-up was conducted for all the patients via telephone or in the Outpatient Department. The patients were subjected to follow-ups every 3 to 6 months, which comprised liver ultrasound, laboratory tests (including blood routine, biochemical function, AFP, coagulation tests, and other indicators), and physical examinations. Magnetic resonance imaging (MRI) or CECT scans were routinely performed for every 6 months.
The trial’s primary endpoint was recurrence-free survival (RFS) which was defined from the date of curative treatment till the date of relapse or death from any cause.
Statistical Analysis and Nomogram Development
Continuous variables were expressed as mean and standard deviation (mean ± SD) and compared between two groups using an independent-sample t-test. For non-normal distribution, non-parametric tests were used. Categorical variables were reported as frequencies and percentages and were contrasted using a chi-squared test. For the prognostic assessment of the two groups of patients, the RFS curve was generated by the Kaplan-Meier (KM) method to predict median RFS (mRFS), and the log-rank method was used for comparison. In order to build a reliable and robust model, patients undergoing CECT examination after ablation were randomly split into the training set (N= 227) and validation set (N = 227) in the ratio of 1:1, and demographic characteristics, laboratory data, and prognosis were compared between the two groups of patients.
The least absolute shrinkage and selection operator (LASSO) regression was used to screen the risk factors, and then variables were included to generate nomograms estimating 1, 3-, and 5-year RFS. Patient recurrence risk scores were calculated based on a nomogram. The risk grouping of patients was done according to the total score categorized into low, intermediate, and high-risk groups. The KM method was applied to plot RFS curves for different risk groups. We tested the model differentiation by the Area Under the Curve (AUC) curve. Calibration was assessed using calibration curves. Decision curve analysis (DCA) was implemented to evaluate the clinical usefulness by quantifying the net benefits of the nomogram model in both the training and validation cohorts.
Statistical and Analysis Software Analyses were conducted using the R software [v.4.1.2]. Statistical significance was set to p<0.05 (two-tailed).
Results
Characteristics of Patients
A total of 711 HCC patients at an early stage who received TACE combined locoregional ablation in our hospital from January 1, 2015, to December 31, 2022, were enrolled. The patients enrolled in this study were all patients with Nodular-type hepatocellular carcinoma. According to the imaging methods applied after ablation, they were categorized into the CECT group (454 patients) or the NECT (257 patients) group.
The baseline patient characteristics were similar between the two groups (Table 1). In the two cohorts, a majority of patients were male (NECT cohort, 82.5%; CECT cohort, 80.2%). The average age was 57.56 years (standard deviation, 8.524 years) in the NECT cohort and 57.22 years (standard deviation, 8.693 years) in the CECT cohort. Most patients suffer from cirrhosis (NECT cohort, 85.6%; CECT cohort, 84%). About characteristics of the tumor, tumor size is less than 3 cm (NECT cohort, 63.8%; CECT cohort, 66.5%) and more than half of the patients had solitary tumors (NECT cohort, 65.8%; CECT cohort, 71.1%). A vast majority of the patients were Child-Pugh A (NECT cohort, 72.4%; CECT cohort, 71.4%), which indicates good liver function. The total number of patients in BCLC stages 0 and A was 211 in the NECT cohort and 382 in the CECT cohort.
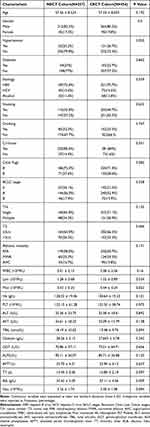 |
Table 1 Baseline Characteristics of the Patients |
Prognostic Differences Between HCC Patients in the NECT and CECT Groups
Up to the last follow-up date of January 1, 2023, the median follow-up time was 4.5 years (95% CI 3.7–5.2 years). A time-dependent recurrence curve was plotted based on the K-M method (Figure 1), showing that the median RFS was 2.95 years (95% CI 1.69–4.21) and 4.5 years (95% CI 3.76–5.24) in the NECT cohort and CECT cohort. The differences in RFS between these two groups were statistically significant (HR =0.759, 95% CI 0.606–0.951, P=0.016).
Factors Associated with Recurrence
Lasso regression was used to screen parameters, and the variation characteristics of the coefficient of these variables are shown in Figure 2A. The 10-fold cross-validation method was applied to the iterative analysis. When λ was 0.077 (Log λ=−1.113), a model with excellent performance but a minimum number of variables was obtained (Figure 2B).
Nomogram as a Tool for Visualization
A nomogram integrating the independent risk factors described above was constructed (Figure 3). Each selected risk factor was assigned a corresponding score according to its value on the nomogram. The point where the score was located was the origin, and a vertical line was drawn to the “score“ line segment to obtain the score of this factor. With these steps, the scores of the six high-risk factors were calculated, and a total score was calculated by summing the points from these variables. Based on the position of the “total score”, and taking the point where the total score is located at the origin, a vertical line was drawn to the ”early recurrence probability” line segment to obtain the patient’s probability of early recurrence. The higher the score, the higher the probability of recurrence.
Evaluation of Predictive Models
We calculate the area under the AUC. In the training cohort, the AUCs for 1-year, 3-year, and 5-year of their time-dependent ROC curves were 0.867, 0.731, and 0.773, respectively (Figure 4A). The calibration curves for the nomogram-predicted probability of 1-year, 3-year, and 5-year RFS showed great agreement between the actual observation and nomogram prediction (Figure 5A–C). DCA was further used to evaluate the clinical values of this nomogram (Figure 6A–C), which showed encouraging net benefits in reasonable threshold probability with the nomogram.
From the nomogram, patients were categorized into three groups by KM curves: low-risk(N=86), intermediate-risk(N=114), and high-risk groups(N=27) (Figure 7A). There were significant differences among the three groups (P<0.0001).
To further confirm the reliability of the nomogram, we verified it internally. In the validation cohort, AUCs of 1-, 3-, and 5-year were 0.896, 0.784, and 0.773 (Figure 4B). Calibration curves also indicated good calibration of the model in the validation cohorts (Figure 5D–F), and the DCA curves of 1-, 3-, and 5-year had good clinical practicability (Figure 6D–F). Patients were also categorized into low-risk(N=91), intermediate-risk(N=98), and high-risk groups(N=38) (Figure 7B). In agreement with the training cohort, the risk of recurrence was significantly higher in the high-risk group than in the low-risk and intermediate-risk groups(P<0.0001).
Discussion
In line with the established study cohort, we first assessed the prognostic difference between CECT and NECT applied immediately after TACE combined with ablation in HCC patients at an early stage. In our long-term follow-up study, we observed superior postoperative outcomes in patients who underwent postoperative CECT evaluation compared to those who had NECT. Secondly, on the basis of this finding, we screened a cohort of patients undergoing CECT for relevant risk factors affecting postoperative RFS and developed a nomogram, which demonstrates a great degree of discrimination, calibration, and clinical applicability.
In clinical practice, physicians often observe differences in the prognosis among different cancer patients with the same stage of disease, pathological type, and treatment regimen. The treatment of malignant tumors has entered the era of “precision medicine”.19 As new tools for prognosis prediction, novel statistical methods have promoted the rapid development of individualized accurate treatment of tumors. In the context of the advantage of up to 9 years of follow-up, this study randomized patients undergoing CECT in a one-to-one ratio into a training and validation set. In the training set, LASSO regression was used to screen RFS-related variables for establishing the risk model. Lasso regression, a machine learning algorithm, is effective in filtering out the independent variables that have the strongest explanatory power for the dependent variable, while also acting on other insignificant variables. According to this precise screening modality, a nomogram was created to predict the RFS of patients at 1, 3, and 5 years. For further precision, after the nomogram was established, the validation set cohort was utilized for overall validation in three dimensions, namely, differentiation, precision, and clinical applicability. The results showed that two cohort models exhibited powerful predictive performance. It is worth noting that with the nomogram we have developed, patients can be accurately categorized into low-, intermediate-, and high-risk groups of recurrence. Consequently, we can follow patients more effectively during the postoperative follow-up period by targeting their risk factors in order to reduce the risk of disease progression and improve prognosis.
Clinical indicators involved in the nomogram include cirrhosis, GGT, tumor number, BCLC stage, tumor size, and globulin. Hepatic impairment in cirrhotic patients is a major risk factor for HCC recurrence.20 A previous study showed that cirrhosis promotes the accumulation of genetic aberrations and cellular transformations due to an accelerated hepatocyte renewal induced by a chronic inflammatory state. Thus, high rates of genetic errors and subsequent uncontrolled cell proliferation promote the development of HCC.21 A study confirmed that elevated levels of GGT are an independent risk factor for early HCC recurrence after radiofrequency ablation, as the expression of GGT provides tumor cells with an additional source of cysteine and cystine thereby increasing tumor recurrence rates.22,23 A key feature of cancer is tumor size and number (solitary or multiple tumors). Several studies have identified tumor size and number as important predictors of vascular invasion in HCC, which may influence patients’ recurrence after ablation.24–26 In the long-term follow-up we found that higher BCLC stage predicted poorer RFS in HCC patients. BCLC staging system integrates factors such as liver function, tumor extension, and physical status, which can help predict the prognostic status.27 Globulins comprise several proinflammatory proteins, including C-reactive protein, α2-macroglobulin, fibrinogen, thrombospondin, and serum amyloid A.28 Due to the primary metabolism of human immunoglobulins by the liver, individuals with severe hepatic insufficiency may experience a diminished capacity for immunoglobulin clearance, leading to hyperglobulinemia. Inflammation can alter tumor cell biology and disrupt immune function, leading to poor clinical prognosis in patients with malignant tumors.29,30
CECT is a means of improving diagnostic accuracy by focusing on suspicious areas found based on CT scans after intravenous injection of contrast agent. CECT has significant advantages over NECT in the following three situations. First is the incomplete accumulation of iodized oil after TACE. Although HCC is usually a multivessel tumor, some HCC tumors have a low blood supply so the flow of arterial blood into the tumor is low. In addition, non-hepatic artery-fed HCC is a major cause of incomplete accumulation of iodized oils. As a result, image-guided local ablation would not be able to visualize the target lesion based on iodine oil deposition, leading to incomplete ablation. Immediate post-ablation CECT can compensate for the limited iodine oil deposition after TACE treatment, thus allowing better visualization of the ablation effect and whether the ablation site is the preoperatively desired target lesion, or whether contrast enhancement is detected in or around the tumor. If incomplete tumor ablation was detected by CECT, secondary ablation was performed. The second context is that the tumor capsule is usually missing or incomplete. HCCcan invade the surrounding tissues by direct infiltration, which can lead to incomplete tumor capsules that are indistinguishable from the surrounding tissues. Immediately post-ablation CECT can be used to determine the presence of residual lesions by observing the enhancement from the surrounding tissues. The third factor is tumors with a diameter of over 3cm. Because of the large size of the tumor, it is more likely for the doctor to cause residual lesions during the ablation process, resulting in incomplete ablation. Immediate postoperative enhanced CT can observe whether the tumor is residual or not, and further ablation will be performed if there are residual lesions, which can effectively reduce the rate of tumor recurrence in the future.
TACE is an important therapy for unresectable HCC.31 It could mark tumors that are not shown clearly on imaging, degrade the tumor by embolizing tumor blood vessels, reduce ablation times, and increase the success rate of ablation.32 Local ablation is a therapy in which electrodes are inserted into the tumor tissue under the guidance of ultrasound, CT, or MRI, and high-frequency electrodes are applied to the tissue in order to damage and kill HCC cells.33,34 However, either single TACE or local ablation has certain limitations in completely inactivating tumors, especially tumors that have a rich blood supply, making TACE combined with local ablation has become a new treatment mode for HCC. A long-term follow-up cohort study reported that TACE combined with local ablation had significantly better overall survival than local ablation alone. TACE combined with ablation was associated with a 45% reduction in the risk of death and a 34% reduction in the risk of HCC recurrence.35 Previous studies from our team have also shown that the combination of TACE and ablation improves RFS compared to TACE alone.36 Accordingly, we adopted TACE sequential ablation therapy, which can be further confirmed in multiple centers for its efficacy in improving the quality of life and prolonging the RFS of more patients with HCC.
Nevertheless, several limitations exist in the study. First of all, despite a large sample size, the generalization ability of the model established was slightly weak since this study was a retrospective, single-center study. Thus, prospective randomized controlled trials and multicenter clinical applications will be necessary to validate our conclusions. This would allow for a broader application of the nomogram and provide more robust evidence of its predictive accuracy in different clinical settings. Another limitation of this study was that the population receiving TACE combined with locoregional therapy was the subject of our study, and the applicability of this nomogram to patients undergoing surgery and liver transplantation is uncertain. Therefore, more studies are needed to explore the application of our nomogram in patients receiving other treatments. Finally, our study focused solely on tumor recurrence without delving into specific subtypes, such as local recurrence, and intrahepatic distant recurrence. Further data analysis in these areas is crucial to validate our findings.
Conclusion
In conclusion, we demonstrated that HCC patients who underwent CECT evaluation after ablation had a better prognosis, making this evaluation method highly recommended for guiding clinical management. We also successfully developed a straightforward nomogram that predicts long-term RFS in HCC patients with early-stage who apply CECT immediately after ablation. The nomogram demonstrated good predictive ability, which could be instrumental in guiding therapeutic decisions.
Data Sharing Statement
All relevant data are available within the manuscript and its supplementary material files. Further enquiries can be directed to the corresponding author (Caixia Hu, [email protected]).
Ethics Statement
The study protocol was approved by the Ethics Committee of Beijing Youan Hospital and conducted following the ethical principles outlined in the Helsinki Declaration of 1964 and its subsequent amendments, or other ethical standards with equivalent requirements. As a retrospective study as well as to ensure patient confidentiality, the identities of the individuals included in this study were anonymized using computer-generated ID numbers, and thus, patient consent was waived.
Acknowledgments
The authors highly appreciate all patients who participated in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Beijing Bethune Public Welfare Foundation(QZHX-21-ZQN-014).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188314. doi:10.1016/j.bbcan.2019.188314
2. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi:10.1016/S0140-6736(22)01200-4
3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
4. European association for the study of the liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
5. Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181–223. doi:10.1159/000514174
6. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatol. 2018;67(1):358–380. doi:10.1002/hep.29086
7. Izzo F, Granata V, Grassi R, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 2019;24(10):e990–e1005. doi:10.1634/theoncologist.2018-0337
8. Kim JW, Shin SS, Heo SH, et al. Ultrasound-guided percutaneous radiofrequency ablation of liver tumors: how we do it safely and completely. Korean J Radiol. 2015;16(6):1226–1239. doi:10.3348/kjr.2015.16.6.1226
9. Ahmed M, Kumar G, Moussa M, et al. Hepatic radiofrequency ablation-induced stimulation of distant tumor growth is suppressed by c-met inhibition. Radiology. 2016;279(1):103–117. doi:10.1148/radiol.2015150080
10. Ruzzenente A, Manzoni GD, Molfetta M, et al. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10(8):1137–1140. doi:10.3748/wjg.v10.i8.1137
11. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
12. Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res. 2005;127(2):208–223. doi:10.1016/j.jss.2005.02.009
13. Wu D, Yang Y, Chen J, Cai H, Duan Y, Sun D. Three different ways of treating primary hepatocellular carcinoma at an early stage: a prospective comparative study. Gastroenterol Res Pract. 2020;2020:7802498. doi:10.1155/2020/7802498
14. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
15. Jang JY, Kim MY, Jeong SW, et al. Current consensus and guidelines of contrast enhanced ultrasound for the characterization of focal liver lesions. Clinic Molec Hepatol Mar. 2013;19(1):1–16. doi:10.3350/cmh.2013.19.1.1
16. Vestito A, Dajti E, Cortellini F, Montagnani M, Bazzoli F, Zagari RM. Can liver ultrasound elastography predict the risk of hepatocellular carcinoma recurrence after radiofrequency ablation? A systematic review and Meta-Analysis. Ultraschall Med. 2023;44(3):e139–e147. doi:10.1055/a-1657-8825
17. Singal AG, Llovet JM, Yarchoan M, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatol. 2023;78(6):1922–1965. doi:10.1097/HEP.0000000000000466
18. Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398(10308):1359–1376. doi:10.1016/S0140-6736(21)01374-X
19. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526–549. doi:10.1016/j.jhep.2017.09.016
20. Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. 2020;9(4):875. doi:10.3390/cells9040875
21. Jung KS, Kim SU, Choi GH, et al. Prediction of recurrence after curative resection of hepatocellular carcinoma using liver stiffness measurement (fibroscan®). Ann Surg Oncol. 2012;19(13):4278–4286. doi:10.1245/s10434-012-2422-3
22. Yang Y, Xin Y, Ye F, et al. Early recurrence after radiofrequency ablation for hepatocellular carcinoma: a multicenter retrospective study on definition, patterns and risk factors. Int J Hyperthermia. 2021;38(1):437–446. doi:10.1080/02656736.2020.1849828
23. Hanigan MH. Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Adv Cancer Res. 2014;122:103–141. doi:10.1016/b978-0-12-420117-0.00003-7
24. Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis b virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surgery. 2016;151(4):356–363. doi:10.1001/jamasurg.2015.4257
25. Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surgery. 2019;154(3):209–217. doi:10.1001/jamasurg.2018.4334
26. Zhang SW, Zhang NN, Zhu WW, et al. A novel nomogram model to predict the recurrence-free survival and overall survival of hepatocellular carcinoma. Front Oncol. 2022;12:946531. doi:10.3389/fonc.2022.946531
27. M-c H, Hasegawa K, Chen X-P, et al. Surgery for intermediate and advanced hepatocellular carcinoma: a consensus report from the 5th Asia-pacific primary liver cancer expert meeting (APPLE 2014). Liver Cancer. 2016;5(4):245–256. doi:10.1159/000449336
28. Deng Y, Pang Q, Miao R-C, et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther. 2016;9:5317–5328. doi:10.2147/OTT.S109736
29. Wang S, Deng Y, Yu X, et al. Prognostic significance of preoperative systemic inflammatory biomarkers in patients with hepatocellular carcinoma after microwave ablation and establishment of a nomogram. Sci Rep. 2021;11(1):13814. doi:10.1038/s41598-021-93289-3
30. Li J, Li Z, Hao S, et al. Inversed albumin-to-globulin ratio and underlying liver disease severity as a prognostic factor for survival in hepatocellular carcinoma patients undergoing transarterial chemoembolization. Diagn Interv Radiol. 2023;29(3):520–528. doi:10.5152/dir.2022.211166
31. Ikeda M, Arai Y, Inaba Y, et al. Conventional or drug-eluting beads? Randomized controlled study of chemoembolization for hepatocellular carcinoma: JIVROSG-1302. Liver Cancer. 2022;11(5):440–450. doi:10.1159/000525500
32. Raoul J-L, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi:10.1016/j.ctrv.2018.11.002
33. Deng M, Li S-H, Guo R-P. Recent advances in local thermal ablation therapy for hepatocellular carcinoma. Am Surg. 2023;89(5):1966–1973. doi:10.1177/00031348211054532
34. Kloeckner R, Galle PR, Bruix J. Local and regional therapies for hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):137–149. doi:10.1002/hep.31424
35. Zhang YJ, Chen MS, Chen Y, Lau WY, Peng Z. Long-term outcomes of transcatheter arterial chemoembolization combined with radiofrequency ablation as an initial treatment for early-stage hepatocellular carcinoma. JAMA network open. 2021;4(9):e2126992. doi:10.1001/jamanetworkopen.2021.26992
36. Wang Q, Qiao W, Zhang H, et al. Nomogram established on account of lasso-cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol. 2022;13:1019638. doi:10.3389/fimmu.2022.1019638
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







